Thymus vulgaris Essential Oil and Hydro-Alcoholic Solutions to Counteract Wooden Artwork Microbial Colonization
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Wooden Artwork
2.2. Microbial Taxa Identification
2.3. In Vitro Antimicrobial Activity Assays
2.4. T. vulgaris EO and HA Solutions
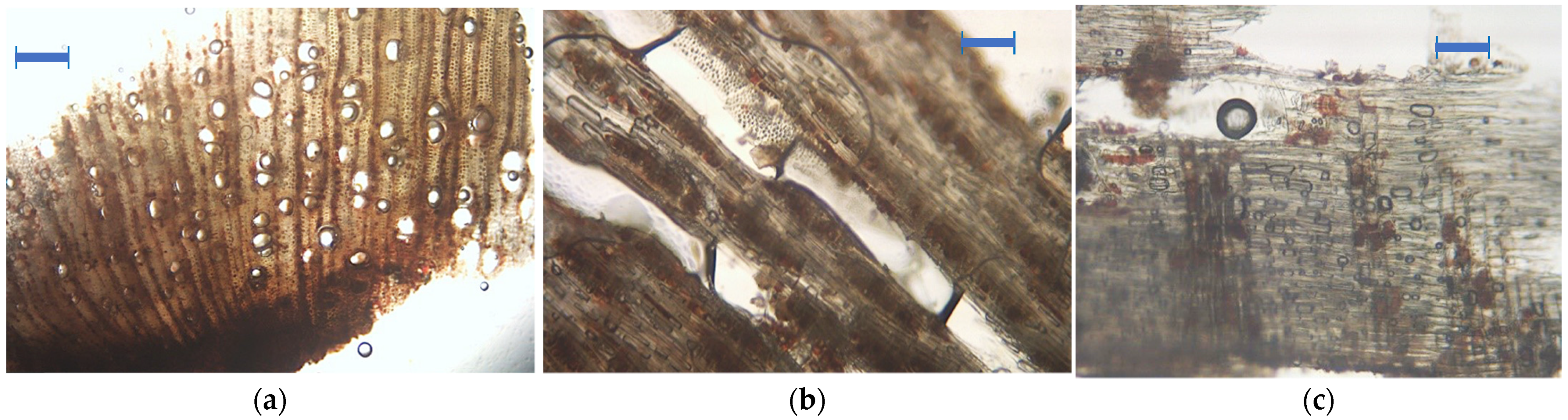
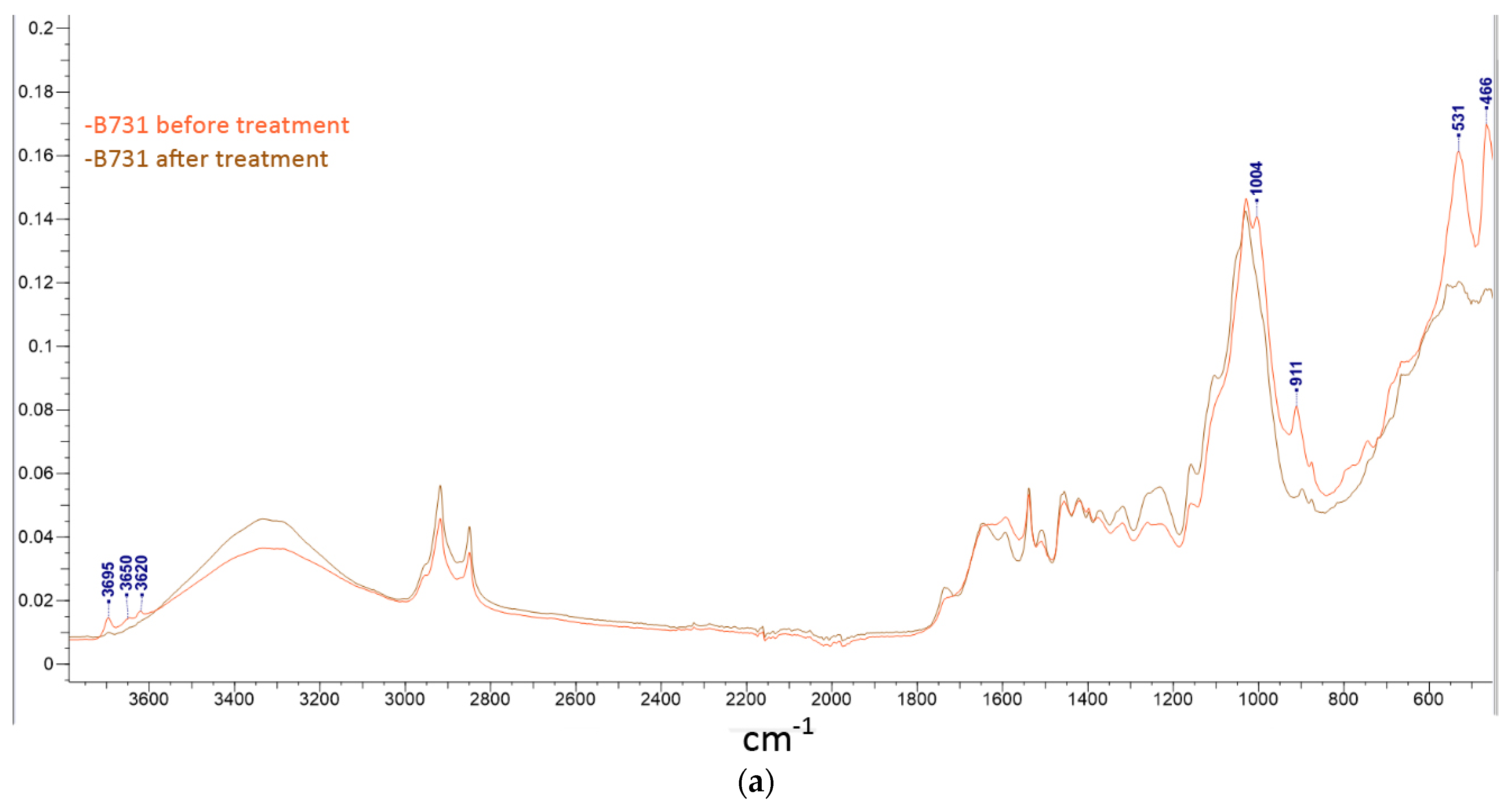
2.5. Artwork Surface Analysis
2.6. Microbial Colonies’ Direct Removal by HA Solution
2.7. Exposure to T. vulgaris EO Volatile Compounds
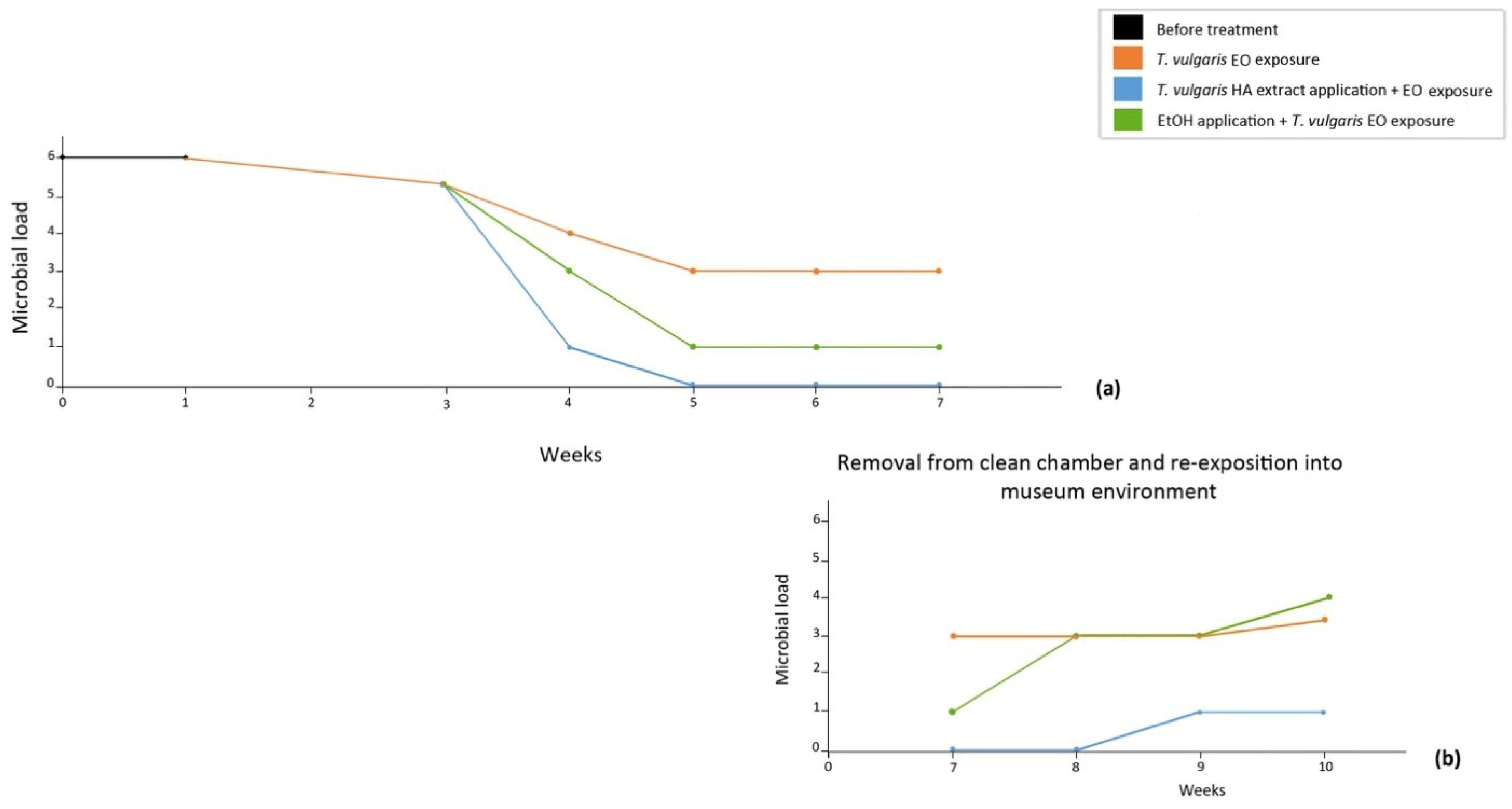
2.8. Microbial Monitoring
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Arnoldi, M.J. Playing the Puppets: Innovation and Rivalry in Bamana Youth Theatre of Mali. Drama Rev. 1988, 32, 2. [Google Scholar] [CrossRef]
- Linking, W.W. Marionnettes du Mali, Paris. 1980. Available online: http://www.gourcuff-gradenigo.com/mali.html (accessed on 24 September 2020).
- Arnoldi, M.J.; Curator Emerita, Africa Department of Anthropology, Smithsonian Institution, Washington, DC, USA. Personal communication, 2021.
- Pournou, A. Biology of wood deteriogens. In Biodeterioration of Wooden Cultural Heritage; Springer Nature: Basingstoke, UK, 2020; Chapter 3; p. 99. [Google Scholar]
- Allsopp, D.; Seal, K.J.; Gaylarde, C.C. Introduction to Biodeterioration; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Dakal, T.C.; Cameotra, S.S. Microbially induced deterioration of architectural heritages: Routes and mechanisms involved. Environ. Sci. Eur. 2012, 24, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ranalli, G.; Zanardini, E.; Sorlini, C. Biodeterioration—Including Cultural Heritage, in Encyclopedia of Microbiology. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Di Carlo, E.; Barresi, G.; Palla., F. Biodeterioration. In Biotechnology and Conservation of Cultural Heritage; Palla, F., Barresi, G., Eds.; Springer International Publishing: Basel, Switzerland, 2017; pp. 1–30. [Google Scholar]
- Blanchette, R.A.; Nilsson, T.; Daniel, G.; Abad, A. Biological degradation of wood. In Archaeological Wood; Rowell, R.M., Barbour, R.J., Eds.; American Chemical Society: Washington, DC, USA, 1990; Volume 74, pp. 141–174. [Google Scholar]
- Reinprect, L. Wood Deterioration, Protection and Maintenance; John Wiley & Sons, Ltd.: Chichester, UK, 2016. [Google Scholar]
- Ljaljević Grbić, M.; Stupar., M.; Vukojević, J.; Maričić, I.; Bungur, N. Molds in Museum Environments: Biodeterioration of Art Photographs and Wooden Sculptures. Arch. Biol. Sci. 2013, 65, 955–962. [Google Scholar] [CrossRef]
- Valentin, N. Microbial contamination in museum collections: Organic materials. In Molecular Biology and Cultural Heritage; Saiz-Jimenez, C., Ed.; Swets & Zeitlinger: Lisse, The Netherlands, 2003; pp. 85–91. [Google Scholar]
- Palla, F. Biological risks for the conservation and exhibition of historical artistic artefacts in confined spaces. In Risk Management in the Cultural Heritage Sector: Museums, Libraries and Archives; Lorusso, S., Natali, A., Palla, F., Eds.; MIMESIS Edizioni: Milano, Italy, 2015; pp. 95–108. [Google Scholar]
- Biological Deterioration & Damage to Furniture & Wooden Objects. Available online: https://www.si.edu/mci/english/learn_more/taking_care/biodetwood.html (accessed on 29 March 2018).
- Tornari, V.; Basset, T.; Andrianakis, M.; Kosma, K. Impact of Relative Humidity on Wood Sample: A Climate Chamber Experimental Simulation Monitored by Digital Holographic Speckle Pattern Interferometry. J. Imaging 2019, 5, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clausen, A.C. Bacterial association with decaying wood: A review. Int. Biodeterior. Biodegrad. 1996, 37, 101–107. [Google Scholar] [CrossRef]
- Blanchette, R.A. A review of microbial deterioration found in archaeological wood from different environments. Int. Biodeterior. Biodegrad. 2000, 46, 189–204. [Google Scholar] [CrossRef]
- Pangallo, D.; Šimonovičová, A.; Chovanová, K.; Ferianc, P. Wooden art objects and the museum environment: Identification and biodegradative characteristics of isolated microflora. Lett. Appl. Microbiol. 2007, 45, 87–94. [Google Scholar] [CrossRef]
- Romero, S.; Giudicessi, S.L.; Vitale, R.G. Is the fungus Aspergillus a threat to cultural heritage? J. Cult. Her. 2021, 51, 107–124. [Google Scholar] [CrossRef]
- Hyde, K.D.; Jones, E.B.G. Introduction to fungal succession. Fungal Divers. 2002, 10, 241–253. [Google Scholar]
- Chojnackah, M.K. Biocides. In Encyclopedia of Toxicology, 3rd ed.; Biomedical Sciences, Elsevier: Amsterdam, The Netherlands, 2014; pp. 461–463. [Google Scholar]
- Young, M.E.; Alakomi, H.L.; Fortune, A.A.; Goburshina, A.A.; Krumbein, W.E.; Maxwell, I.; McCullagh, C.; Robertson, P.; Saarela, M.; Valero, J.; et al. Development of a biocidal treatment regime to inhibit biological growths on cultural heritage: BIODAM. Environ. Geol. 2008, 56, 631–641. [Google Scholar] [CrossRef]
- Marcotte, S.; Estel, L.; Leboucher, S.; Minchin, S. Occurrence of organic biocides in the air and dust at the Natural History Museum of Rouen, France. J. Cult. Herit. 2014, 15, 68–72. [Google Scholar] [CrossRef]
- Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J.; Juan-Mejuto, J.C.; García-Río, L. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar] [CrossRef]
- Hernández, A.F.; Parron, T.; Tsatsakis, M.A.; Requena, M.; Alarcón, R.; López-Guarnido, O. Toxic effects of pesticide mixtures at molecular level: Their relevance to human health. Toxicology 2013, 307, 136–145. [Google Scholar] [CrossRef]
- Hernández, A.F.; Gil, F.; Lacasagña, M. Toxicological interactions of pesticide mixtures: An update. Arch. Toxicol. 2017, 91, 3211–3223. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Wang, Z.; Huang, Q.; Zhang, D. Antifungal activity of several essential oils and major components against wood-rot fungi. Ind. Crop. Prod. 2017, 108, 278–285. [Google Scholar] [CrossRef]
- Fernandez-Lopes, J.; Viuda-Martos, M. Application of essential oils in food systems. Foods 2018, 7, 1–4. [Google Scholar]
- Abu-Shanab, B.; Adwan, G.; Abu-Safiya, D.; Jarrar, N.; Adwan, K. Antibacterial Activities of Some Plant Extracts Utilized in Popular Medicine in Palestine. Turk. J. Biol. 2004, 28, 99–102. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averback, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties—An overview. Complement. Med. Res. 2009, 16, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Afifi, H.A.M. Comparative efficacy of some plant extracts against fungal deterioration of stucco ornaments in the Mihrab of Mostafa Pasha, Ribat, Cairo, Egypt. Am. J. Biochem. Mol. Biol. 2012, 2, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Gatti, L.; Troiano, F.; Vacchini, V.; Cappitelli, F.; Balloi, A. An In Vitro Evaluation of the Biocidal Effect of Oregano and Cloves’ Volatile Compounds against Microorganisms Colonizing an Oil Painting—A Pioneer Study. Appl. Sci. 2021, 11, 78. [Google Scholar] [CrossRef]
- Fierascu, I.; Ion, R.M.; Radu, M.; Bunghez, I.R.; Avramescu, S.M.; Fierascu, R.C. Comparative study of antifungal effect of natural extracts and essential oils of Ocimum basilicum on selected artefacts. Rev. Roum. Chim. 2014, 59, 207–211. [Google Scholar]
- Liu, Z.; Zhang, Y.; Zhang, F.; Hu, C.; Liu, G.; Pan, J. Microbial community analyses of the deteriorated storeroom objects in the Tianjin Museum using culture-independent and culture-dependent approaches. Front. Microbiol. 2018, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Rakotonirainy, M.S.; Lavédrine, B. Screening for antifungal activity of essential oils and related compounds to control the biocontamination in libraries and archives storage areas. Int. Biodeter. Biodegrad. 2005, 55, 141–147. [Google Scholar] [CrossRef]
- Barresi, G.; Di Carlo, E.; Trapani, M.R.; Parisi, M.G.; Chille, C.; Mulè, M.F.; Cammarata, M.; Palla, F. Marine organisms as source of bioactive molecules applied in restoration projects. Herit. Sci. 2015, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Rotolo, V.; Barresi, G.; Di Carlo, E.; Giordano, A.; Lombardo, G.; Crimi, E.; Costa, E.; Bruno, M.; Palla, F. Plant extracts as green potential strategies to control the biodeterioration of cultural heritage. Int. J. Conserv. Sci. 2016, 7, 839–846. [Google Scholar]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential oil as natural biocides in conservation of cultural heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential oils extracted from different species of the Lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef] [Green Version]
- Palla, F.; Caruana, E.; Di Carlo, E.; Rotolo, V. Plant essential oils in controlling fungal colonization on wooden substrate. Borziana 2021, 2, 5–14. [Google Scholar]
- Di Carlo, E.; Chisesi, R.; Barresi, G.; Barbaro, S.; Lombardo, G.; Rotolo, V.; Sebastianelli, M.; Travagliato, G.; Palla, F. Fungi and bacteria in indoor cultural heritage environments: Microbial related risks for artworks and human health. Environ. Ecol. Res. 2016, 4, 257–264. [Google Scholar] [CrossRef]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [Green Version]
- Cardinale, M.; Brusetti, L.; Quatrini, P.; Borin, S.; Puglia, A.M.; Rizzi, A.; Zanardini, E.; Sorlini, C.; Corselli, C.; Daffonchio, D. Comparison of different primer sets for use in automated ribosomal intergenic spacer analysis of complex bacterial communities. Appl. Environ. Microbiol. 2004, 70, 6147–6156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.J.; Rygiewicz, P.T. Fungal-specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiol. 2005, 5, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barresi, G.; Cammarata, M.; Palla, F. Biocide. In Biotechnology and Conservation of Cultural Heritage; Palla, F., Barresi, G., Eds.; Springer International Publishing: Basel, Switzerland, 2017; pp. 49–66. [Google Scholar]
- European Pharmacopoeia 10th Edition, 10.2 n.0865, July 2020. Available online: www.edqm.eu (accessed on 24 September 2020).
- Timofeeff, M.N.; Lowestein, T.K.; Blackburn, W.H. ESEM-EDS: An improved technique for major element chemical analysis of fluid inclusions. Chem. Geol. 2000, 164, 171–181. [Google Scholar] [CrossRef]
- Rotolo, V.; De Caro, M.L.; Giordano, A.; Palla, F. Solunto archaeological park in Sicily: Life under tesserae. Flora Medit 2018, 28, 233–245. [Google Scholar]
- Barry, A.L. The Antimicrobic Susceptibility Test: Principles and Practices; Lea and Febiger: Philadelphia, PA, USA, 1976. [Google Scholar]
- Reichling, J.; Suschke, U.; Schneele, J.; Geiss, H.K. Antibacterial activity and irritation potential of selected essential oil components structure activity relationship. Nat. Prod. Commun. 2006, 1, 1003–1012. [Google Scholar] [CrossRef]
- Popovici, R.A.; Vaduva, D.A.; Pinzaru, I.; Dehelean, C.A.; Farcas, C.G.; Coricovac, D.; Danciu, C.; Popescu, I.; Alexa, E.; Lazureanu, V.; et al. A comparative study on the biological activity of essential oil and total hydro-alcoholic extract of Satureja hortensis L. Exp. Ther. Med. 2019, 18, 932–942. [Google Scholar] [CrossRef]
- Saad, N.; Muller, C.D.; Lobstein, A. Major bioactivities and mechanism of action of essential oil and their components. Fragance Flavours J. 2013, 28, 269–279. [Google Scholar] [CrossRef]
- Cappitelli, F.; Cattò, C.; Villa, F. The control of cultural heritage microbial deterioration. Microorganisms 2020, 8, 1542. [Google Scholar] [CrossRef] [PubMed]
- Fidanza, M.R.; Caneva, G. Natural biocides for the conservation of stone cultural heritage: A review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Lavin, P.; Gómez de Saravia, S.; Guiamet, P. Scopulariopsis sp. and Fusarium sp. in the Documentary Heritage: Evaluation of their biodeterioration ability and antifungal effect of two essential oils. Microb. Ecol. 2016, 71, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Nuță, D.C.; Limban, C.; Ioniță, C.C.; Nicolau, I.; Zarafu, I. The Use of Essential Oils as a Strategy to Combat Microbial Biofilms. A Review. Processes 2021, 9, 537. [Google Scholar] [CrossRef]
- Borrego, S.; Valdés, O.; Vivar, I.; Lavin, P.; Guiamet, P.; Battistoni, P.; Gómez de Saravia, S.G.; Borges, P. Essential oils of plants as biocides against microorganisms isolated from Cuban and Argentine documentary heritage. ISRN Microbiol. 2012, 2012, 826786. [Google Scholar] [CrossRef] [Green Version]
- Sakr, A.A.; Ghaly, M.F.; El-Sayed, F.; Abdel-Haliem, M. The efficacy of specific essential oils on yeasts isolated from the royal tomb paintings at Tanis, Egypt. Int. J. Conserv. Sci. 2012, 3, 87–92. [Google Scholar]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical profile, antioxidant and antibacterial activity of Thyme and Oregano essential oils, Thymol and Carvacrol and their possible synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Klarić, M.Š.; Kosaleć, I.; Mastelić, J.; Piecková, E.; Pepeljnak, S. Antifungal activity of thyme (Thymus vulgaris L.) essential oil and thymol against moulds from damp dwellings. Lett. Appl. Microbiol. 2007, 44, 36–42. [Google Scholar] [CrossRef]
- D’Agostino, G.; Giambra, B.; Palla, F.; Bruno, M.; Badalamenti, N. The Application of the Essential Oils of Thymus vulgaris L. and Crithmum maritimum L. as Biocidal on Two Tholu Bommalu Indian Leather Puppets. Plants 2021, 10, 1508. [Google Scholar] [CrossRef]
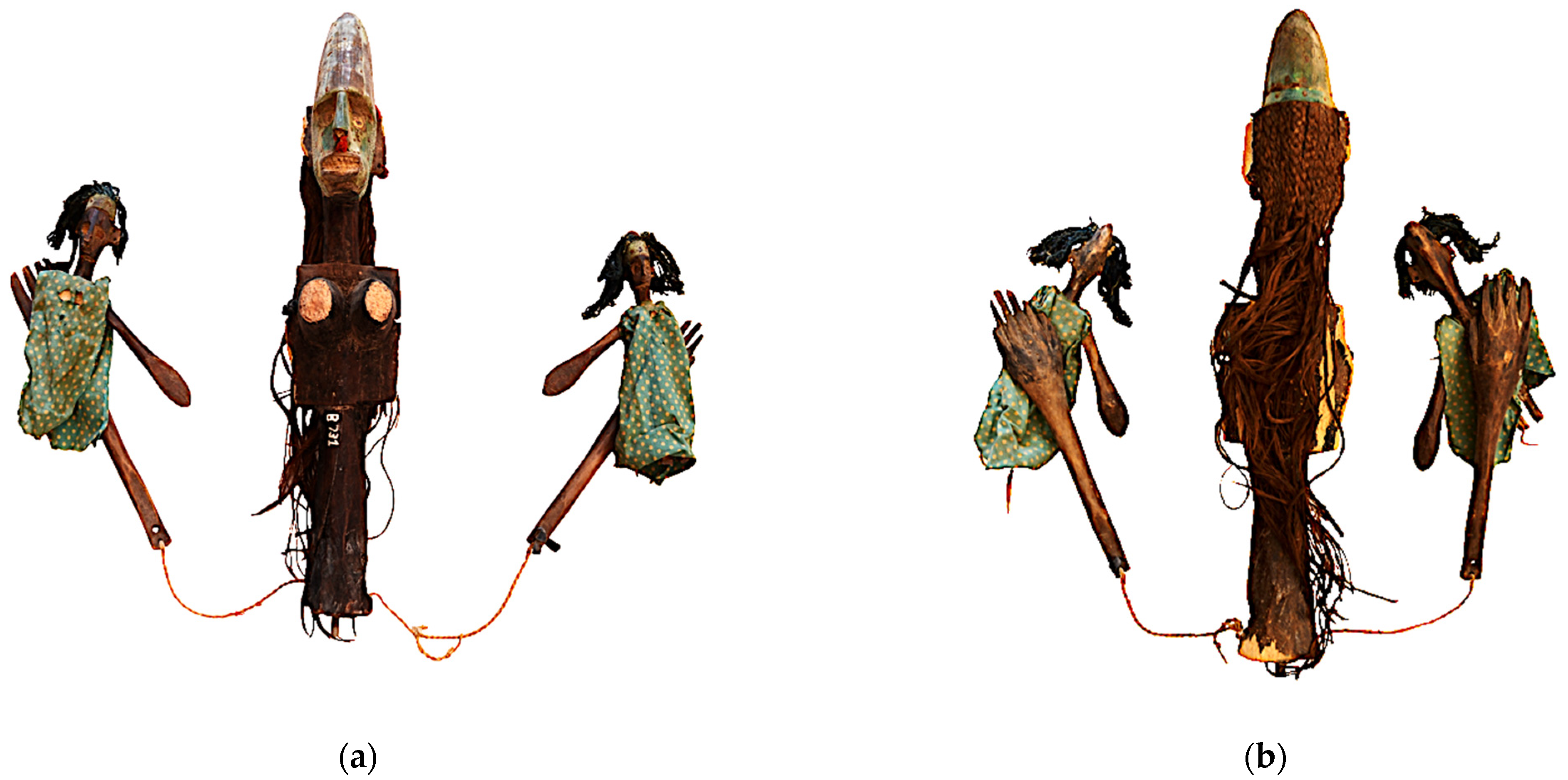

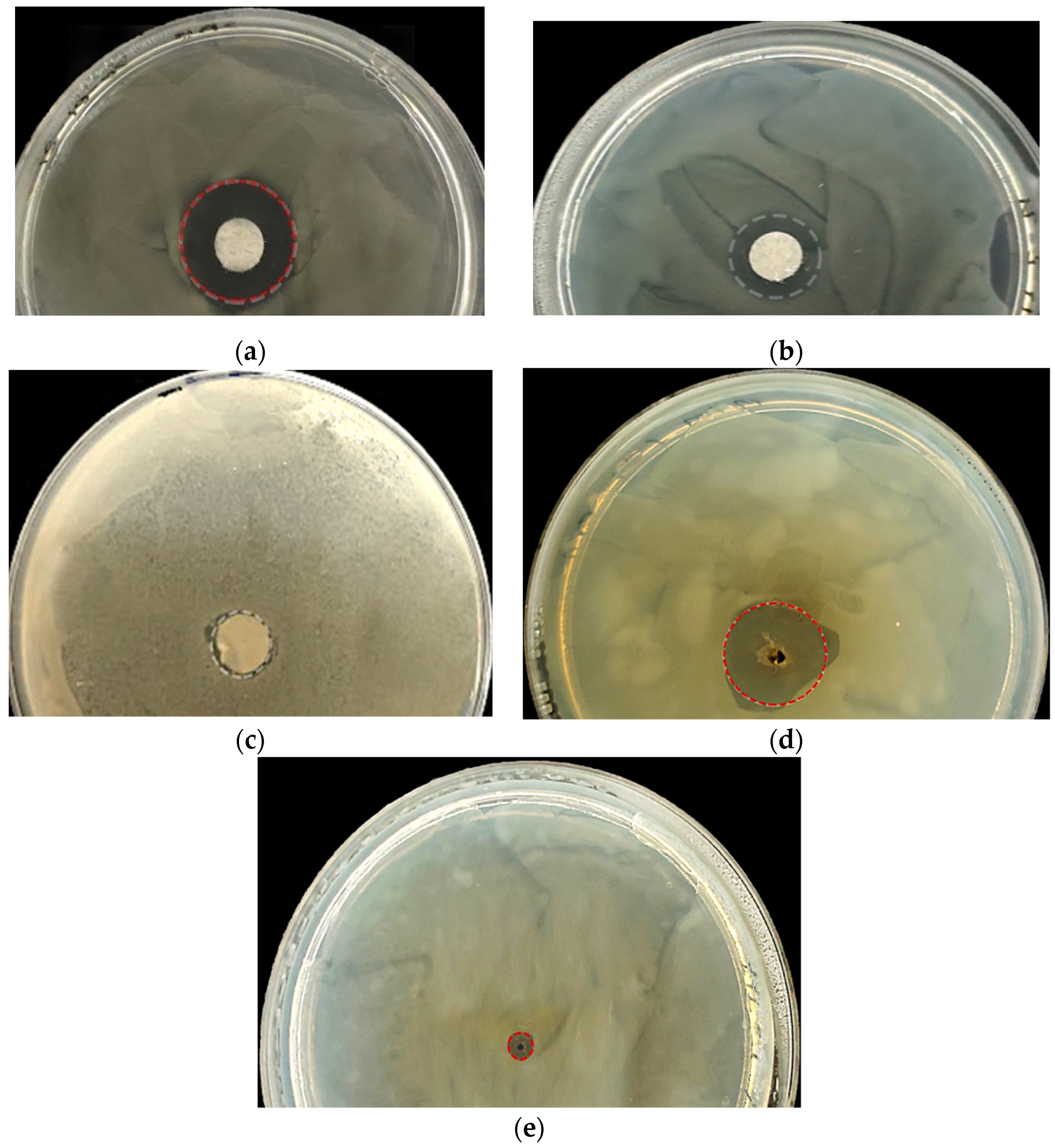

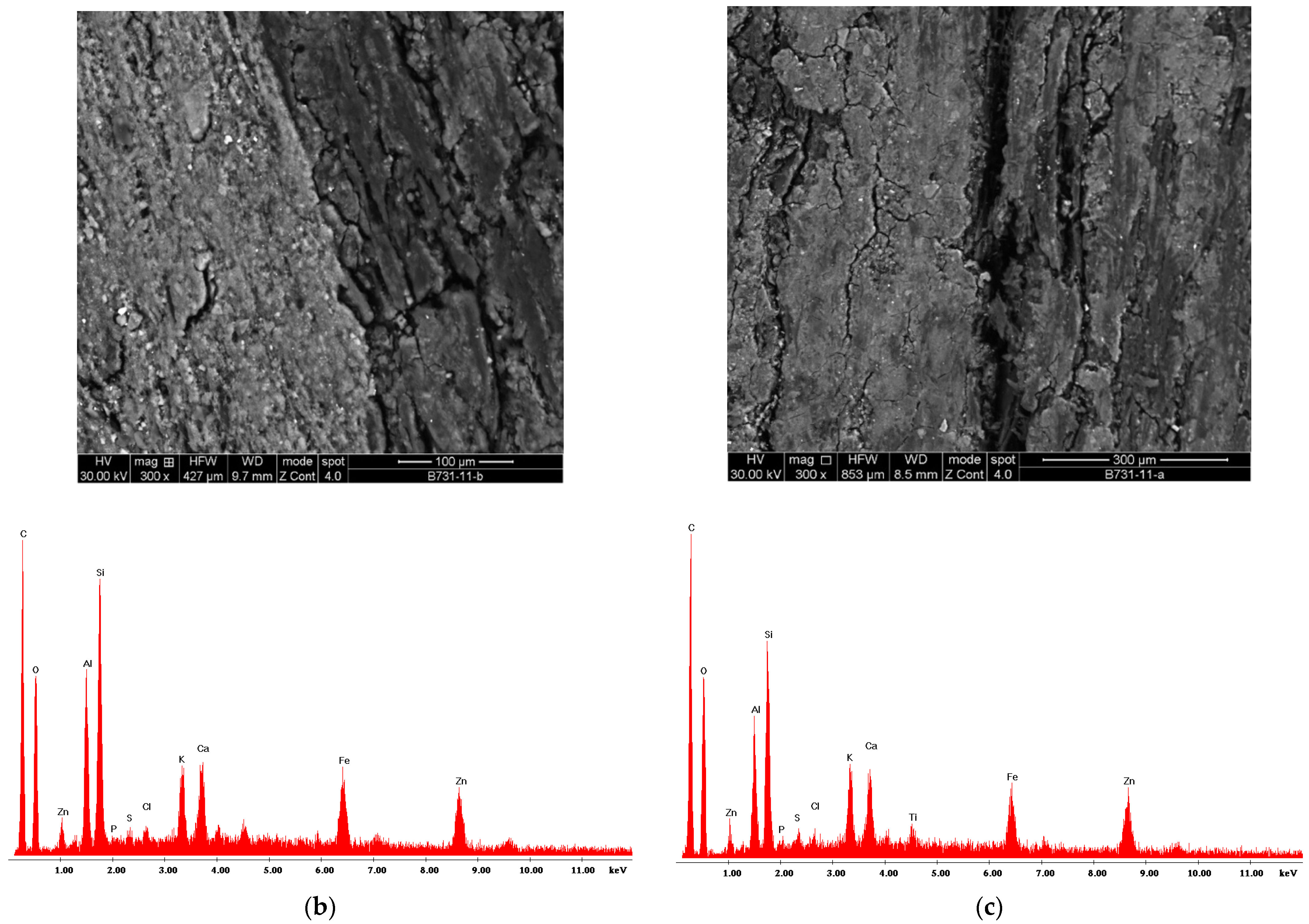

| Biocide Solution | Microbial Taxa | ADD Method Inhibition Halo (mm) |
|---|---|---|
| E.O. 12.5% | Bacillus sp. | 21.5–22.0 |
| Streptococcus sp. | 17.4–18.0 | |
| Aspergillus sp. | 8.5–9.0 | |
| Penicillum sp. | 12.0–12.5 | |
| WPD method Inhibition halo (mm) | ||
| H.A. 100%. | Bacillus sp. | 11.0–11.0 |
| Streptococcus sp. | 9.3–10.0 | |
| Aspergillus sp. | 8.7–9.0 | |
| Penicillum sp. | 8.1–9.0 | |
| Control solutions | All bacterial and fungal isolated colonies | ADD or WPD method Inhibition halo (mm) |
| Et-OH 70% | 1.8–2.0 0.4–0.5 | |
| Benz. Clor. 3% | 5.5–6.0 3.8–4.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparacello, S.; Gallo, G.; Faddetta, T.; Megna, B.; Nicotra, G.; Bruno, B.; Giambra, B.; Palla, F. Thymus vulgaris Essential Oil and Hydro-Alcoholic Solutions to Counteract Wooden Artwork Microbial Colonization. Appl. Sci. 2021, 11, 8704. https://doi.org/10.3390/app11188704
Sparacello S, Gallo G, Faddetta T, Megna B, Nicotra G, Bruno B, Giambra B, Palla F. Thymus vulgaris Essential Oil and Hydro-Alcoholic Solutions to Counteract Wooden Artwork Microbial Colonization. Applied Sciences. 2021; 11(18):8704. https://doi.org/10.3390/app11188704
Chicago/Turabian StyleSparacello, Silvia, Giuseppe Gallo, Teresa Faddetta, Bartolomeo Megna, Giovanna Nicotra, Beatrice Bruno, Belinda Giambra, and Franco Palla. 2021. "Thymus vulgaris Essential Oil and Hydro-Alcoholic Solutions to Counteract Wooden Artwork Microbial Colonization" Applied Sciences 11, no. 18: 8704. https://doi.org/10.3390/app11188704
APA StyleSparacello, S., Gallo, G., Faddetta, T., Megna, B., Nicotra, G., Bruno, B., Giambra, B., & Palla, F. (2021). Thymus vulgaris Essential Oil and Hydro-Alcoholic Solutions to Counteract Wooden Artwork Microbial Colonization. Applied Sciences, 11(18), 8704. https://doi.org/10.3390/app11188704








