The Effect of Enzymatic Crosslink Degradation on the Mechanics of the Anterior Cruciate Ligament: A Hybrid Multi-Domain Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Collagen Fibril Coarse-Grained Model
2.2. Fibril Hyper-Elastoplastic Model
2.3. Connective Tissue Model
2.4. Molecular Dynamic-Softening Hyperelasticity Approach
2.5. ACL Model
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen fibril formation. Biochem. J. 1996, 316, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.A. The molecular fibrillar structure of collagen and its relationship to the mechanical properties of connective tissue. Biophys. Chem. 1988, 29, 195–209. [Google Scholar] [CrossRef]
- Kramer, R.Z.; Venugopal, M.G.; Bella, J.; Mayville, P.; Brodsky, B.; Berman, H. Staggered molecular packing in crystals of a collagen-like peptide with a single charged pair. J. Mol. Biol. 2000, 301, 1191–1205. [Google Scholar] [CrossRef]
- Bella, J.; Eaton, M.; Brodsky, B.; Berman, H. Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science 1994, 266, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Walter, P.; Roberts, K. Cell Chemistry and Biosynthesis. In Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Walter, P.; Roberts, K. Cell Junctions, Cell Adhesion, and the Extracellular Matrix. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Bailey, A.J. Molecular mechanisms of ageing in connective tissues. Mech. Ageing Dev. 2001, 122, 735–755. [Google Scholar] [CrossRef]
- Catanese III, J.; Iverson, E.P.; Ng, R.K.; Keaveny, T.M. Heterogeneity of the mechanical properties of demineralized bone. J. Biomech. 1999, 32, 1365–1369. [Google Scholar] [CrossRef]
- Akizuki, S.; Mow, V.C.; Muller, F.; Pita, J.C.; Howell, D.S.; Manicourt, D.H. Tensile properties of human knee joint cartilage: I. Influence of ionic conditions, weight bearing, and fibrillation on the tensile modulus. J. Orthop. Res. 1986, 4, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Diamant, J.; Keller, A.; Baer, E.; Litt, M.; Arridge, R.G.C. Collagen; ultrastructure and its relation to mechanical properties as a function of ageing. Proc. R. Soc. Ser. B Boil. Sci. 1972, 180, 293–315. [Google Scholar] [CrossRef]
- Abrahams, M. Mechanical behaviour of tendonIn vitro. Med. Biol. Eng. Comput. 1967, 5, 433–443. [Google Scholar] [CrossRef]
- Shen, Z.L.; Dodge, M.R.; Kahn, H.; Ballarini, R.; Eppell, S. Stress-strain Experiments on Individual Collagen Fibrils. Biophys. J. 2008, 95, 3956–3963. [Google Scholar] [CrossRef]
- Shen, Z.L.; Kahn, H.; Ballarini, R.; Eppell, S.J. Viscoelastic Properties of Isolated Collagen Fibrils. Biophys. J. 2011, 100, 3008–3015. [Google Scholar] [CrossRef]
- Lorenzo, A.C.; Caffarena, E.R. Elastic properties, Young’s modulus determination and structural stability of the tropocollagen molecule: A computational study by steered molecular dynamics. J. Biomech. 2004, 38, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, S.; Raabe, D. Hierarchical modeling of the elastic properties of bone at submicron scales: The role of extrafibrillar mineralization. Biophys. J. 2008, 94, 4220–4232. [Google Scholar] [CrossRef] [PubMed]
- Veld, P.J.I.; Stevens, M.J. Simulation of the Mechanical Strength of a Single Collagen Molecule. Biophys. J. 2008, 95, 33–39. [Google Scholar] [CrossRef]
- Buehler, M.J. Nature designs tough collagen: Explaining the nanostructure of collagen fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 12285–12290. [Google Scholar] [CrossRef]
- Chang, S.-W.; Shefelbine, S.J.; Buehler, M.J. Structural and Mechanical Differences between Collagen Homo-and Heterotrimers: Relevance for the Molecular Origin of Brittle Bone Disease. Biophys. J. 2012, 102, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Depalle, B.; Qin, Z.; Shefelbine, S.J.; Buehler, M.J. Large Deformation Mechanisms, Plasticity, and Failure of an Individual Collagen Fibril with Different Mineral Content. J. Bone Miner. Res. 2015, 31, 380–390. [Google Scholar] [CrossRef]
- Liu, Y.; Thomopoulos, S.; Chen, C.; Birman, V.; Buehler, M.; Genin, G.M. Modelling the mechanics of partially mineralized collagen fibrils, fibres and tissue. J. R. Soc. Interface 2014, 11, 20130835. [Google Scholar] [CrossRef]
- Tang, Y.; Ballarini, R.; Buehler, M.J.; Eppell, S. Deformation micromechanisms of collagen fibrils under uniaxial tension. J. R. Soc. Interface 2009, 7, 839–850. [Google Scholar] [CrossRef]
- Buehler, M.J.; Ballarini, R. Microelectromechanical Systems (MEMS) Platforms for Testing the Mechanical Properties of Collagen Fibrils. Materiomics: Multiscale Mechanics of Biological Materials and Structures; Springer: Berlin, Germany, 2013. [Google Scholar]
- Weiss, J.A.; Gardiner, J.C. Computational Modeling of Ligament Mechanics. Crit. Rev. Biomed. Eng. 2001, 29, 303–371. [Google Scholar] [CrossRef]
- Ateshian, G.A.; Ellis, B.J.; Weiss, J.A. Equivalence Between Short-Time Biphasic and Incompressible Elastic Material Responses. J. Biomech. Eng. 2006, 129, 405–412. [Google Scholar] [CrossRef]
- Gardiner, J.C.; Weiss, J.A. Subject-specific finite element analysis of the human medial collateral ligament during valgus knee loading. J. Orthop. Res. 2003, 21, 1098–1106. [Google Scholar] [CrossRef]
- Gardiner, J.C.; Weiss, J.A.; Rosenberg, T.D. Strain in the Human Medial Collateral Ligament During Valgus Loading of the Knee. Clin. Orthop. Relat. Res. 2001, 391, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Quapp, K.M.; Weiss, J.A. Material Characterization of Human Medial Collateral Ligament. J. Biomech. Eng. 1998, 120, 757–763. [Google Scholar] [CrossRef]
- Limbert, G.; Middleton, J. A transversely isotropic viscohyperelastic material—Application to the modeling of biological soft connective tissues. Int. J. Solids Struct. 2004, 41, 4237–4260. [Google Scholar] [CrossRef]
- Limbert, G.; Taylor, M.; Middleton, J. Three-dimensional finite element modelling of the human ACL: Simulation of passive knee flexion with a stressed and stress-free ACL. J. Biomech. 2004, 37, 1723–1731. [Google Scholar] [CrossRef]
- Adouni, M.; Mbarki, R.; Al Khatib, F.; Eilaghi, A. Multiscale modeling of knee ligament biomechanics. Int. J. Numer. Methods Biomed. Eng. 2021, 37, e3413. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P. Collagen: Structure and Mechanics; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Nessler, T.; Denney, L.; Sampley, J. ACL Injury Prevention: What Does Research Tell Us? Curr. Rev. Musculoskelet. Med. 2017, 10, 281–288. [Google Scholar] [CrossRef]
- Kiapour, A.M.; Murray, M.M. Basic science of anterior cruciate ligament injury and repair. Bone Jt. Res. 2014, 3, 20–31. [Google Scholar] [CrossRef]
- Boorman, R.S.; Thornton, G.M.; Shrive, N.G.; Frank, C.B. Ligament grafts become more susceptible to creep within days after surgery: Evidence for early enzymatic deg-radation of a ligament graft in a rabbit model. Acta Orthop. Scand. 2002, 73, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Orgel, J.P.R.O.; Irving, T.C.; Miller, A.; Wess, T.J. Microfibrillar structure of type I collagen in situ. Proc. Natl. Acad. Sci. USA 2006, 103, 9001–9005. [Google Scholar] [CrossRef]
- Depalle, B.; Qin, Z.; Shefelbine, S.J.; Buehler, M.J. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J. Mech. Behav. Biomed. Mater. 2014, 52, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dépalle, B.; Duarte, A.G.; Fiedler, I.A.; Pujo-Menjouet, L.; Buehler, M.J.; Berteau, J.-P. The different distribution of enzymatic collagen cross-links found in adult and children bone result in different mechanical behavior of collagen. Bone 2018, 110, 107–114. [Google Scholar] [CrossRef]
- Sweeney, S.M.; Orgel, J.P.; Fertala, A.; McAuliffe, J.D.; Turner, K.R.; Di Lullo, G.A.; Chen, S.; Antipova, O.; Perumal, S.; Ala-Kokko, L.; et al. Candidate Cell and Matrix Interaction Domains on the Collagen Fibril, the Predominant Protein of Vertebrates. J. Biol. Chem. 2008, 283, 21187–21197. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Holl, M.B. Variation in type I collagen fibril nanomorphology: The significance and origin. Bone Key Rep. 2013, 2, 394. [Google Scholar] [CrossRef] [PubMed]
- Gautieri, A.; Vesentini, S.; Redaelli, A.; Buehler, M. Hierarchical Structure and Nanomechanics of Collagen Microfibrils from the Atomistic Scale Up. Nano Lett. 2011, 11, 757–766. [Google Scholar] [CrossRef]
- Watanabe-Nakayama, T.; Itami, M.; Kodera, N.; Ando, T.; Konno, H. High-speed atomic force microscopy reveals strongly polarized movement of clostridial collagenase along collagen fibrils. Sci. Rep. 2016, 6, 28975. [Google Scholar] [CrossRef]
- Buehler, M.J. Nanomechanics of collagen fibrils under varying cross-link densities: Atomistic and continuum studies. J. Mech. Behav. Biomed. Mater. 2008, 1, 59–67. [Google Scholar] [CrossRef]
- Saito, M.; Marumo, K.; Fujii, K.; Ishioka, N. Single-column high-performance liquid chromatographic–fluorescence detection of immature, mature, and senescent cross-links of collagen. Anal. Biochem. 1997, 253, 26–32. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18. [Google Scholar] [CrossRef]
- Asaro, R.; Rice, J.R. Strain localization in ductile single crystals. J. Mech. Phys. Solids 1977, 25, 309–338. [Google Scholar] [CrossRef]
- Lee, E.H. Elastic-Plastic Deformation at Finite Strains. J. Appl. Mech. 1969, 36, 1–6. [Google Scholar] [CrossRef]
- Buehler, M.J.; Ballarini, R. Multiscale Modeling of Biomaterials and Tissues. In Materiomics: Multiscale Mechanics of Biological Materials and Structures; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Yeo, J.; Jung, G.; Tarakanova, A.; Martín-Martínez, F.J.; Qin, Z.; Cheng, Y.; Zhang, Y.-W.; Buehler, M.J. Multiscale modeling of keratin, collagen, elastin and related human diseases: Perspectives from atomistic to coarse-grained molecular dynamics simulations. Extrem. Mech. Lett. 2018, 20, 112–124. [Google Scholar] [CrossRef]
- Tang, H.; Buehler, M.J.; Moran, B. A Constitutive Model of Soft Tissue: From Nanoscale Collagen to Tissue Continuum. Ann. Biomed. Eng. 2009, 37, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, Y.; Lu, Y.; Peng, C.; Lu, H.; Ballarini, R.; Lou, J. Development and application of a novel microfabricated device for the in situ tensile testing of 1-D nanomaterials. J. Microelectromech. Syst. 2010, 19, 675–682. [Google Scholar] [CrossRef]
- Malaspina, D.C.; Szleifer, I.; Dhaher, Y. Mechanical properties of a collagen fibril under simulated degradation. J. Mech. Behav. Biomed. Mater. 2017, 75, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Bessa, M.; Elkhodary, K.I.; Liu, W.K.; Belytschko, T.; Moran, B. Nonlinear Finite Elements for Continua and Structures, 2nd ed.; Solution Manual; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Gasser, T.C.; Holzapfel, G.A. A rate-independent elastoplastic constitutive model for biological fiber-reinforced composites at finite strains: Continuum basis, algorithmic formulation and finite element implementation. Comput. Mech. 2002, 29, 340–360. [Google Scholar] [CrossRef]
- Martin-Martinez, F.J.; Qin, Z.; Jung, G.; Buehler, M. Multi-scale modeling of nanomaterials: From DFT to molecular dynamics simulations. In Abstracts of Papers of the American Chemical Society; Amer Chemical Soc: Washington, DC, USA, 2017. [Google Scholar]
- Erdemir, A. Open knee: Open source modeling & simulation to enable scientific discovery and clinical care in knee biomechanics. J. Knee Surg. 2016, 29, 107. [Google Scholar]
- Wenger, M.P.; Bozec, L.; Horton, M.A.; Mesquida, P. Mechanical Properties of Collagen Fibrils. Biophys. J. 2007, 93, 1255–1263. [Google Scholar] [CrossRef]
- Misof, K.; Rapp, G.; Fratzl, P. A new molecular model for collagen elasticity based on synchrotron X-ray scattering evidence. Biophys. J. 1997, 72, 1376–1381. [Google Scholar] [CrossRef]
- Gupta, H.S.; Messmer, P.; Roschger, P.; Bernstorff, S.; Klaushofer, K.; Fratzl, P. Synchrotron Diffraction Study of Deformation Mechanisms in Mineralized Tendon. Phys. Rev. Lett. 2004, 93, 158101. [Google Scholar] [CrossRef]
- Brodsky, B.; Persikov, A.V. Molecular Structure of the Collagen Triple Helix. Adv. Protein Chem. 2005, 70, 301–339. [Google Scholar] [CrossRef]
- Uzel, S.G.M.; Buehler, M. Nanomechanical sequencing of collagen: Tropocollagen features heterogeneous elastic properties at the nanoscale. Integr. Biol. 2009, 1, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.L.; Kay, M.D.; Stouffer, D.C. Comparison of material properties in fascicle-bone units from human patellar tendon and knee ligaments. J. Biomech. 1986, 19, 425–432. [Google Scholar] [CrossRef]
- Bull, A.M.J. Measurement and Computer Simulation of Knee Kinematics; Imperial College London: London, UK, 1999. [Google Scholar]
- Bach, J.M.; Hull, M.L. Strain Inhomogeneity in the Anterior Cruciate Ligament Under Application of External and Muscular Loads. J. Biomech. Eng. 1998, 120, 497–503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bach, J.; Hull, M.; Patterson, H. Direct measurement of strain in the posterolateral bundle of the anterior cruciate ligament. J. Biomech. 1997, 30, 281–283. [Google Scholar] [CrossRef]
- Woo, S.L.-Y.; Fox, R.J.; Sakane, M.; Livesay, G.A.; Rudy, T.W.; Fu, F.H. Biomechanics of the ACL: Measurements of in situ force in the ACL and knee kinematics. Knee 1998, 5, 267–288. [Google Scholar] [CrossRef]
- Adouni, M.; Faisal, T.R.; Dhaher, Y.Y. Computational frame of ligament in situ strain in a full knee model. Comput. Biol. Med. 2020, 126, 104012. [Google Scholar] [CrossRef]
- Hansen, P.; Kovanen, V.; Hölmich, P.; Krogsgaard, M.; Hansson, P.; Dahl, M.; Hald, M.; Aagaard, P.; Kjaer, M.; Magnusson, S.P. Micromechanical Properties and Collagen Composition of Ruptured Human Achilles Tendon. Am. J. Sports Med. 2012, 41, 437–443. [Google Scholar] [CrossRef]
- Noyes, F.R.; Delucas, J.L.; Torvik, P.J. Biomechanics of Anterior Cruciate Ligament Failure. J. Bone Jt. Surg.-Am. 1974, 56, 236–253. [Google Scholar] [CrossRef]
- Noyes, F.R.; Torvik, P.J.; Hyde, W.B.; DeLucas, J.L. Biomechanics of ligament failure. II. An analysis of immobilization, exercise, and reconditioning effects in primates. J. Bone Jt. Surg.-Am. 1974, 56, 1406–1418. [Google Scholar] [CrossRef]
- Viidik, A. Elasticity and tensile strength of the anterior cruciate ligament in rabbits as influenced by training. Acta Physiol. Scand. 1968, 74, 372–380. [Google Scholar] [CrossRef]
- Schenck, R.C., Jr.; Kovach, I.S.; Agarwal, A.; Brummett, R.; Ward, R.A.; Lanctot, D.; Athanasiou, K.A. Cruciate injury patterns in knee hyperextension: A cadaveric model. Arthroscopy 1999, 15, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.S.R. Patellar Tendon Rupture. In StatPearls; 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513275/ (accessed on 12 January 2019).
- Aalbersberg, S.; Kingma, I.; Ronsky, J.L.; Frayne, R.; Van Dieën, J.H. Orientation of tendons in vivo with active and passive knee muscles. J. Biomech. 2005, 38, 1780–1788. [Google Scholar] [CrossRef]
- Ireland, M.L. The female ACL: Why is it more prone to injury? Orthop. Clin. 2002, 33, 637–651. [Google Scholar] [CrossRef]
- Powell, B.S.; Dhaher, Y.Y.; Szleifer, I.G. Review of the Multiscale Effects of Female Sex Hormones on Matrix Metalloprotein-ase−Mediated Collagen Degradation. Crit. Rev. Biomed. Eng. 2015, 43, 401–428. [Google Scholar] [CrossRef]
- Slauterbeck, J.; Hardy, D. Sex Hormones and Knee Ligament Injuries in Female Athletes. Am. J. Med. Sci. 2001, 322, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Couppe, C.; Hansen, P.; Kongsgaard, M.; Kovanen, V.; Suetta, C.; Aagaard, P.; Kjær, M.; Magnusson, S.P. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J. Appl. Physiol. 2009, 107, 880–886. [Google Scholar] [CrossRef]
- Adouni, M.; Shirazi-Adl, A.; Shirazi, R. Computational biodynamics of human knee joint in gait: From muscle forces to cartilage stresses. J. Biomech. 2012, 45, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Dhaher, Y.Y.; Salehghaffari, S.; Adouni, M. Anterior laxity, graft-tunnel interaction and surgical design variations during anterior cruciate ligament reconstruction: A probabilistic simulation of the surgery. J. Biomech. 2016, 49, 3009–3016. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.R.; Wu, J.-J. Collagen Cross-Links. In Collagen: Primer in Structure, Processing and Assembly; Brinckmann, J., Notbohm, H., Müller, P.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 207–229. [Google Scholar]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos. Int. 2005, 17, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Banse, X.; Sims, T.J.; Bailey, A.J. Mechanical Properties of Adult Vertebral Cancellous Bone: Correlation with Collagen Intermo-lecular Cross-Links. J. Bone Min. Res. 2002, 17, 1621–1628. [Google Scholar] [CrossRef]
- Nanci, A. Content and Distribution of Noncollagenous Matrix Proteins in Bone and Cementum: Relationship to Speed of Formation and Collagen Packing Density. J. Struct. Biol. 1999, 126, 256–269. [Google Scholar] [CrossRef] [PubMed]
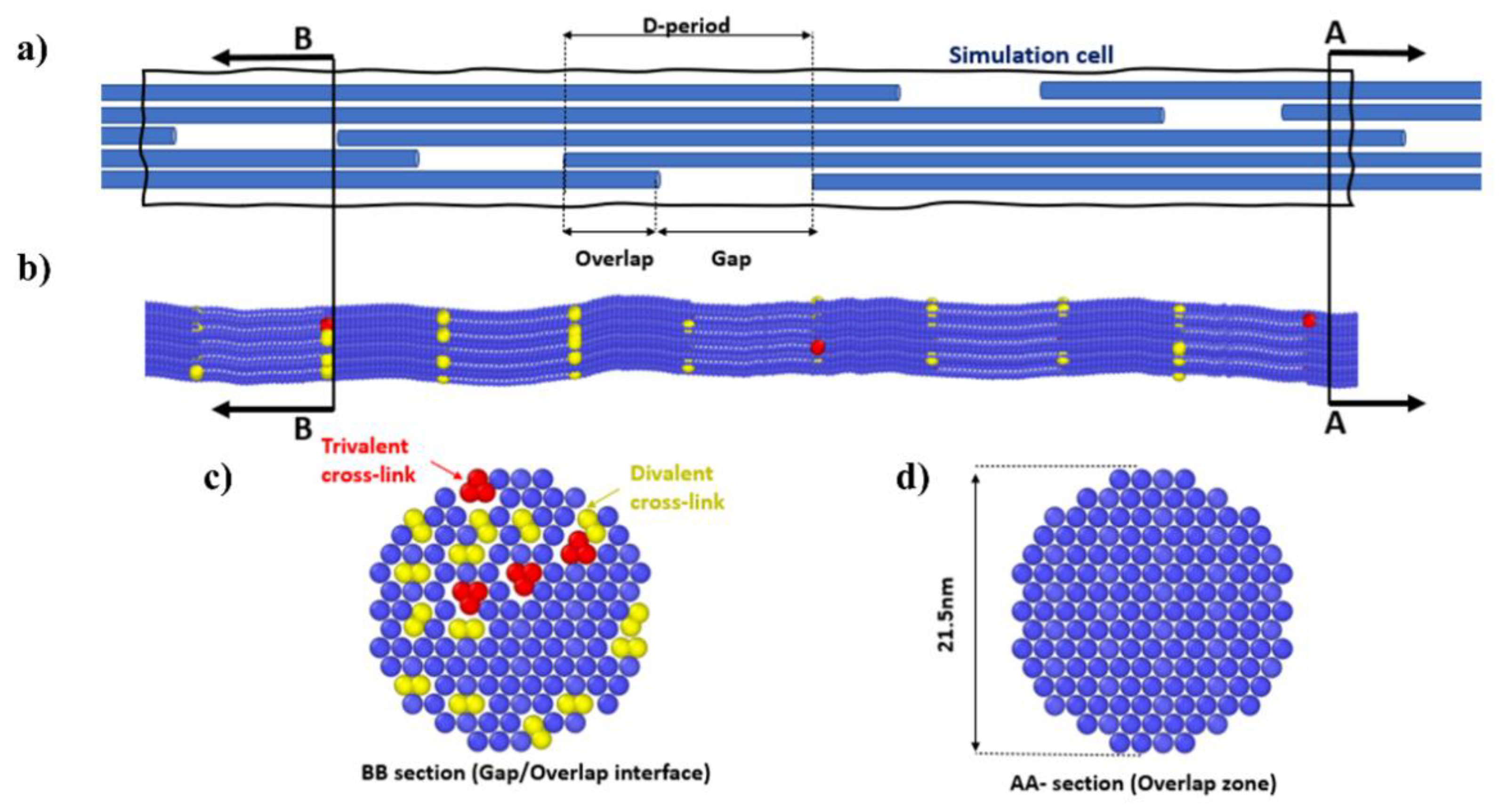
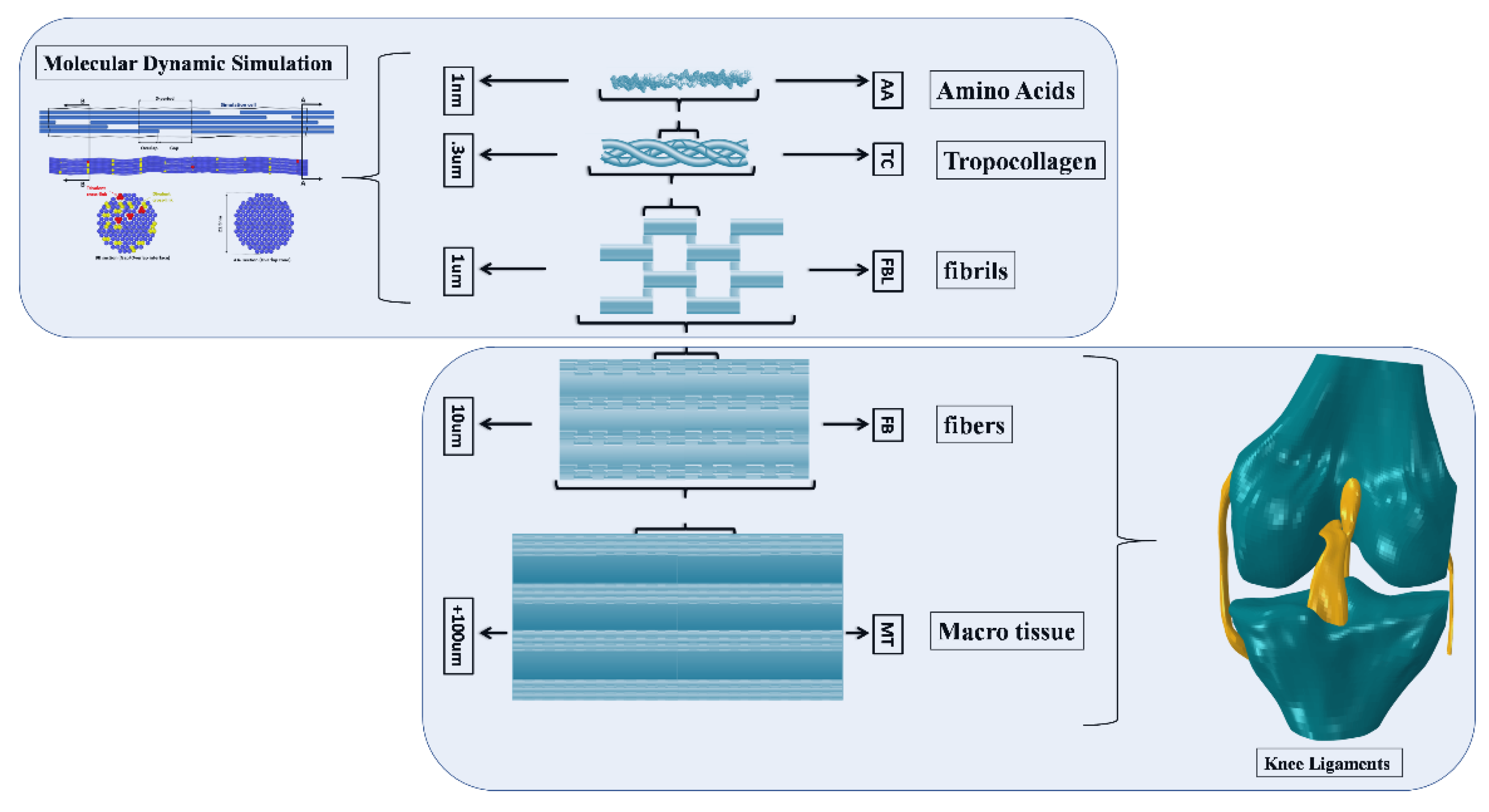
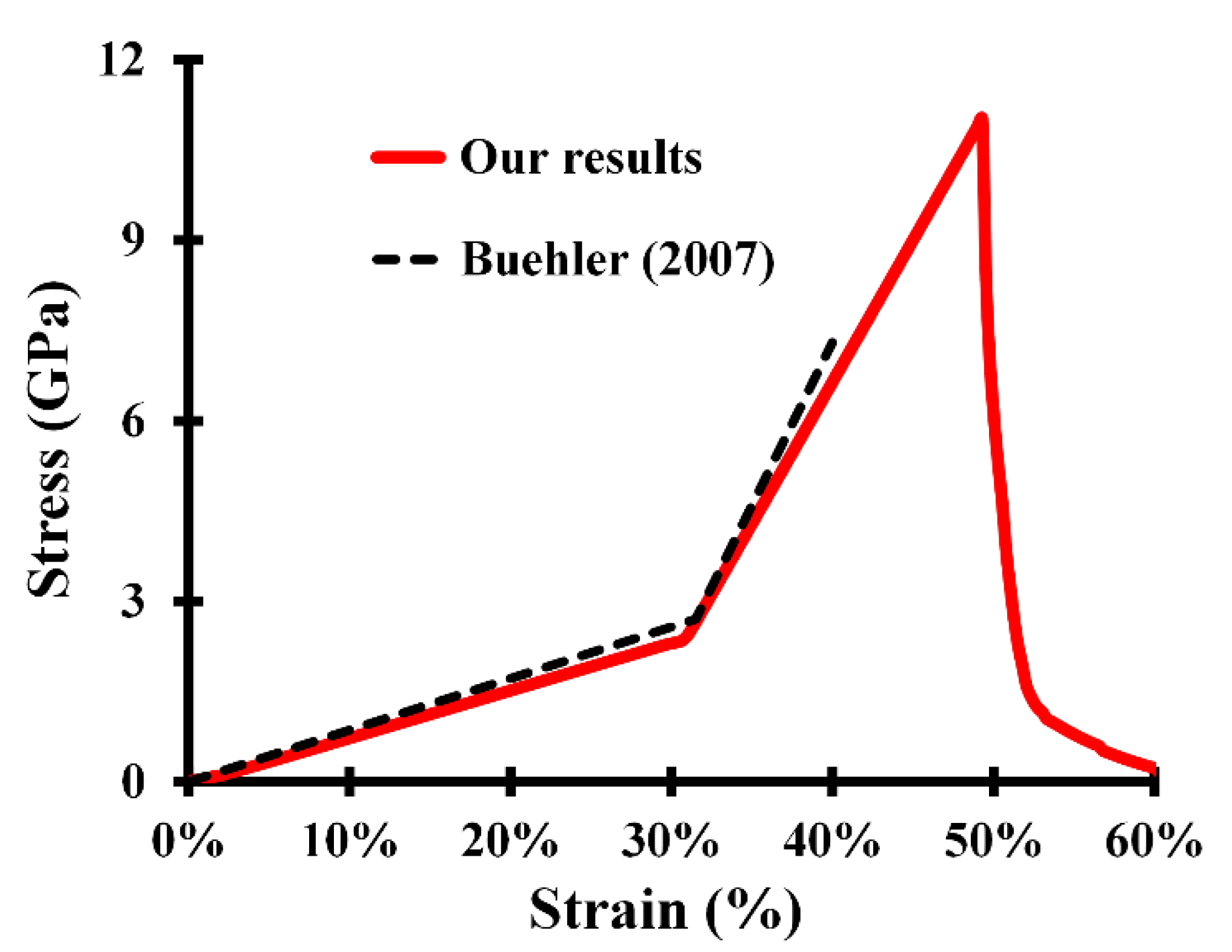





| Molecular Properties | Fiber Properties | ||
|---|---|---|---|
| Parameter | Value | Parameter | Value |
| Molecule number of atoms | 3134 | Gap [Å] | 400 |
| Molecule total mass [g/mol] | 287,000 | Overlap [Å] | ~282 |
| Number of beads per molecule | 218 | D-period [Å] | ~682 |
| Mass of each bead [g/mol] | 1316 | Length of fibril [Å] | 3410 |
| Length along principal axis [Å] | 3011 | Hex. lattice constant [Å] | 16.52 |
| Parameter | Molecule | Divalent | Trivalent |
|---|---|---|---|
| r0—equilibrium distance [å] | 14.00 | 10.00 | 8.60 |
| r1—critical hyperplastic distance [å] | 18.20 | 12.00 | 12.20 |
| rb—bond-breaking distance [å] | 21.00 | 14.68 | 14.89 |
| kt0—stretching-strength constant [kcal/mol] | 17.13 | 0.20 | 0.20 |
| kt1—stretching-strength constant [kcal/mol] | 97.66 | 41.84 | 54.60 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| —Lennard-Jones [Kcal/mol] | 6.87 | —Equilibrium bending angle [degree] | 164–180 |
| —Lennard-Jones [Å] | 14.72 | —Equilibrium bending constant [Kcal/mol/rad2] | 14.98 |
| Parameters | Lower Bound | Upper Bound | Crosslink Failure Parametric Values | |
|---|---|---|---|---|
| Shear modulus of the fibril (MPa) | 1250 | 4250 | 2703.050 (724.298) | |
| Secondary stiffening of the fibril | 1.5 | 2.9 | 2.35 (0.238) | |
| Dimensionless fibril parameter 1 | 0.05 | 2 | 1.651 (0.249) | |
| Dimensionless fibril parameter 2 | 250 | 1000 | 731.002 (93.156) | |
| Dimensionless fibril parameter 3 | 20 | 110 | 50.842 (18.981) | |
| Yield strength of fibril | 411 | 2500 | 985.833 (550.556) | |
| Initial plastic strain rate | 0.01 | 0.025 | 0.016 (0.003) | |
| k | Rate sensitivity | 0.04 | 0.09 | 0.064 (0.014) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatib, F.A.; Gouissem, A.; Eilaghi, A.; Adouni, M. The Effect of Enzymatic Crosslink Degradation on the Mechanics of the Anterior Cruciate Ligament: A Hybrid Multi-Domain Model. Appl. Sci. 2021, 11, 8580. https://doi.org/10.3390/app11188580
Khatib FA, Gouissem A, Eilaghi A, Adouni M. The Effect of Enzymatic Crosslink Degradation on the Mechanics of the Anterior Cruciate Ligament: A Hybrid Multi-Domain Model. Applied Sciences. 2021; 11(18):8580. https://doi.org/10.3390/app11188580
Chicago/Turabian StyleKhatib, Fadi Al, Afif Gouissem, Armin Eilaghi, and Malek Adouni. 2021. "The Effect of Enzymatic Crosslink Degradation on the Mechanics of the Anterior Cruciate Ligament: A Hybrid Multi-Domain Model" Applied Sciences 11, no. 18: 8580. https://doi.org/10.3390/app11188580
APA StyleKhatib, F. A., Gouissem, A., Eilaghi, A., & Adouni, M. (2021). The Effect of Enzymatic Crosslink Degradation on the Mechanics of the Anterior Cruciate Ligament: A Hybrid Multi-Domain Model. Applied Sciences, 11(18), 8580. https://doi.org/10.3390/app11188580






