Featured Application
This paper proposes several quality marker regions that could enable fast FT-IR qualitative screening of lignocellulosic compounds in raw materials such as stems of fiber plants.
Abstract
Plant fibers are sustainable sources of materials for many industries, and can be obtained from a variety of plants. Cellulose is the main constituent of plant-based fibers, and its properties give the characteristics of the fibers obtained. Detailed characterization of cellulosic fibers is often performed after lengthy extraction procedures, while fast screening might bring the benefit of quick qualitative assessment of unprocessed stems. The aim of this research was to define some marker spectral regions that could serve for fast, preliminary qualitative characterization of unprocessed stems from some textile plants through a practical and minimally invasive method without lengthy extraction procedures. This could serve as a screening method for sorting raw materials by providing an accurate overall fingerprint of chemical composition. For this purpose, we conducted comparative Fourier Transform Infrared Spectroscopy (FT-IR) prospecting for quality markers in stems of flax (Linum usitatissimum L.), velvet leaf (Abutilon theophrasti Medik.), hemp (Cannabis sativa L.) and jute (Corchorus olitorius L.). Analysis confirmed the presence of major components in the stems of the studied plants. Fingerprint regions for cellulose signals were attributed to bands at 1420–1428 cm−1 assigned to the crystalline region and 896–898 cm−1 assigned to the amorphous region of cellulose. The optimization of characterization methods for raw materials is important and can find immediate practical applications.
1. Introduction
Natural fibers are renewable resources that can be obtained from a wide range of plants [1]. Some plants have been used for fibers since Neolithic times [2], while others were brought to attention only lately [3,4]. Fibers can be obtained from various organs of certain plants depending on the species, such as stem, bark, leaf, root, fruit or seed [4,5,6]. Today, plant-based natural fibers are considered highly convenient bio-degradable materials due to their low cost, recycling possibilities and lack of polluting emissions into the environment [7,8]. Due to both health and environmental concerns, natural fibers are more sought than synthetic ones and seen as a sustainable solution for most fiber-related applications [9]. Plant fibers have been used traditionally for fabrics, ropes, cordages, strings, canvas and mats [4]. However, lately, plant fibers are seen as versatile materials that can be used as fillers for reinforced composites, providing significantly increased durability and desired physical properties to matrices [6,10,11]. Such composites have wide applications today from automotive, aerospace to various other industrial and household applications [7,8,9,12]. Besides their primary importance as a source of fibers, some of these plant species can present other secondary valorization opportunities, which increases their economic significance (Table 1).

Table 1.
Primary and secondary importance of some commonly cultivated fiber species.
Plant fibers from stems and leaves are elongated sclerenchyma cells with lignified walls that confer mechanical strength to the plant [35]. The main component of the plant’s lignocellulosic fibers is cellulose in the form of semi-crystalline microfibrils (Figure 1) that are embedded within a matrix of hemicellulose and lignin [36,37,38,39].
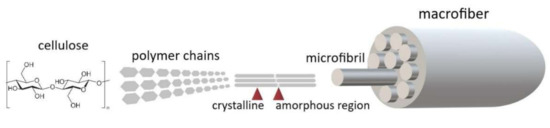
Figure 1.
Structural organization of lignocellulosic plant fibers (original drawing).
Cellulose is a versatile biopolymer representing the most abundant polysaccharide on Earth and an inexhaustible renewable material [40,41]. Its utilization is diverse from the textile and paper industries to pharmaceutical and food industries [42]. Cellulose comprises repeating unbranched subunits of glucose linked through glycosidic bonds that are arranged in parallel chains [42,43]. Between the chains, strong hydrogen bonds are formed that hold them together. The cellulose microfibrils display high tensile strength that constitutes their fundamental property and ensures the structural role they have in plants, while also making them useful in industry [37,43]. By comparison, lignin comprises three types of benzene–propane units that have a high molecular weight. It exhibits increased thermal stability while also decomposing slower. Hemicellulose has a structure that is random and amorphous [40]. The lignin and hemicellulose content in plants tends to increase with plant maturity [39].
The characterization of lignocellulosic fibers can be conveniently made based on analysis of the three components: cellulose, lignin and hemicellulose [40]. These constituents contain the function group -OH in their structure and explain the strong hydrophilic property of plant-based fibers [44]. In practice, lignocellulosic plant fibers are usually characterized after extraction. Parameters that present importance refer to chemical and physical properties [19]. However, characterization conducted after extraction depends to a great extent on the extraction procedure employed, with results varying with conditions and the isolation method used due to their qualitative influence over the sample [19,38,42]. In addition, these methods are most often lengthy procedures and do not allow quick analysis. A fast method would be highly suitable for raw materials such as stems to be quickly analyzed. However, so far, insufficient attention has been given to optimizing analysis methods for these materials. Particularly, there is need to clearly define spectral marker regions that could be considered in the qualitative assessment of such raw materials. The optimization of characterization methods for raw materials is important and could find immediate practical applications. Fast characterization might render a precise overall quality measurement that could be particularly helpful for fast qualitative screening with applications for the preliminary sorting of the raw materials represented by unprocessed plant stems before undergoing lengthy extraction and isolation procedures.
FT-IR (Fourier Transform Infrared Spectroscopy) is a highly useful tool that relies on the measurements of molecular bonds vibration modes, providing an informative fingerprint of the general chemical composition for a given sample. It has been used successfully for the characterization of plants and their constituents [45,46]. Defining fingerprint FT-IR spectral regions could serve for fast qualitative analysis of stems from fiber plants without the need for lengthy sample preparation. This could serve as a preliminary screening method for qualitative sorting, while also eliminating the influence of extraction and thus providing an accurate overall fingerprint for the raw material analyzed. Because cellulose is the main constituent of plant fibers, identification and characterization based on assigned spectral regions can enable inferences about its presence including certain parameters such as abundance, crystallinity or structural particularities of this constituent directly in stem tissue before any treatment that could alter or change the structural integrity of native cellulose.
The aim of this research was to screen the stems of four plant species that are commonly used for fibers (Linum usitatissimum L., Abutilon theophrasti Medik., Cannabis sativa L. and Corchorus olitorius L.) using the FT-IR method in order to provide fingerprint characterization and potential quality marker regions of fiber stems. To reach this goal, three objectives were established:
- Identification and assignment of functional groups to main bands and peaks common among the analyzed species;
- Identification of regions with highest variability as well as any particularities based on comparative assessment of spectra;
- Defining common spectral quality marker regions of cellulosic fibers among the four species.
From the species selected for this study, two have been traditionally cultivated in temperate conditions of Romania (flax and hemp), one is a common fiber crop from tropical–subtropical regions (jute) and the other has been less explored so far but might have good potential (velvet leaf).
2. Materials and Methods
2.1. Biologic Material
Biologic material for this study was represented by stems from plants cultivated in Cluj County Romania in the year 2019. Provenance of the seeds is mentioned in Table 2.

Table 2.
Samples analyzed through FT-IR method.
Plants were obtained from seeds and field-grown in Agro-Botanical Garden UASVM Cluj-Napoca. Seeds were obtained through International Plant Exchange Network, except Cannabis sativa seeds, which were procured from Agricultural Research and Development Station from Secuieni (Table 2). Voucher specimens were deposited at the Department of Botany—UASVM Cluj-Napoca, Romania (Voucher numbers 30083–30089).
2.2. Cultivation
Linum usitatissimum and Abutilon theophrasti species were sown directly in the field on 15 April 2019. The plants sprouted after about 15 days. The plants developed normally during the growing season. Flax cultivar ‘Domnești’ is among the first ones created in Romania.
Cannabis sativa ‘Zenit’ and ‘Succesiv’ are monoecious cultivars suitable for both seed and fiber production. These were created and are maintained by the Agricultural Research and Development Station from Secuieni from Romania (ARDS Secuieni, Neamț, Romania). The first one is the most widely cultivated in Romania, while the second one is a newly released cultivar. The growing season length for hemp is between 110 and 120 days for ‘Zenit’ and around 100 days for ‘Succesiv’. Both varieties were sown on 13 May 2019 and the plants started to grow after 9 days (22 April 2019) for ‘Succesiv’ variety and after 11 days (24 April 2019) for ‘Zenit’. The experimental field was organized according to a Latin rectangle design with subdivided plots with a sowing distance between rows of 60 cm and between plants in a row of 30 cm. Even if the rainfall was low, in the hemp field, no irrigation was carried out.
The crop of Corchorus olitorius was established by seedlings. Seeds were sown in a heated greenhouse. The sowing was conducted on 13 March 2019. Seeds sprouted after about 10 days (23 March 2019) in all accessions studied. Pricking of seedlings into larger pots was performed when the plantlets had 3–4 fully developed leaves. The field crop was established in early June (2 June 2019), as weather conditions due to excessive rainfall did not allow planting in May, which is considered the optimal period. Planting was performed at a distance of 50 cm between rows and 30 cm between plants in the row. After planting and during the summer, for jute watering was applied daily for a week after planting, then every 10 days during the months of June and July.
The establishment of the plants in the field was considered good because plants had a healthy aspect and development followed the normal phenological succession for the species.
2.3. Climatic Conditions and Soil
Romania is situated within temperate climatic zone with four seasons. Climatic conditions during the year 2019 when plants were cultivated are presented in Figure 2. Overall, the experimental year was drier and hotter than the average of previous decade. Frost occurred in winter and spring until April, and again in autumn starting with the month of October. Temperatures reached over 30 °C throughout June to September, but highest monthly average temperatures were registered for June and August. High precipitation levels occurred in May followed by decreased rainfall until the end of the year.
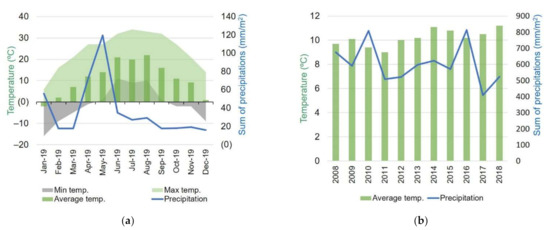
Figure 2.
Climatic conditions in Cluj County in (a) 2019, and (b) previous decade [50,51].
In Agro-Botanical Garden UASVM Cluj-Napoca, the soil has a clay-loam texture with a good humus content and the following macro-nutrient supply: Nitrogen 0.46%, Phosphorus 68 ppm, Potassium 312 ppm [52].
2.4. Spectroscopic Assay
Mature stems of the fiber plants were collected from the experimental field in September 2019 and placed for slow drying at room temperature in laboratory. Stems were randomly collected from ten plants per species/cultivar/accession, were cut into small pieces and were ground together (having equal contribution) to obtain a composed sample for each species/cultivar/accession. A small quantity of powder was taken for analysis.
Analysis was conducted at spectroscopy laboratory from Life Sciences Institute “King Michael I of Romania” from Cluj-Napoca. Samples were prepared by mixing 200 calcinated KBr with 3 mg of sample [52]. The sample and KBr were ground again together with an agate mortar and pestle until the mix was fully homogenized and a very fine powder was obtained. The mix was placed into steel spectral pellet kit and vitrification of the pellet was obtained with Specac hydraulic press (Figure 3).

Figure 3.
Spectral pellets, macroscopic aspect of ground samples, microscopic observation of coarse ground samples magnified 64× showing fibrous texture for the samples: (a) cellulose (standard), (b) Linum usitatissimum ‘Domneşti’, (c) Cannabis sativa ‘Succesiv’, (d) Cannabis sativa ‘Zenit’, (e) Corchorus olitorius GZU, (f) Corchorus olitorius NTM, (g) Corchorus olitorius SZTE, (h) Abutilon theophrasti.
Each pellet was immediately loaded into cassette of Fourier Transform Infrared Spectrometer Jasco FT/IR 4100 for measurements. Scanning range was 4000–350 cm−1 and resolution 4.0 cm−1. Each spectrum represents the average of 256 scans performed on one sample pellet per species/cultivar/accession. All spectra obtained received five corrections for CO2 and five corrections for H2O using Spectra Manager. Peak reading for detailed analysis was conducted with Origin by OriginLab (Figure 4).
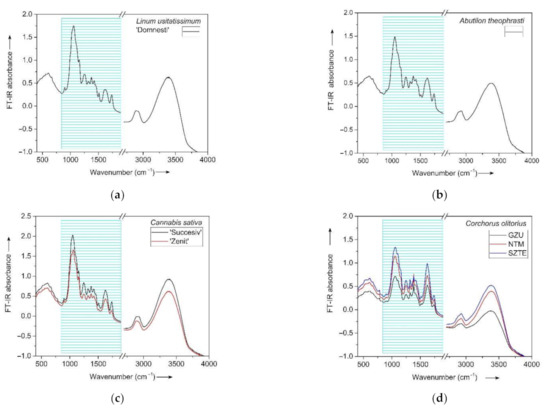
Figure 4.
FT-IR spectra for stems of flax (a), velvet leaf (b), hemp (c) and jute (d), showing the regions selected for detailed analysis.
For characterization of the bands of each species (Figure 4), the following regions were defined: 650–1200 cm−1, 1200–1800 cm−1 and 2500–3500 cm−1. Based on data from the literature, spectral features were attributed to functional groups and corresponding compounds. Bands were also compared against a cellulose standard. For detailed analysis, a spectral range was established as well (Figure 4).
3. Results and Discussion
3.1. Characterization of Main Spectral Regions
3.1.1. Spectral Region 650–1200 cm−1
Within region 650–750 cm−1, samples from stems of the four species analyzed in this study as well as the cellulose standard presented a broad band with several weak peaks (Figure 4). Previous studies attributed the band from ≈660 cm−1 to the out-of-plane bending vibration [3,53], while the one at 710 cm−1 was attributed to CH2 rocking vibration [53].
For the cellulose standard, besides the peak at 897 cm−1, we observed another one at 876 cm−1 that was not present in the stems of the four species analyzed (Figure 5). The peak situated in this region (≈890 cm−1) was attributed to glycosidic bonds of monosaccharides [3,53,54,55,56] as well as to the amorphous region of cellulose [40].
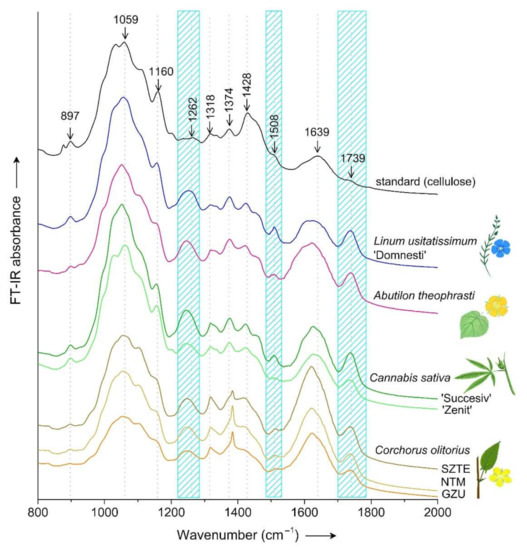
Figure 5.
Detail of FT-IR spectral region 800–2000 cm−1 for stems of flax, velvet leaf, hemp and jute compared to cellulose; cyan strips mark regions of high variability compared to standard.
Similarly, in Abutilon indicum lignocellulosic fibers, a peak around 899 cm−1 was identified and attributed to the vibration of functional group C–O–C [10]. FT-IR investigation of Gossypium hirsutum fiber development revealed that the intensity of bands at 897 cm−1, besides those at 667 cm−1, has a relationship with cellulose content [53]. The fact that the signal was stronger for the cellulose standard sample suggests that these are in fact due to cellulose. The signal was the weakest in jute samples and strongest in flax and hemp, out of the four species studied (Figure 5).
3.1.2. Spectral Region 1200–1800 cm−1
Within region 1244–1252 cm−1, plant stem samples displayed a strong band that showed a very low intensity in the cellulose standard (Figure 5). According to the literature, the peak close to this range, at 1230 cm−1, was attributed to methoxyl groups from the structure of lignin [43]. The peak occurring within a similar range was attributed to C–O stretching vibration of the acyl group of lignin [3,8,36,43,54,56,57]. The disappearance of the peak at 1239 cm−1 from kenaf fibers [36] as well of the peak at 1243 cm−1 from corn stem fibers [8] was attributed to the removal of lignin following chemical treatments. Considering that the peak within this region was missing in the standard sample, it can be assigned to other components than cellulose, such as lignin.
Within region 1374–1384 cm−1, a peak of varying intensity was observed in the four species analyzed as well as in the cellulose standard sample. Among samples and species, jute samples displayed the highest intensity of this peak, and showed a particular sharp shape compared to the other samples (Figure 5). This could be considered a species particularity, as it was observed in all three jute accessions. According to the literature, the peak in this region based on FT-IR analysis of bamboo samples was assigned to C–H deformation vibration [3,53,58] from cellulose and hemicellulose [58] (Table 3).

Table 3.
Summary of peak positions for FT-IR spectra of stems from four fiber species within region 800–1800 cm−1.
Within range 1420–1428 cm−1, a band displaying a peak and shoulder was observed in all samples, but was most prominent in the cellulose standard and least distinguishable in jute samples (Figure 5). The investigation of vegetal and wood fibers defined the band at 1420–1430 cm−1 as representative for crystalline structural parameters of cellulose [40]. Since crystallinity has an influence on the fiber mechanical property [39], this region represents a good candidate as a marker region. Within the same range, a study of bamboo stems assigned the peaks of this region to CH2 scissoring as well as bending vibration of olefin, as characteristics both for cellulose as well as hemicellulose [58].
In bamboo samples, the characteristic peak of lignin was detected at 1230 cm−1 and 1506 cm−1 [43]. In the case of Epipremnum aureum stem fibers, the lignin characteristic peaks were found at 1240 cm−1 and caused by C–O stretching vibration, while the peak of low intensity around 1500 cm−1 was determined to be due to C=C stretching vibration of the benzene ring of lignin [7]. In this study, the decrease in intensity of peaks between 1245–1252 cm−1 and 1508–1509 cm−1 can be attributed to a lower of lignin content, and could be used as a purity criterion for cellulose-based products and materials. A similar decrease in peaks at 1506 cm−1 and 1230 cm−1 was identified in bamboo samples after treatment for the isolation of the cellulose fraction, which was due to the dissociation of functional groups from components of lignin such as the dissolution of aromatic rings [43].
The peak situated within region 1620–1628 cm−1 was identified in all four species analyzed, and could be attributed to both C=C vibration from lignin as assigned based on Abutilon indicum fibers [10], superimposed with C=O vibration corresponding to amide I as assigned based on FT-IR analysis of cotton fibers [53] (Table 3). In jute plants from this study, the band of this region was situated at a lower wavenumber, but also displayed the highest intensity out of the species analyzed, particularly the accession from Hungary (SZTE). For flax and velvet leaf samples, the broad band of this region was situated at a slightly higher wavenumber and the shape of the band was more rounded compared to jute and hemp, which displayed a band with a more acute shape (Figure 5). In the standard sample represented by cellulose paper, there was a band at 1638 cm−1, which could be attributed to O–H bending vibration from absorbed water [53].
The stems of all four species presented a peak between 1738 and 1740 cm−1 (Figure 5). Similarly, intense peaks in region 1715–1756 cm−1 were identified in Fimbristylis globulosa following FT-IR analysis and were assigned to C=O of ketone and carbonyl groups of hemicellulose [54]. The peak assigned as well to C=O stretching vibration was situated at 1737 cm−1 for Abutilon indicum fibers [10] and Chrysanthemum morifolium [56]. For Epipremnum aureum fibers, the C=O stretching vibration of the acetyl group from hemicellulose produced a peak at a higher wavenumber: 1757 cm−1 [7]. For bamboo, the peak was found at 1735 cm−1 [43,58], as well as for Thespesia populnea [39], while for banana fibers the peak was at 1730 cm−1 [44] (Table 3). An increased proportion of hemicellulose causes this peak to become narrow and sharp and as the plant matures and ages [39]. The decrease in intensity of this peak following treatments was attributed to the cleavage of the molecular bonds [43]. The disappearance of the peak in this region was attributed to the removal of hemicellulose [8,36,54]. This peak was not observed in the cellulose standard (Figure 5), since hemicellulose and lignin are mostly removed from the paper during the production process. Cannabis sativa ‘Succesiv’ compared to ‘Zenit’ presented a stronger intensity of bands at 1244–1245 cm−1 and 1739 cm−1 (Figure 5), a fact that could indicate a higher lignin and, respectively, higher hemicellulose content in the first cultivar compared to the second one. Out of the analyzed samples, jute samples had the sharpest peak, which would suggest a higher hemicellulose content, while the jute accession from Hungary (SZTE) showed the highest peak intensity out of all samples analyzed. It should be noticed that the peak intensity among jute samples also varied.
3.1.3. Spectral Region 2500–3500 cm−1
A broad band around 2900 cm−1 was identified in all samples—both in stems as well as the standard cellulose sample (Figure 4). The band from this range was attributed to the C–H stretching from the aromatic methyl group of cellulose and hemicellulose also identified following FT-IR analysis of Abutilon indicum fibers [10]. Mauritia flexuosa fibers were shown to present two bands around 2918 and 2849 cm−1 that were associated with increased levels of organic extractives such as phenolic acid methyl esters and fatty acid methyl esters. This, in addition to increased water content, might indicate an easier degradation onset of these fibers [40], which is not a desired characteristic. At 2922 cm−1, Thespesia populnea fibers also displayed a band that was attributed to stretching vibration of the C–H molecular bond from functional groups CH2 and CH3 (Table 3). This band was shown to shrink once with stem maturity [39].
A broad band at 3000–3700 cm−1 was present in all samples (Figure 4). A similar broad band was identified in cotton samples and was attributed to OH stretching vibration that is due to the hydrogen bonds of cellulose. Particularly, the peaks at 3286 cm−1 and 3336 cm−1 were assigned to intra-molecular bonds from cellulose fibers, with the first one becoming evident earlier and second one becoming evident later during cotton fiber development [53]. Peaks at 3432 cm−1 and 3342 cm−1 were attributed to intramolecular hydrogen bonds from cellulose, while the band at 3568–3577 cm−1 was assigned to the signal of intramolecular hydrogen bonds from a phenolic functional group of lignin [40].
3.2. Quality Marker Regions and Their Significance
Comparing the stem spectra with those of a cellulose standard, we identified several spectral areas with high variability among samples. Particularly, we identified three regions present in all four species analyzed that were either weaker or missing in the cellulose standard. These were situated between 1245–1252 cm−1, 1508–1509 cm−1 and 1738–2740 cm−1. Because these were very weak or absent in the cellulose standard sample, they can be attributed to other constituents. The comparison of FT-IR spectra from bamboo stems and isolated cellulose nano-fibers has also shown the presence of several peaks in stems that are not due to cellulose’s presence but are attributed to other constituents. In the case of bamboo, these were situated at 1230 cm−1, 1456 cm−1, 1506 cm−1 and 1735 cm−1 [43].
The investigation into lignocelluloses of bamboo proved the existence of a strong correlation between their concentration and IR spectroscopic measurements [8], a fact that further confirms that the intensity of spectral absorbance can reflect the quantity of those components in the sample.
The study conducted on bamboo defined characteristic FT-IR spectral regions for cellulose and hemicellulose between 1363 and 1893 cm−1, and between 1315 and 1942 cm−1 for lignin. Within this overlapping range, specific bands for each of the three constituents were selected and defined as characteristic fingerprints of lignocelluloses [8].
The proportion of cellulose in plant fibers plays the definitive role in fiber characteristics, and due to this the study of cellulose from fibers is important before being considered for use [39]. Defining criteria for cellulose characteristics are the distribution of functional groups within the repeating subunits and along the chains, the number of inter and intra hydrogen bounds at the molecular level, the length of the chains and their distribution and crystallinity [40].
Based on band assignment, for cellulose key marker ranges proposed for species analyzed in the current study correspond to a signal for the amorphous region from cellulose at 896–898 cm−1, for the crystalline region at 1420–1428 cm−1 and, respectively, inter and intra hydrogen bonds occurring at ≈3000 cm−1. Hydrogen bonds are responsible for certain properties of lignocellulosic fibers, considering that closer packed cellulose chains due to stronger and more hydrogen links would translate into increased mechanical strength [40]. Because the quality criteria sought to meet for fibers are high crystallinity and high tensile strength, the specific bands from the last two spectral ranges mentioned should be associated with desired characteristics with increasing corresponding band intensity.
During the processing of fibrous plant materials, various constituents such as lignin, hemicellulose, pectin and extractives such as waxes are removed because they are responsible for undesired qualitative characteristics. However, lignin is a constituent that increases in plants with the ageing process [39], and when present within plants it has been associated with pathogen resistance [60]. From this perspective, an increased absorption signal for lignin in stems of plants could indicate the older age of the plant or a higher resistance to pathogens. However, for fiber quality, lignin is not a desired constituent, and should not be present after cellulosic fiber extraction. Additionally, for extractive processes, lower levels and thus a weaker signal for this constituent are sought. Based on the four plant species analyzed, the signature lignin regions were assigned to spectral intervals 1245–1252 cm−1 and 1508–1509 cm−1. Other constituents that are undesired in fibers are hemicellulose, which displayed a characteristic signal in region 1738–1740 cm−1, and extractives such as fatty acids, which were assigned to spectral region ≈2800–2900 cm−1.
Both IR and Raman spectroscopy have been among the most widely used instruments employed in the characterization of plant constituents including cellulose [61,62].
FT-IR spectroscopy has been successfully used to confirm the crystalline structure or to analyze chemical constituents of various ligno-cellulosic materials, not limited to textile plants but wood as well. In this sense, the study of wood powder from Pinus sylvestris var. mongolica after alkali treatment displayed changes in crystalline structure marked by spectral changes including a decrease in intensity of the band from 1428 cm−1 and a shift at 1418 cm−1. Additionally, the disappearance of the peak at 1507 cm−1 from purified wood pulp was attributed to the removal of lignin, while the peak at 1737 cm−1 was attributed to the leaching of hemicellulose after treatments with alkali [63].
Spectra of pure cellulose Iβ film analyzed comparatively through vibrational methods IR and Raman allowed the identification of vibrational modes of crystalline cellulose, while also highlighting differences and correspondence among bands of spectra obtained though these techniques. The spectral region below 1000 cm−1 can be considered as a domain of skeletal deformation vibration modes both for IR and Raman, followed until 1500 cm−1 with regions that exhibit bands associated with highly delocalized skeletal stretching. However, C–O stretches around 1000 cm−1 as well as polar group vibration modes (OH stretches) in 3200–3600 cm−1 were shown to be stronger in IR than in Raman spectra, while CH and CH2 stretches around 2800–3000 cm−1 are prominent in both [62].
Vibrational spectroscopy remains a facile analysis technique for plant samples. The optimization of these characterization methods for plant-based raw materials can extend their practical applications.
Cellulose from textile plants is the starting material for many derivatives [64] depending on the destination of use. Chemically unaltered cellulose, known as cellulose I, is characterized by a specific crystal form. After treatments, cellulose experiences changes in properties and characteristics, particularly its crystal structure, leading to so-called cellulose II, III and IV being obtained. These treatments are meant to restructure the cellulose to obtain lattice structuring or to stretch the fibers [35], all these being required for processing and various optimized uses.
The analysis of cellulose in the native state as it is found in textile plants before any significant structural modifications take place through processing, such as changes in the orientation of chains that occur following mercerization [65], can provide a characterization at the point “0” of the primary state in which cellulose is found in raw material. The identification of cellulose-specific regions in stems of given textile plants provides the basis and starting point for qualitative sorting and preliminary qualitative characterization. This could prove useful for deciding the destination of use of the raw material according to desired cellulose parameters or to select for further processes a specific type of stem according to pre-established criteria.
4. Conclusions
Fibers obtained from textile plants are versatile materials with many applications. Cellulose is the main constituent of fibers obtained from textile plants. The detailed characterization of cellulosic fibers is often performed after lengthy extraction procedures. The optimization of a fast analysis method for unprocessed stems requires defined spectral marker regions that could be considered in the qualitative assessment of such raw materials.
This research used FT-IR for a comparative screening of unprocessed stems from four species (flax, hemp, jute and velvet leaf) in order to identify candidate quality marker regions that could enable fast qualitative characterization of these raw materials.
Based on the obtained spectra and literature data, the presence of major constituents was confirmed for the four species, and several regions were proposed as potential quality maker candidates. The bands at 1420–1428 cm−1 were assigned to the crystalline region of cellulose, and those at 896–898 cm−1 were assigned to the amorphous region of cellulose.
FT-IR could be used as a preliminary screening method for the quick analysis of stems from textile plants before the extraction and isolation of the fibers.
Author Contributions
Conceptualization, R.V., D.V., A.O. and I.C.; methodology, R.Ș.; software, R.Ș.; validation, R.Ș. and L.O.; formal analysis, I.C.; investigation, I.C., R.Ș. and L.O.; resources, R.V. and A.O.; data curation, R.V. and A.S.; writing—original draft preparation, I.C. and R.V.; writing—review and editing, R.V., D.V., A.O., A.S., L.O., R.Ș.; visualization, I.C.; supervision, R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Voucher plant specimens were deposited at the Department of Botany—UASVM Cluj-Napoca, Romania. Spectroscopic data records are digitally stored at the Department of Biophysics, Life Sciences Institute King Michael I of Romania from Cluj-Napoca.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Madhu, P.; Sanjay, M.R.; Senthamaraikannan, P.; Pradeep, S.; Saravanakumar, S.S.; Yogesha, B. A Review on Synthesis and Characterization of Commercially Available Natural Fibers: Part-I. J. Nat. Fibers 2019, 16, 1132–1144. [Google Scholar] [CrossRef]
- Fisher, C.H. History of Natural Fibers. J. Macromol. Sci. Part Chem. 1981, 15, 1345–1375. [Google Scholar] [CrossRef]
- Maache, M.; Bezazi, A.; Amroune, S.; Scarpa, F.; Dufresne, A. Characterization of a Novel Natural Cellulosic Fiber from Juncus effusus L. Carbohydr. Polym. 2017, 171, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Jebadurai, S.G.; Raj, R.E.; Sreenivasan, V.S.; Binoj, J.S. Comprehensive Characterization of Natural Cellulosic Fiber from Coccinia grandis Stem. Carbohydr. Polym. 2019, 207, 675–683. [Google Scholar] [CrossRef]
- Ramawat, K.G.; Ahuja, M.R. Fiber Plants: An Overview. In Fiber Plants: Biology, Biotechnology and Applications; Ramawat, K.G., Ahuja, M.R., Eds.; Sustainable Development and Biodiversity; Springer International Publishing: Cham, The Netherlands, 2016; pp. 3–15. ISBN 978-3-319-44570-0. [Google Scholar]
- Manimaran, P.; Prithiviraj, M.; Saravanakumar, S.S.; Arthanarieswaran, V.P.; Senthamaraikannan, P. Physicochemical, Tensile, and Thermal Characterization of New Natural Cellulosic Fibers from the Stems of Sida cordifolia. J. Nat. Fibers 2018, 15, 860–869. [Google Scholar] [CrossRef]
- Maheshwaran, M.V.; Hyness, N.R.J.; Senthamaraikannan, P.; Saravanakumar, S.S.; Sanjay, M.R. Characterization of Natural Cellulosic Fiber from Epipremnum aureum Stem. J. Nat. Fibers 2018, 15, 789–798. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, J.; Wu, N.; Ma, Y.; Menon, C.; Tong, J. Characterization of Natural Cellulose Fiber from Corn Stalk Waste Subjected to Different Surface Treatments. Cellulose 2019, 26, 4707–4719. [Google Scholar] [CrossRef]
- Indran, S.; Raj, R.E. Characterization of New Natural Cellulosic Fiber from Cissus quadrangularis Stem. Carbohydr. Polym. 2015, 117, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hassan, E.A.M.; Memon, H.; Elagib, T.H.H.; Abad AllaIdris, F. Characterization of Natural Composites Fabricated from Abutilon-Fiber-Reinforced Poly (Lactic Acid). Processes 2019, 7, 583. [Google Scholar] [CrossRef] [Green Version]
- Bhuvaneshwaran, M.; Subramani, S.P.; Palaniappan, S.K.; Pal, S.K.; Balu, S. Natural Cellulosic Fiber from Coccinia indica Stem for Polymer Composites: Extraction and Characterization. J. Nat. Fibers 2021, 18, 644–652. [Google Scholar] [CrossRef]
- Vignesh, V.; Balaji, A.N.; Karthikeyan, M.K.V. Extraction and Characterization of New Cellulosic Fibers from Indian Mallow Stem: An Exploratory Investigation. Int. J. Polym. Anal. Charact. 2016, 21, 504–512. [Google Scholar] [CrossRef]
- Botanic Classification and Taxonomy. Available online: http://www.worldfloraonline.org/classification (accessed on 6 July 2021).
- Jhala, A.J.; Hall, L.M.; Hall, J.C. Potential Hybridization of Flax with Weedy and Wild Relatives: An Avenue for Movement of Engineered Genes? Crop Sci. 2008, 48, 825–840. [Google Scholar] [CrossRef]
- Yu, J.; Jin, W.; Wang, Y.; Yuan, Q. The Complete Chloroplast Genome of a Medicinal Plant, Abutilon theophrasti Medik. (Malvaceae). Mitochondrial DNA Part B 2020, 5, 3759–3760. [Google Scholar] [CrossRef]
- McPartland, J.M.; Hegman, W.; Long, T. Cannabis in Asia: Its Center of Origin and Early Cultivation, Based on a Synthesis of Subfossil Pollen and Archaeobotanical Studies. Veg. Hist. Archaeobot. 2019, 28, 691–702. [Google Scholar] [CrossRef]
- Benor, S. Molecular Phylogeny of the Genus Corchorus (Grewioideae, Malvaceae s.l.) Based on Nuclear RDNA ITS Sequences. Crop J. 2018, 6, 552–563. [Google Scholar] [CrossRef]
- Zommere, G.; Viļumsone, A.; Kalniņa, D.; Soliženko, R.; Stramkale, V. Comparative Analysis of Fiber Structure and Cellulose Contents in Flax and Hemp Fibres. Mater. Sci. Text. Cloth. Technol. 2013, 8, 96–104. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Characterizing Natural Cellulose Fibers from Velvet Leaf (Abutilon theophrasti) Stems. Bioresour. Technol. 2008, 99, 2449–2454. [Google Scholar] [CrossRef] [PubMed]
- Tanmoy, A.; Alum, M.; Islam, M.; Farzana, T.; Khan, H. Jute (Corchorus olitorius Var. O-72) Stem Lignin: Variation in Content with Age. Bangladesh J. Bot. 2014, 43, 309–314. [Google Scholar] [CrossRef]
- Palla, A.H.; Khan, N.A.; Bashir, S.; Ur-Rehman, N.; Iqbal, J.; Gilani, A.-H. Pharmacological Basis for the Medicinal Use of Linum usitatissimum (Flaxseed) in Infectious and Non-Infectious Diarrhea. J. Ethnopharmacol. 2015, 160, 61–68. [Google Scholar] [CrossRef]
- Leson, G.; Pless, P. Hemp seed and hemp oil. In Cannabis and Cannabinoids: Pharmacology, Toxicology, and Therapeutic Potential; Haworth Press: Philadelphia, PA, USA, 2002; p. 411. [Google Scholar]
- Chigurupati, S.; Aladhadh, H.; Alhowail, A.; Krishnan Selvarajan, K.; Bhatia, S. Phytochemical Composition, Antioxidant and Antidiabetic Potential of Methanolic Extract from Corchorus olitorius Linn. Grown in Saudi Arabia. Med. Plants Int. J. Phytomed. Relat. Ind. 2020, 12, 71. [Google Scholar] [CrossRef]
- Jhala, A.J.; Hall, L.M. Flax (Linum usitatissimum L.): Current Uses and Future Applications. Aust. J. Basic Appl. Sci. 2010, 4, 4304–4312. [Google Scholar]
- Traoré, K.; Parkouda, C.; Savadogo, A.; Ba/Hama, F.; Kamga, R.; Traoré, Y. Effect of Processing Methods on the Nutritional Content of Three Traditional Vegetables Leaves: Amaranth, Black Nightshade and Jute Mallow. Am. J. Food Sci. Technol. 2019, 7, 223–226. [Google Scholar] [CrossRef]
- Samuel, F.O.; Ayoola, P.B.; Ejoh, S.I. Nutrient, Antinutrient and Sensory Evaluation of Corchorus olitorius Fruit. Ife J. Agric. 2020, 32, 13–20. [Google Scholar]
- Dinu, M.; Uivarosi, V.; Popescu, M.-L.; Radulescu, V.; Arama, C.C.; Nicolescu, O.; Ancuceanu, R.V. Proximate Composition and Some Physico-Chemical Properties of Abutilon theophrasti (Velvetleaf) Seed Oil. Rev. Chim. 2021, 61, 50–54. [Google Scholar]
- Ibrahim, T.A.; Fagbohun, E.D. Physicochemical Properties and In Vitro Antibacterial Activity of Corchorus olitorius Linn. Seed Oil. Life Sci. Leafl. 2011, 15, 499–505. [Google Scholar]
- Saleem, M.H.; Ali, S.; Hussain, S.; Kamran, M.; Chattha, M.S.; Ahmad, S.; Aqeel, M.; Rizwan, M.; Aljarba, N.H.; Alkahtani, S.; et al. Flax (Linum Usitatissimum L.): A Potential Candidate for Phytoremediation? Biological and Economical Points of View. Plants 2020, 9, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linger, P.; Müssig, J.; Fischer, H.; Kobert, J. Industrial Hemp (Cannabis sativa L.) Growing on Heavy Metal Contaminated Soil: Fibre Quality and Phytoremediation Potential. Ind. Crops Prod. 2002, 16, 33–42. [Google Scholar] [CrossRef]
- Ndlovu, S.; Pullabhotla, R.V.S.R.; Ntuli, N.R. Response of Corchorus olitorius Leafy Vegetable to Cadmium in the Soil. Plants 2020, 9, 1200. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Green Biosynthesis of Flaxseed Gold Nanoparticles (Au-NPs) as Potent Anti-Cancer Agent Against Breast Cancer Cells. J. Saudi Chem. Soc. 2021, 25, 101243. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Garnæs, J.; Tunjic, S.; Mokkapati, V.R.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green Synthesis of Gold and Silver Nanoparticles from Cannabis sativa (Industrial Hemp) and Their Capacity for Biofilm Inhibition. Int. J. Nanomed. 2018, 13, 3571–3591. [Google Scholar] [CrossRef]
- El-Rafie, H.; Abd, S. Bioactivities of Gold and Iron Oxide Nanoparticles Biosynthesized from the Edible Plant Corchorus olitorius. Pharm. Lett. 2016, 8, 156–164. [Google Scholar]
- Smole, M.S.; Hribernik, S.; Kleinschek, K.S.; Kreže, T. Plant Fibres for Textile and Technical Applications; Intech Open: London, UK, 2013; ISBN 978-953-51-1184-9. [Google Scholar]
- Jonoobi, M.; Harun, J.; Tahir, P.M.; Shakeri, A.; SaifulAzry, S.; Makinejad, M.D. Physicochemical Characterization of Pulp and Nanofibers from Kenaf Stem. Mater. Lett. 2011, 65, 1098–1100. [Google Scholar] [CrossRef]
- Thomas, S.; Paul, S.A.; Pothan, L.A.; Deepa, B. Natural Fibres: Structure, Properties and Applications. In Cellulose Fibers: Bio- and Nano-Polymer Composites: Green Chemistry and Technology; Kalia, S., Kaith, B.S., Kaur, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–42. ISBN 978-3-642-17370-7. [Google Scholar]
- Célino, A.; Freour, S.; Jacquemin, F.; Casari, P. The Hygroscopic Behavior of Plant Fibers: A Review. Front. Chem. 2014, 1, 43. [Google Scholar] [CrossRef] [Green Version]
- Kathirselvam, M.; Kumaravel, A.; Arthanarieswaran, V.P.; Saravanakumar, S.S. Assessment of Cellulose in Bark Fibers of Thespesia populnea: Influence of Stem Maturity on Fiber Characterization. Carbohydr. Polym. 2019, 212, 439–449. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi Júnior, H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [Green Version]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Ventura-Cruz, S.; Tecante, A. Extraction and Characterization of Cellulose Nanofibers from Rose Stems (Rosa spp.). Carbohydr. Polym. 2019, 220, 53–59. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.-Y.; De Hoop, C.F.; Hu, T.; Qi, J.; Shupe, T.F. Isolation and Characterization of Cellulose Nanofibers from Bamboo Using Microwave Liquefaction Combined with Chemical Treatment and Ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Gañán, P.; Cruz, J.; Garbizu, S.; Arbelaiz, A.; Mondragon, I. Stem and Bunch Banana Fibers from Cultivation Wastes: Effect of Treatments on Physico-Chemical Behavior. J. Appl. Polym. Sci. 2004, 94, 1489–1495. [Google Scholar] [CrossRef]
- Gorgulu, S.T.; Dogan, M.; Severcan, F. The Characterization and Differentiation of Higher Plants by Fourier Transform Infrared Spectroscopy. Appl. Spectrosc. 2007, 61, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Dai, D.; Huang, B. Fourier Transform Infrared Spectroscopy for Natural Fibres; Intech Open: London, UK, 2012; ISBN 978-953-51-0594-7. [Google Scholar]
- Academic Press. Hortus Agrobotanicus Napocensis—Index Seminum; Academic Press: Cluj-Napoca, Romania, 2021. [Google Scholar]
- EU Common Catalogue of Varieties of Agricultural Plant Species. Available online: https://ec.europa.eu/food/system/files/2021-02/plant-variety-catalogues_agricultural-plant-species.pdf (accessed on 11 July 2021).
- The International Plant Exchange Network. Available online: https://www.bgci.org/our-work/policy-and-advocacy/access-and-benefit-sharing/the-international-plant-exchange-network/ (accessed on 4 July 2021).
- Climate Data for Cluj-Napoca, Romania Weather History. Available online: https://www.wunderground.com/history/daily/ro/cluj-napoca (accessed on 11 July 2021).
- Tutiempo Network Climate Data for Cluj-Napoca, Romania Weather Station 151200. Available online: https://en.tutiempo.net/climate/ws-151200.html (accessed on 11 July 2021).
- Crișan, I.; Vidican, R.; Olar, L.; Stoian, V.; Morea, A.; Ștefan, R. Screening for Changes on Iris germanica L. Rhizomes Following Inoculation with Arbuscular Mycorrhiza Using Fourier Transform Infrared Spectroscopy. Agronomy 2019, 9, 815. [Google Scholar] [CrossRef] [Green Version]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the Cell Wall and Cellulose Content of Developing Cotton Fibers Investigated by FTIR Spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Suryanto, H.; Marsyahyo, E.; Irawan, Y.S.; Soenoko, R. Morphology, Structure, and Mechanical Properties of Natural Cellulose Fiber from Mendong Grass (Fimbristylis globulosa). J. Nat. Fibers 2014, 11, 333–351. [Google Scholar] [CrossRef]
- Longaresi, R.H.; de Menezes, A.J.; Pereira-da-Silva, M.A.; Baron, D.; Mathias, S.L. The Maize Stem as a Potential Source of Cellulose Nanocrystal: Cellulose Characterization from Its Phenological Growth Stage Dependence. Ind. Crops Prod. 2019, 133, 232–240. [Google Scholar] [CrossRef]
- Dalmis, R.; Kilic, G.B.; Seki, Y.; Koktas, S.; Keskin, O.Y. Characterization of a Novel Natural Cellulosic Fiber Extracted from the Stem of Chrysanthemum morifolium. Cellulose 2020, 27, 8621–8634. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Dhlamini, M.S.; Mothudi, B.M.; Kommula, V.P.; Zhang, J.; Zhang, J.; Rajulu, A.V. Extraction and Characterization of Cellulose Single Fibers from Native African Napier Grass. Carbohydr. Polym. 2018, 188, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, Y.; Xu, J.; Xu, N.; He, Y. Quantitative Visualization of Lignocellulose Components in Transverse Sections of Moso Bamboo Based on FTIR Macro- and Micro-Spectroscopy Coupled with Chemometrics. Biotechnol. Biofuels 2018, 11, 263. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, T.; Kumaravel, A.; Palanivelu, R. Extraction and Characterization of Natural Cellulosic Fiber from Passiflora foetida Stem. Int. J. Polym. Anal. Charact. 2016, 21, 478–485. [Google Scholar] [CrossRef]
- Bhuiyan, N.H.; Selvaraj, G.; Wei, Y.; King, J. Role of Lignification in Plant Defense. Plant Signal. Behav. 2009, 4, 158–159. [Google Scholar] [CrossRef] [Green Version]
- Cael, J.; Gardner, K.; Koenig, J.; Blackwell, J. Infrared and Raman Spectroscopy of Carbohydrates. Paper V. Normal Coordinate Analysis of Cellulose I. J. Chem. Phys. 1975, 62, 1145–1153. [Google Scholar] [CrossRef]
- Makarem, M.; Lee, C.M.; Kafle, K.; Huang, S.; Chae, I.; Yang, H.; Kubicki, J.D.; Kim, S.H. Probing Cellulose Structures with Vibrational Spectroscopy. Cellulose 2019, 26, 35–79. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Wu, T.; Wang, X.; Cheng, X.; Li, D. A Comparative Study on the Characterization of Nanofibers with Cellulose I, I/II, and II Polymorphs from Wood. Polymers 2019, 11, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferro, M.; Mannu, A.; Panzeri, W.; Theeuwen, C.H.J.; Mele, A. An Integrated Approach to Optimizing Cellulose Mercerization. Polymers 2020, 12, 1559. [Google Scholar] [CrossRef]
- Zlenko, D.V.; Vtyurina, D.N.; Usachev, S.V.; Skoblin, A.A.; Mikhaleva, M.G.; Politenkova, G.G.; Nikolsky, N.S.; Stovbun, V.S. On the orientation of the chains in the mercerized cellulose. Sci. Rep. 2021, 11, 8765. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




