Vitamin C-Assisted Fabrication of Aerogels from Industrial Graphene Oxide for Gaseous Hexamethyldisiloxane Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
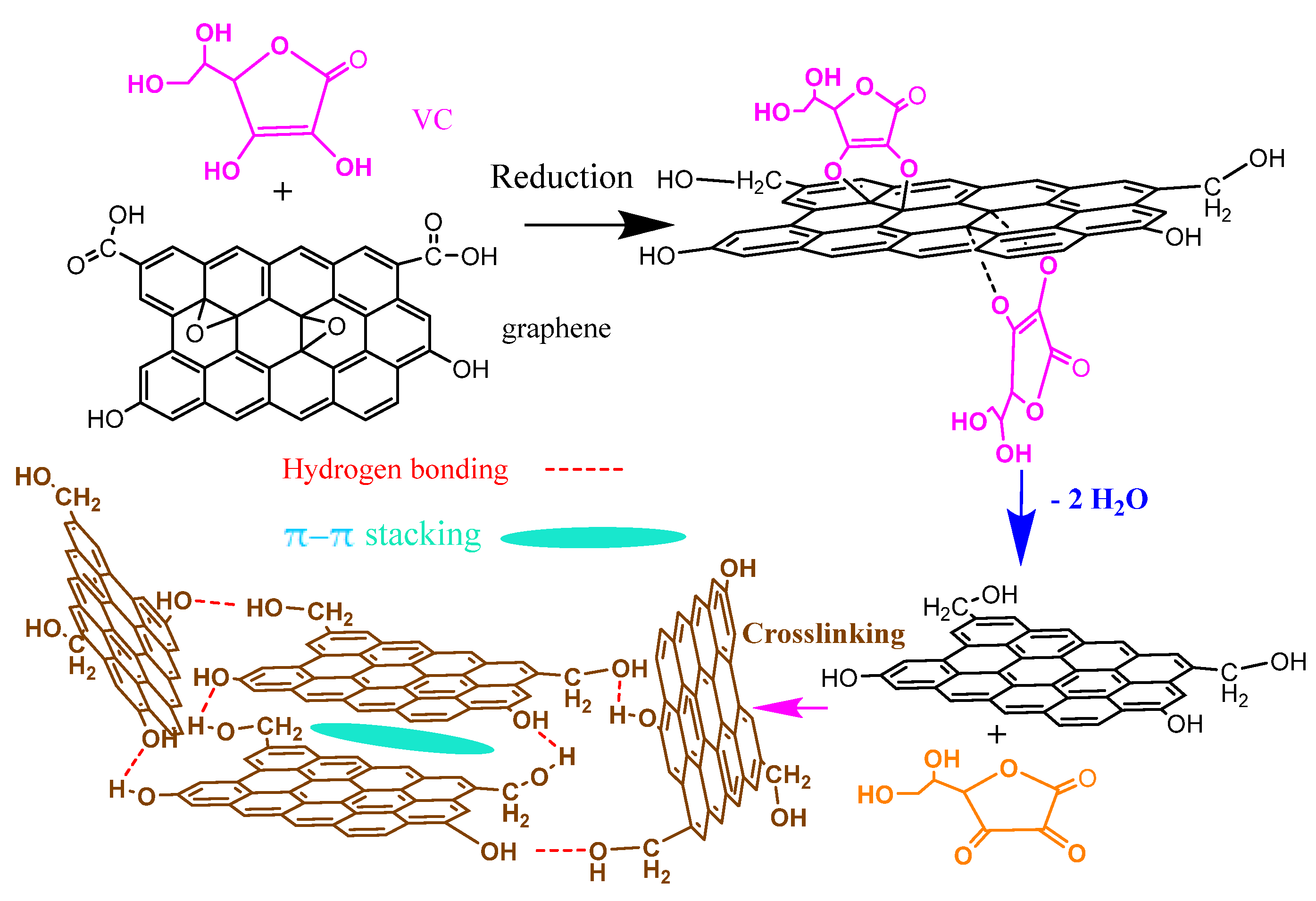
2.2. Preparation of Reduced Graphene-Oxide Aerogels (rGOAs)
2.3. Characterization
2.4. L2 Adsorption and Regeneration
2.5. Model of the Breakthrough Curves
3. Results
3.1. Effect of the Vitamin C (VC) Amount on Textural Properties and Hydrophobicity
3.2. Scanning Electron Microscopy (SEM), X-ray Diffraction (XRD), Fourier-Transform Infrared (FTIR), and Raman Analyses of Industrial-Grade Graphene Oxide (IGGO), rGOA-0, and rGOA-1
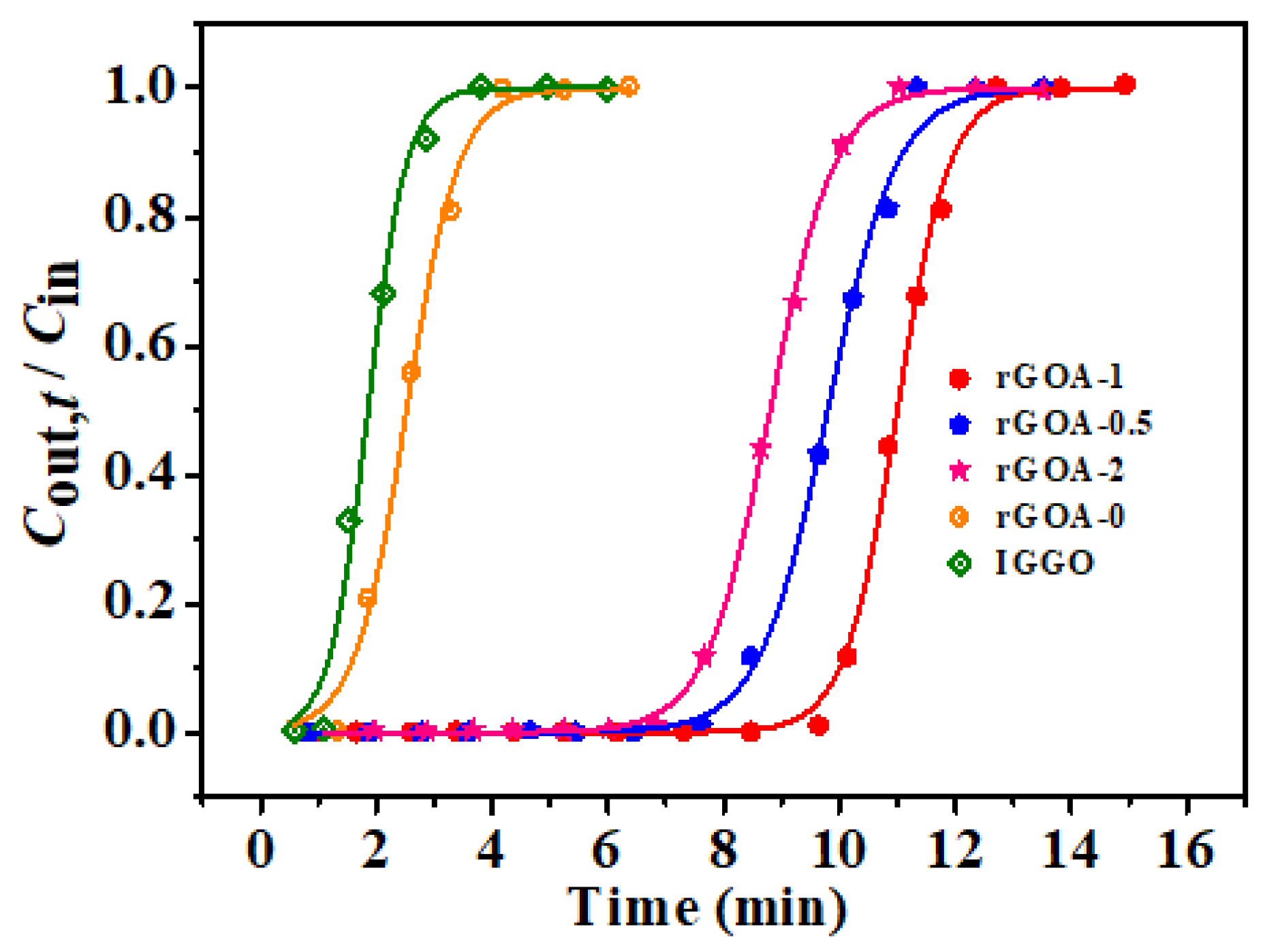
3.3. Comparison of Dynamic Adsorption Performances of rGOAs
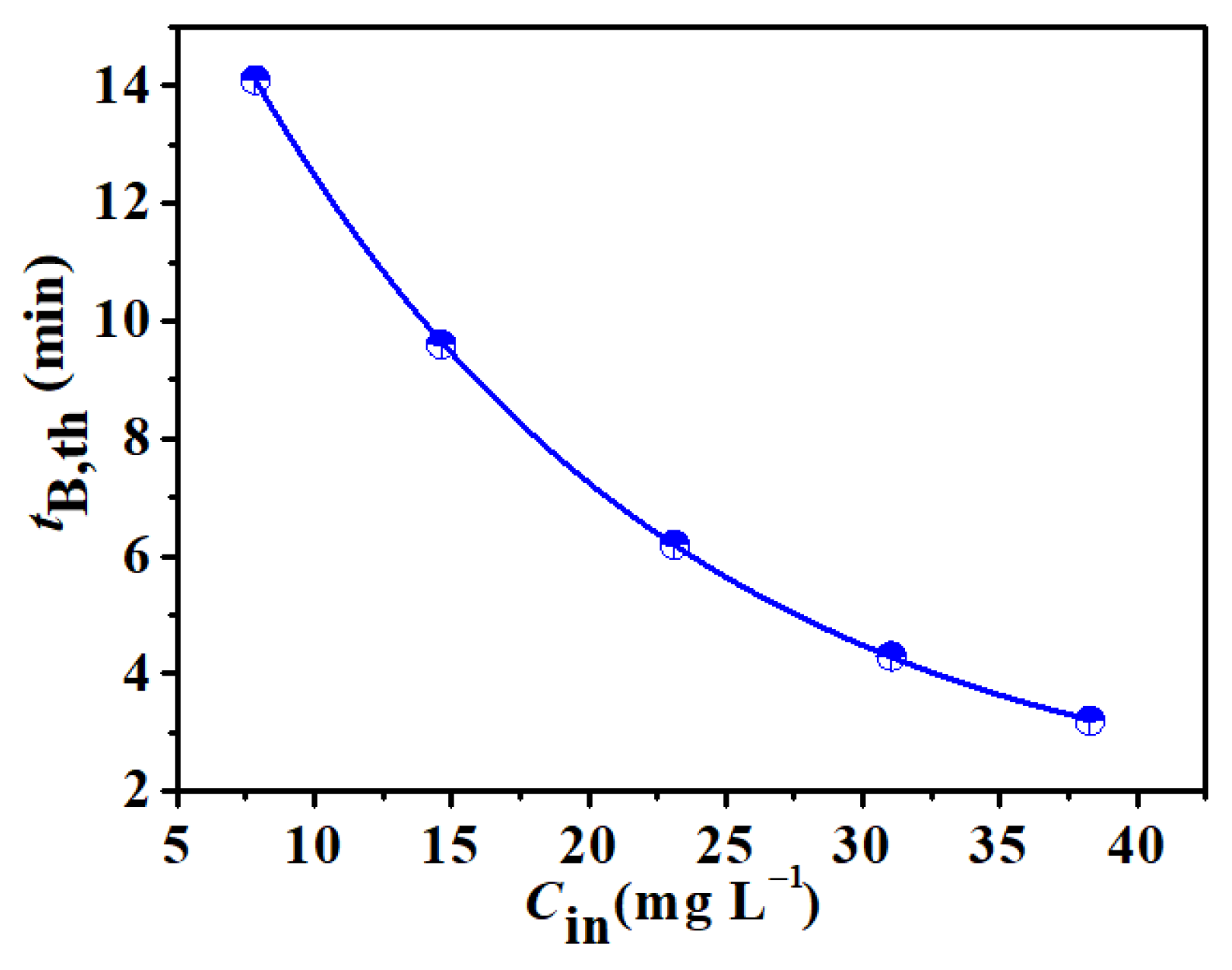
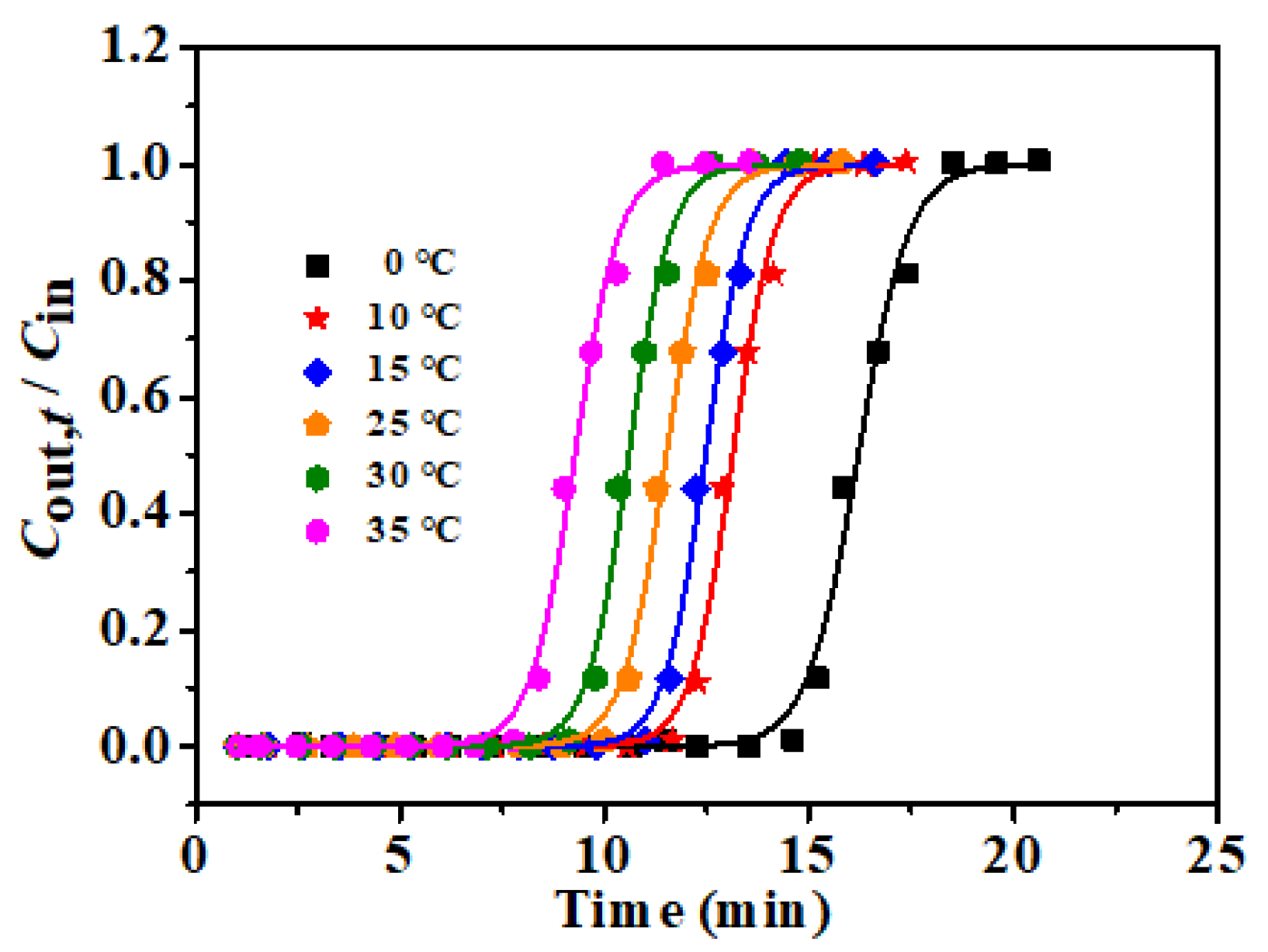
3.4. Influence of Process Conditions on Adsorption Performance of rGOA-1
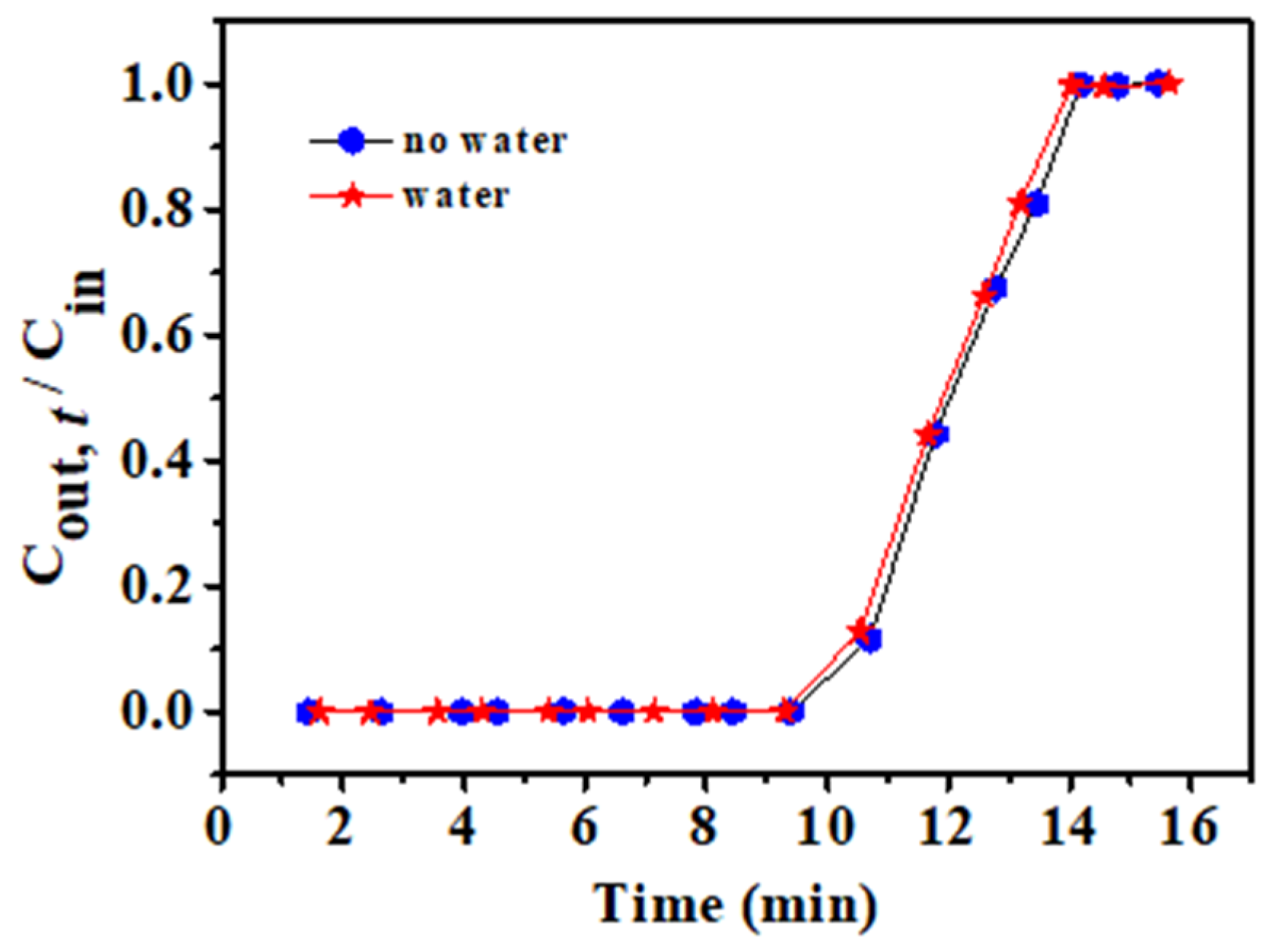
3.5. Influence of Water on the rGOA-1 Adsorbent
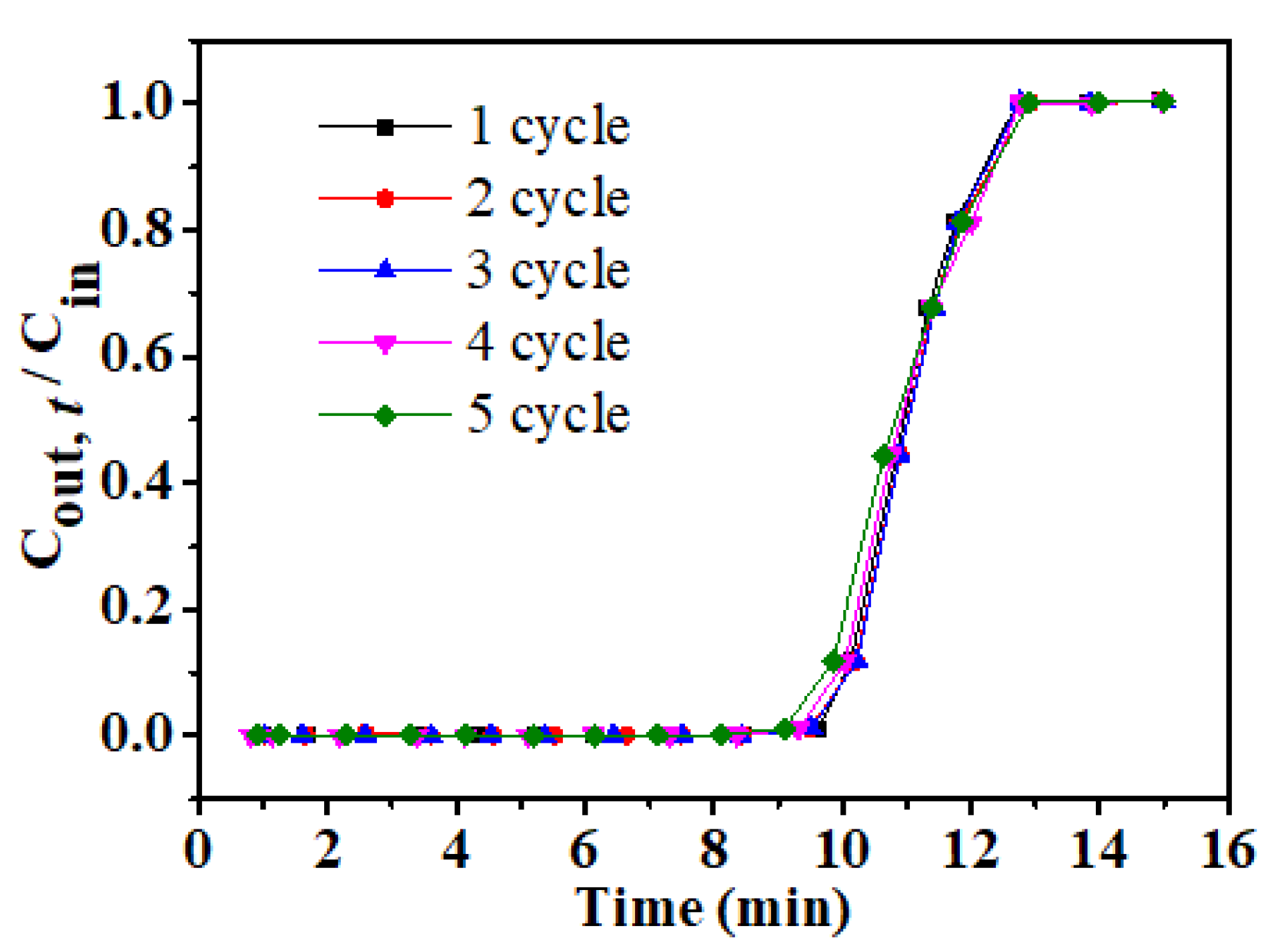
3.6. Recycling Performance of rGOA-1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ajhar, M.; Travesset, M.; Yuce, S.; Melin, T. Siloxane removal from landfill and digester gas—A technology overview. Bioresour. Technol. 2010, 101, 2913–2923. [Google Scholar] [CrossRef]
- Nair, N.; Zhang, X.W.; Gutierrez, J.; Chen, J.; Egolfopoulos, F.; Tsotsis, T. Impact of Siloxane Impurities on the Performance of an Engine Operating on Renewable Natural Gas. Ind. Eng. Chem. Res. 2012, 51, 15786–15795. [Google Scholar] [CrossRef]
- de Arespacochaga, N.; Valderrama, C.; Raich-Montiu, J.; Crest, M.; Mehta, S.; Cortina, J.L. Understanding the effects of the origin, occurrence, monitoring, control, fate and removal of siloxanes on the energetic valorization of sewage biogas-A review. Renew. Sustain. Energy Rev. 2015, 52, 366–381. [Google Scholar] [CrossRef] [Green Version]
- Santos-Clotas, E.; Cabrera-Codony, A.; Castillo, A.; Martín, M.; Poch, M.; Monclús, H. Environmental Decision Support System for Biogas Upgrading to Feasible Fuel. Energies 2019, 12, 1546. [Google Scholar] [CrossRef] [Green Version]
- Slupek, E.; Makos-Chelstowska, P.; Gebicki, J. Removal of Siloxanes from Model Biogas by Means of Deep Eutectic Solvents in Absorption Process. Materials 2021, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Nyamukamba, P.; Mukumba, P.; Chikukwa, E.S.; Makaka, G. Biogas Upgrading Approaches with Special Focus on Siloxane Removal—A Review. Energies 2020, 13, 6088. [Google Scholar] [CrossRef]
- Papurello, D.; Gandiglio, M.; Kafashan, J.; Lanzini, A. Biogas Purification: A Comparison of Adsorption Performance in D4 Siloxane Removal between Commercial Activated Carbons and Waste Wood-Derived Char Using Isotherm Equations. Processes 2019, 7, 774. [Google Scholar] [CrossRef] [Green Version]
- Jafari, T.; Jiang, T.; Zhong, W.; Khakpash, N.; Deljoo, B.; Aindow, M.; Singh, P.; Suib, S.L. Modified Mesoporous Silica for Efficient Siloxane Capture. Langmuir 2016, 32, 2369–2377. [Google Scholar] [CrossRef]
- Bletsou, A.A.; Asimakopoulos, A.G.; Stasinakis, A.S.; Thomaidis, N.S.; Kannan, K. Mass loading and fate of linear and cyclic siloxanes in a wastewater treatment plant in Greece. Environ. Sci. Technol. 2013, 47, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Gislon, P.; Galli, S.; Monteleone, G. Siloxanes removal from biogas by high surface area adsorbents. Waste Manag. 2013, 33, 2687–2693. [Google Scholar] [CrossRef]
- Finocchio, E.; Montanari, T.; Garuti, G.; Pistarino, C.; Federici, F.; Cugino, M.; Busca, G. Purification of Biogases from Siloxanes by Adsorption: On the Regenerability of Activated Carbon Sorbents. Energy Fuels 2009, 23, 4156–4159. [Google Scholar] [CrossRef]
- Cabrera-Codony, A.; Gonzalez-Olmos, R.; Martin, M.J. Regeneration of siloxane-exhausted activated carbon by advanced oxidation processes. J. Hazard. Mater. 2015, 285, 501–508. [Google Scholar] [CrossRef]
- Jung, H.; Jurng, J. Purification of wastewater digester biogas from siloxanes via adsorption-desorption with NaOH-reformed SiO2 adsorbent. Renew. Energy 2020, 156, 459–468. [Google Scholar] [CrossRef]
- Meng, Z.Y.; Hou, X.F.; Liu, Y.H.; Ma, Z.C.; Shen, H.Z. Facile fabrication of iron-modified biochar as a Renewable adsorbent for efficient siloxane (L2) removal. J. Environ. Chem. Eng. 2021, 9, 105799. [Google Scholar] [CrossRef]
- Meng, Z.Y.; Liu, Y.H.; Ma, Z.C.; Hou, X.F. The regulation of micro/mesoporous silica gel by polyethylene imine for enhancing the siloxane removal. Inorg. Chem. Commun. 2020, 112, 107754. [Google Scholar] [CrossRef]
- Tran, V.T.L.; Gélin, P.; Ferronato, C.; Mascunan, P.; Rac, V.; Chovelon, J.M.; Postole, G. Siloxane adsorption on activated carbons: Role of the surface chemistry on sorption properties in humid atmosphere and regenerability issues. Chem. Eng. J. 2019, 371, 821–832. [Google Scholar] [CrossRef]
- Cabrera-Codony, A.; Montes-Moran, M.A.; Sanchez-Polo, M.; Martin, M.J.; Gonzalez-Olmos, R. Biogas upgrading: Optimal activated carbon properties for siloxane removal. Environ. Sci. Technol. 2014, 48, 7187–7195. [Google Scholar] [CrossRef]
- Sigot, L.; Ducom, G.; Benadda, B.; Labouré, C. Adsorption of octamethylcyclotetrasiloxane on silica gel for biogas purification. Fuel 2014, 135, 205–209. [Google Scholar] [CrossRef]
- Sigot, L.; Ducom, G.; Germain, P. Adsorption of octamethylcyclotetrasiloxane (D4) on silica gel (SG): Retention mechanism. Microporous Mesoporous Mater. 2015, 213, 118–124. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, X.L.; Jiang, K.; Liu, F.J.; You, F.; Yao, C. Fabrication of Reusable Carboxymethyl Cellulose/Graphene Oxide Composite Aerogel with Large Surface Area for Adsorption of Methylene Blue. Nanomaterials 2021, 11, 1609. [Google Scholar] [CrossRef]
- Hou, X.F.; Zheng, Y.H.; Ma, X.L.; Liu, Y.H.; Ma, Z.C. The Effects of Hydrophobicity and Textural Properties on Hexamethyldisiloxane Adsorption in Reduced Graphene Oxide Aerogels. Molecules 2021, 26, 1130. [Google Scholar] [CrossRef] [PubMed]
- Plastiras, O.E.; Deliyanni, E.; Samanidou, V. Applications of Graphene-Based Nanomaterials in Environmental Analysis. Appl. Sci. 2021, 11, 3028. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.X.; Gao, H.; Liang, D.X. Properties Analysis and Preparation of Biochar–Graphene Composites Under a One-Step Dip Coating Method in Water Treatment. Appl. Sci. 2020, 10, 3689. [Google Scholar] [CrossRef]
- Zhu, B.W.; Zhang, Z.; Song, F.X.; Guo, Z.J.; Liu, B. Efficient Removal Of U(VI) Ions from Aqueous Solutions by Tannic Acid/Graphene Oxide Composites. Appl. Sci. 2020, 10, 8870. [Google Scholar] [CrossRef]
- Lusk, M.T.; Wu, D.T.; Carr, L.D. Graphene Nanoengineering and the Inverse-Stone-Thrower-Wales Defect. Phys. Rev. 2010, 81, 155444.1–155444.9. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.W.; Xie, X.Y.; Cerruti, M.; Szkopek, T. Controlling the Shell Formation in Hydrothermally Reduced Graphene Hydrogel. Langmuir 2015, 31, 5545–5549. [Google Scholar] [CrossRef]
- Hu, K.W.; Xie, X.Y.; Szkopek, T.; Cerruti, M. Understanding Hydrothermally Reduced Graphene Oxide Hydrogels: From Reaction Products to Hydrogel Properties. Chem. Mater. 2016, 28, 1756–1768. [Google Scholar] [CrossRef]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H., Jr.; Baumann, T.F. Synthesis of Graphene Aerogel with High Electrical Conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef]
- Yang, Z.X.; Xing, G.J.; Hou, P.C.; Han, D. Amino acid-mediated N-doped graphene aerogels and its electrochemical properties. Mater. Sci. Eng. B 2018, 228, 198–205. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Hou, X.F.; Liu, Y.H.; Ma, Z.C. Hexamethyldisiloxane removal from biogas using reduced graphene-oxide aerogels as adsorbents. Renew. Energy 2021, 178, 153–161. [Google Scholar] [CrossRef]
- Yu, L.; Wang, L.; Xu, W.C.; Chen, L.M.; Fu, M.L.; Wu, J.L.; Ye, D.Q. Adsorption of VOCs on reduced graphene oxide. J. Environ. Sci. (China) 2018, 67, 171–178. [Google Scholar] [CrossRef]
- Chen, W.F.; Yan, L.F. In situ self-assembly of mild chemical reduction graphene for three-dimensional architectures. Nanoscale 2011, 3, 3132–3137. [Google Scholar] [CrossRef]
- Ai, S.; Chen, Y.X.; Liu, Y.L.; Zhang, Q.; Xiong, L.J.; Huang, H.B.; Li, L.; Yu, X.H.; Wei, L. Facile synthesis of nitrogen-doped graphene aerogels for electrochemical detection of dopamine. Solid State Sci. 2018, 86, 6–11. [Google Scholar] [CrossRef]
- Rahmani, Z.; Rashidi, A.M.; Kazemi, A.; Samadi, M.T.; Rahmani, A.R. N-doped reduced graphene oxide aerogel for the selective adsorption of oil pollutants from water: Isotherm and kinetic study. J. Ind. Eng. Chem. 2018, 61, 416–426. [Google Scholar] [CrossRef]
- Xu, P.; Gao, Q.M.; Ma, L.; Li, Z.Y.; Zhang, H.; Xiao, H.; Liang, X.; Zhang, T.F.; Tian, X.H.; Liu, C.H. A high surface area N-doped holey graphene aerogel with low charge transfer resistance as high performance electrode of non-flammable thermostable supercapacitors. Carbon 2019, 149, 452–461. [Google Scholar] [CrossRef]
- Liu, Y.H.; Meng, Z.Y.; Wang, J.Y.; Dong, Y.F.; Ma, Z.C. Removal of siloxanes from biogas using acetylated silica gel as adsorbent. Pet. Sci. 2019, 16, 920–928. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.Y.; Ma, M.Z.; Liu, Y.H.; Ma, Z.C. A comparative study of the removal of o-xylene from gas streams using mesoporous silicas and their silica supported sulfuric acids. J. Hazard. Mater. 2021, 409, 124965. [Google Scholar] [CrossRef]
- Qin, W.; Zhu, W.H.; Ma, J.; Yang, Y.Z.; Tang, B. Carbon fibers assisted 3D N-doped graphene aerogel on excellent adsorption capacity and mechanical property. Colloids Surf. A 2021, 608, 125602. [Google Scholar] [CrossRef]
- Sui, Z.Y.; Zhang, X.T.; Lei, Y.; Luo, Y.J. Easy and green synthesis of reduced graphite oxide-based hydrogels. Carbon 2011, 49, 4314–4321. [Google Scholar] [CrossRef]
- Tas, M.; Altin, Y.; Celik Bedeloglu, A. Reduction of graphene oxide thin films using a stepwise thermal annealing assisted by l-ascorbic acid. Diamond Relat. Mater. 2019, 92, 242–247. [Google Scholar] [CrossRef]
- Trinh, T.T.P.N.X.; Quang, D.T.; Tu, T.H.; Dat, N.M.; Linh, V.N.P.; Van Cuong, L.; Nghia, L.T.T.; Loan, T.T.; Hang, P.T.; Phuong, N.T.L.; et al. Fabrication, characterization, and adsorption capacity for cadmium ions of graphene aerogels. Synth. Met. 2019, 247, 116–123. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Bao, N.; Wang, X.Q.; Hu, X.D.; Miao, X.H.; Chaker, M.; Ma, D.L. Advanced Fabrication of Chemically Bonded Graphene/TiO2 Continuous Fibers with Enhanced Broadband Photocatalytic Properties and Involved Mechanisms Exploration. Sci. Rep. 2016, 6, 38066. [Google Scholar] [CrossRef]
- Ren, X.H.; Guo, H.H.; Feng, J.K.; Si, P.C.; Zhang, L.; Ci, L.J. Synergic mechanism of adsorption and metal-free catalysis for phenol degradation by N-doped graphene aerogel. Chemosphere 2018, 191, 389–399. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.Y.; Chen, S.F.; Zhang, Y.H.; Li, J.L.; Hong, J.P. Construction of three-dimensional nitrogen-doped graphene aerogel (NGA) supported cobalt catalysts for Fischer-Tropsch synthesis. Catal. Today 2020, 355, 10–16. [Google Scholar] [CrossRef]
- Wan, W.C.; Zhang, F.; Yu, S.; Zhang, R.Y.; Zhou, Y. Hydrothermal formation of graphene aerogel for oil sorption: The role of reducing agent, reaction time and temperature. New J. Chem. 2016, 40, 3040–3046. [Google Scholar] [CrossRef]
- Go, S.H.; Kim, H.; Yu, J.; You, N.H.; Ku, B.C.; Kim, Y.K. Synergistic effect of UV and l-ascorbic acid on the reduction of graphene oxide: Reduction kinetics and quantum chemical simulations. Solid State Sci. 2018, 84, 120–125. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Yoshimura, M. Progress of reduction of graphene oxide by ascorbic acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Gao, J.; Liu, F.; Liu, Y.L.; Ma, N.; Wang, Z.Q.; Zhang, X. Environment-Friendly Method To Produce Graphene That Employs Vitamin C and Amino Acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar] [CrossRef]
- Meng, Z.Y.; Liu, Y.H.; Li, X.; Ma, Z.C. Removal of siloxane (L2) from biogas using methyl-functionalised silica gel as adsorbent. Chem. Eng. J. 2020, 389, 124440. [Google Scholar] [CrossRef]
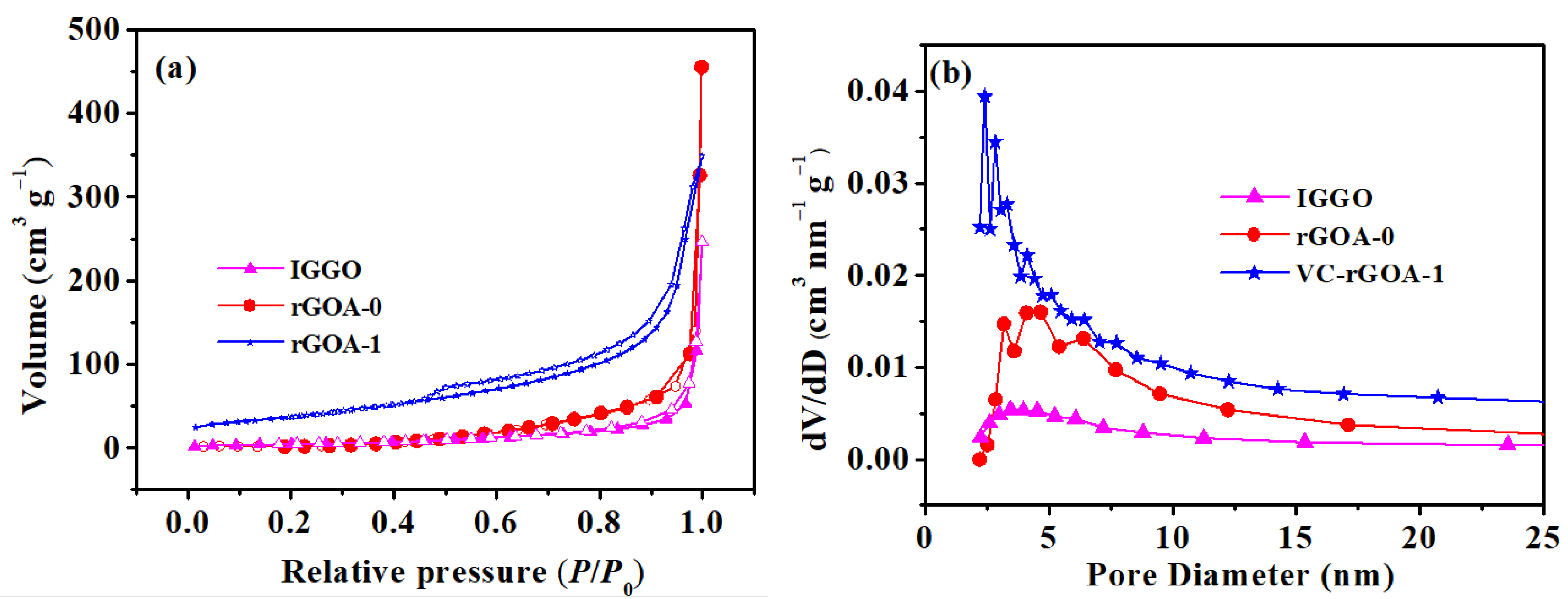
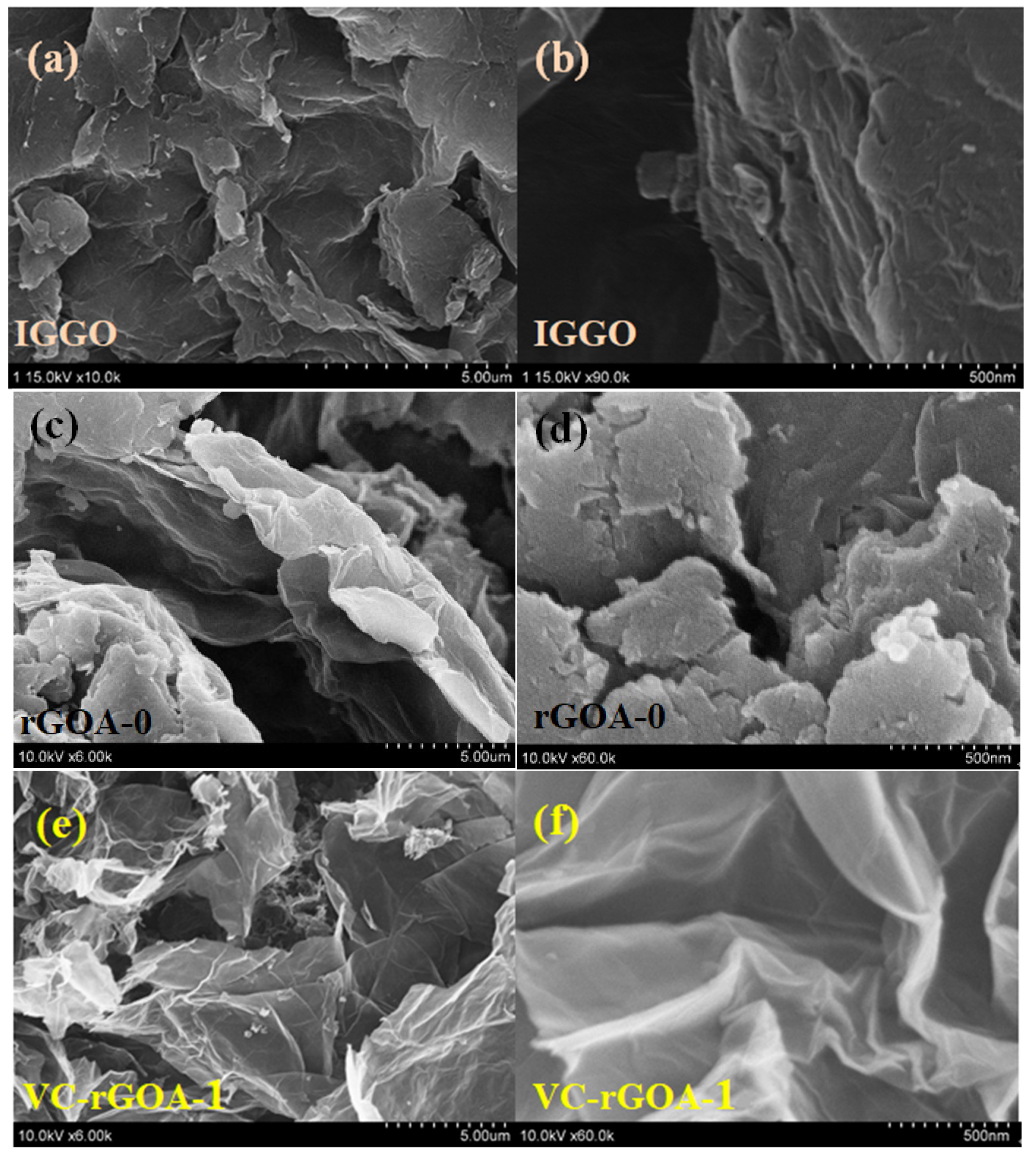
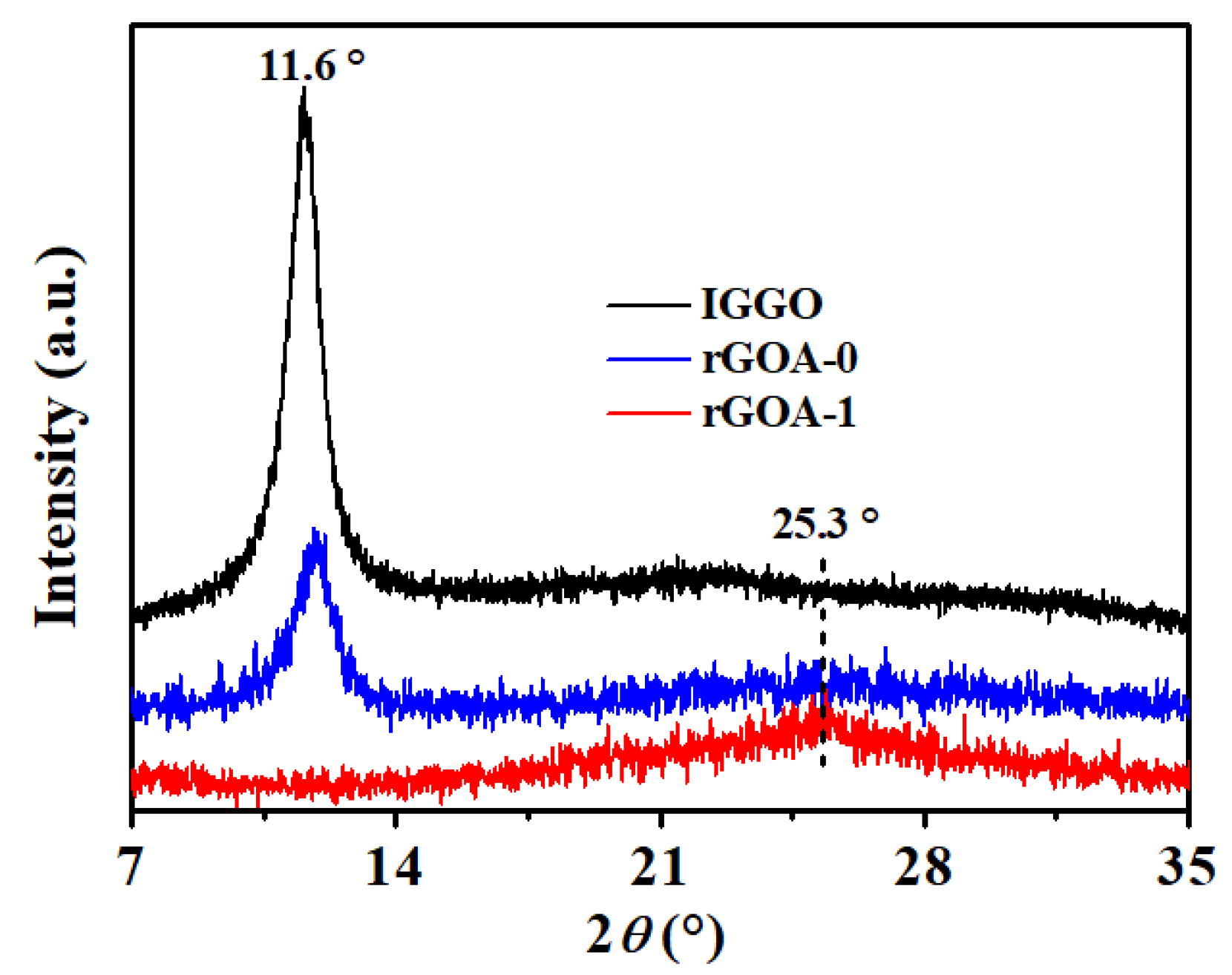

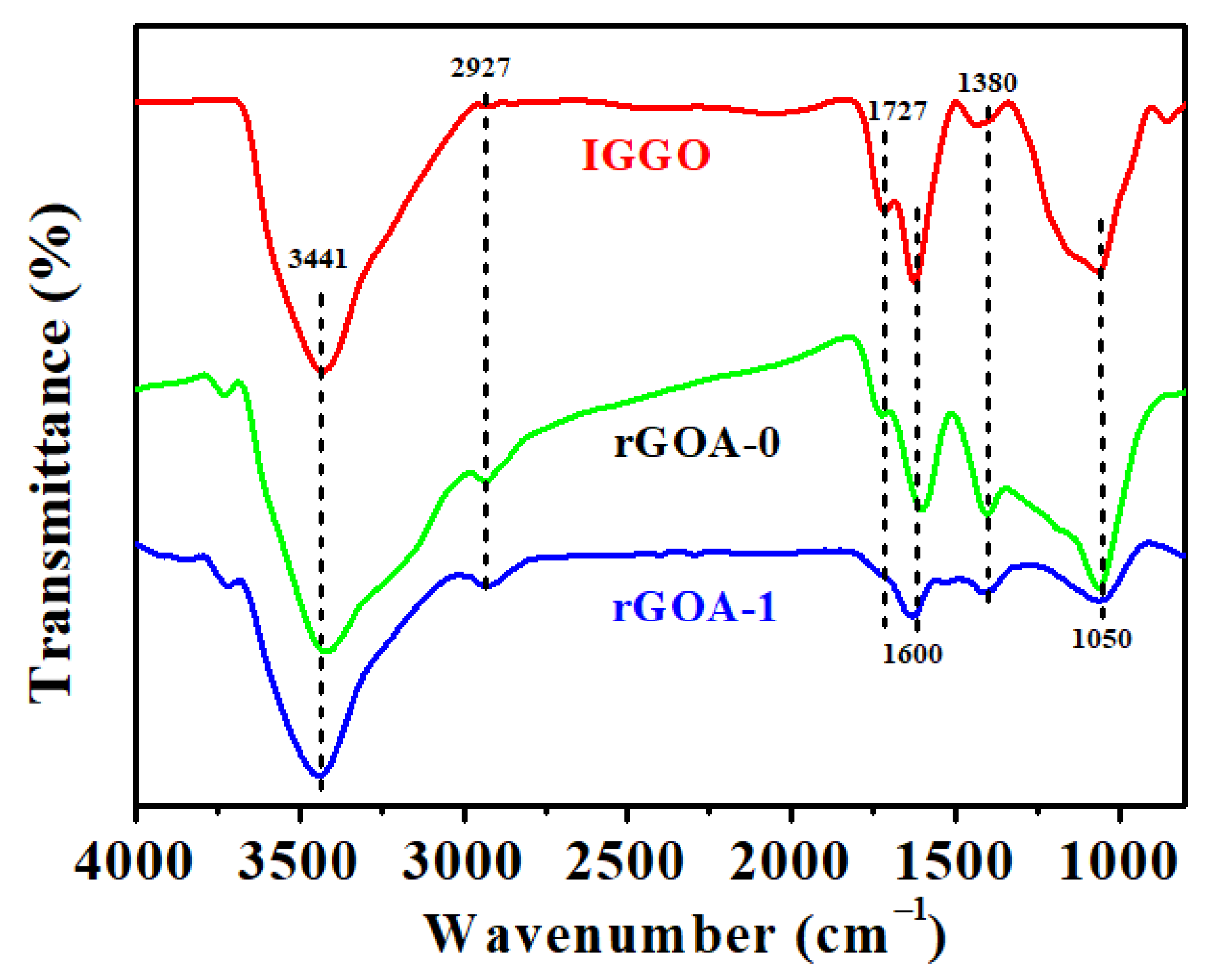
| Adsorbent | SBET (m2 g−1) | Vtot (cm3 g−1) | Daver (nm) | Contact Angle (°) |
|---|---|---|---|---|
| IGGO | 7.4 | 0.23 | 13.18 | 76.6 |
| rGOA-0 | 19.1 | 0.39 | 10.30 | 119.9 |
| rGOA-0.5 | 86.8 | 0.71 | 9.20 | 135.1 |
| rGOA-1 | 137.9 | 0.88 | 5.08 | 143.8 |
| rGOA-2 | 76.1 | 0.65 | 8.76 | 132.0 |
| Absorbent | Experimental | Model | |||||
|---|---|---|---|---|---|---|---|
| tB (min) | QB (mg g−1) | tB,th (min) | QB,th (mg g−1) | KYN | τ (min) | R2 | |
| IGGO | 1.16 | 8.1 | 0.89 | 7.1 | 3.0263 | 1.86 | 0.9901 |
| rGOA-0 | 1.45 | 10.6 | 1.20 | 9.5 | 2.2160 | 2.53 | 0.9942 |
| rGOA-0.5 | 7.96 | 63.6 | 8.06 | 64.8 | 1.6900 | 9.80 | 0.9969 |
| rGOA-1 | 9.82 | 80.3 | 9.66 | 77.7 | 2.2066 | 10.99 | 0.9985 |
| rGOA-2 | 7.18 | 57.9 | 7.17 | 57.6 | 1.8042 | 8.80 | 0.9995 |
| Temp. (°C) | tB,th (min) | QB,th (mg g−1) | KYN | τ min | R2 |
|---|---|---|---|---|---|
| 0 | 14.38 | 104.9 | 1.6164 | 16.20 | 0.9942 |
| 10 | 11.62 | 89.2 | 1.9491 | 13.13 | 0.9956 |
| 15 | 10.99 | 85.2 | 1.9861 | 12.47 | 0.9974 |
| 25 | 9.94 | 76.3 | 1.8750 | 11.51 | 0.9964 |
| 30 | 9.11 | 69.2 | 1.9730 | 10.60 | 0.9957 |
| 35 | 7.67 | 62.1 | 1.8436 | 9.27 | 0.9951 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Hou, X.; Ma, X.; Hao, Z.; Ma, Z. Vitamin C-Assisted Fabrication of Aerogels from Industrial Graphene Oxide for Gaseous Hexamethyldisiloxane Adsorption. Appl. Sci. 2021, 11, 8486. https://doi.org/10.3390/app11188486
Zheng Y, Hou X, Ma X, Hao Z, Ma Z. Vitamin C-Assisted Fabrication of Aerogels from Industrial Graphene Oxide for Gaseous Hexamethyldisiloxane Adsorption. Applied Sciences. 2021; 11(18):8486. https://doi.org/10.3390/app11188486
Chicago/Turabian StyleZheng, Yanhui, Xifeng Hou, Xiaolong Ma, Zelin Hao, and Zichuan Ma. 2021. "Vitamin C-Assisted Fabrication of Aerogels from Industrial Graphene Oxide for Gaseous Hexamethyldisiloxane Adsorption" Applied Sciences 11, no. 18: 8486. https://doi.org/10.3390/app11188486
APA StyleZheng, Y., Hou, X., Ma, X., Hao, Z., & Ma, Z. (2021). Vitamin C-Assisted Fabrication of Aerogels from Industrial Graphene Oxide for Gaseous Hexamethyldisiloxane Adsorption. Applied Sciences, 11(18), 8486. https://doi.org/10.3390/app11188486









