Abstract
Background: The use of bromelain for the removal of eschar in deep burns is considered to be effective because it does not affect the unaffected skin and leaves a clean dermis after use. The main objective of this study is to find out whether bromelain is a good alternative to surgical debridement. In order to achieve that, we aim to evaluate its indications, limitations, and safety measures. Methods: The current study was conducted on a group of 30 patients with deep burn lesions, aged 20 to 56 years, from which 15 underwent enzymatic debridement and 15 patients acted as a control group in which primary surgical debridement was used. The mixture of enzymes enriched in bromelain, meant to dissolve burn eschar, was provided by NexoBrid™. The inclusion criteria were in agreement with the manufacturer’s protocols, but the application protocol was slightly modified in order to implement a better intern protocol and to assess its efficiency. Results: Complete eschar debridement was obtained in 13 of the 15 cases, from which 10 patients went through spontaneous healing and 3 needed to be covered with a skin graft. In the other 2 cases, partial eschar debridement was associated with surgical debridement and coverage with split-thickness skin graft in the same operation. The results obtained in the two groups were assessed with the Vancouver Scar Scale. Conclusions: Even though early excision followed by coverage with split-thickness skin graft remains the gold standard for the treatment of deep burns, enzymatic debridement can provide a series of advantages when the inclusion and exclusion criteria are respected. Bromelain is an alternative to surgical debridement that provides speed, tissue selectivity, safety, and less blood loss.
1. Introduction
The need to preserve as large an area of tissue and viable skin as possible in the case of burns, especially in areas with complex anatomy, raises the question of whether an agent that would effectively ensure eschar debridement without a negative effect on adjacent viable structures is needed. Thus, several surgical and non-surgical methods have been used, such as hydrosurgery, laser therapy, therapy with worms or larvae, and, of course, enzymatic debridement. Since 1970, early surgical excision and grafting in the case of deep burns have been the standard of care (SOC) [1]. In 2012, a new agent used in enzymatic debridement was approved in Europe, with bromelain being its active substance. Previous studies in animals and humans have shown its effectiveness in eschar debridement and viable tissue preservation [2,3].
Bromelain is an extract from pineapple (Ananascomosus) and is a mixture of proteolytic enzymes and non-enzymatic substances. Its anti-edematous, anti-inflammatory, anti-thrombotic, analgesic, and exfoliating effects have been scientifically proven. The mechanisms incriminated in its activity are the kallikrein–kinin pathway, the arachidonic acid pathway, and cell-mediated immunity [4,5,6]. The usefulness of perioperative bromelain therapy has been demonstrated in orthopedics, obstetrics, otorhinolaryngology, dentistry, ophthalmology, sports medicine, and, last but not least, the topical use of bromelain in wound and burn debridement [7,8]. Thus, the enzymatic approach with bromelain is an alternative to surgical debridement [9,10]. The topical application of bromelain avoids skin grafts or reduces the surfaces to be grafted, thus also avoiding the utilization of skin substitutes and preserving more dermis, thanks to the protection of viable tissues. It can be done at bedside and has proven to be very effective and rapid, displaying minimal blood loss compared to classical debridement methods [11]. In 2017, the first European consensus about how to use bromelain for the treatment of deep and partial-thickness skin burns was reached. Since then, the utilization of bromelain in some deep or partial-thickness burns has been included in SOC [1].
This study aims to present our experience with the use of bromelain in a group of 15 patients with burns of different etiologies, with varying surface area and depth, and compare it with a control group of 15 patients.
2. Materials and Methods
The current study included 30 patients aged 20 to 56 years, treated between September 2019 and September 2020. The study patients were diagnosed with flame burns (10 cases) and scald burns (20 cases) covering 5% to 30% of the skin surface, burn degree IIB (deep partial-thickness)—III (full-thickness) (Table 1). The cases included in this study were randomly divided into two groups. The intern protocol of enzymatic debridement was initiated once the patients of the first group were assessed from the point of view of inclusion and exclusion criteria and if they were eligible. The patients of the second group were treated with the actual gold-standard protocol of early surgical excision with or without split-thickness skin grafting (STSG).

Table 1.
Demographics and burn wound characteristics of patients included in the study.
Inclusion criteria: (a) age over 18 years; (b) burn degrees IIB or III that have surgical indication; (c) burns greater than 1% of total body surface area (TBSA); (d) flame or scald burns; (e) patient consent (Figure 1).
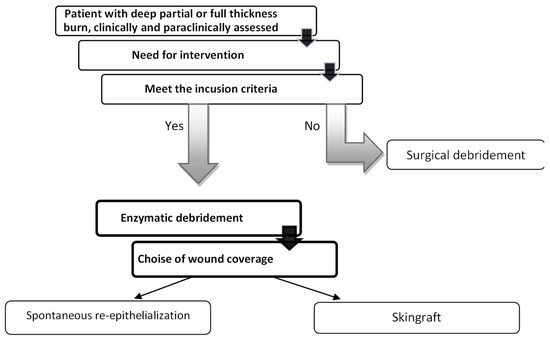
Figure 1.
Flow of study progression.
Exclusion criteria: (a) absence of consent; (b) women during pregnancy or lactation; (c) confirmed allergy to bromelain, pineapple, or papaya; (d) coagulation disorders; (e) patients with chemical or electrical burns; (f) patients on chronic corticosteroid treatment.
Burn depth was determined by clinical examination: color, sensitivity, capillary refilling, thrombotic vessels, skin pliability, and laboratory examination using a skin scanner.
As enzymatic debridement is seen as a painful procedure, it requires pre-, intra-, and postprocedural pain management. The type of analgesia or anesthesia was chosen depending on the anatomical region of the lesions. In most cases, continuous locoregional anesthesia and intravenous sedation, epidural anesthesia, were used.
NexoBrid™ was used for enzymatic debridement. NexoBrid™ is presented as a package of powder and gel that needs to be mixed. It is a concentrate of proteolytic enzymes enriched in bromelain, extracted from the stem of Ananas comosus. It is used in the first 72 h after the burn injury and is limited to an area of no more than 15% TSBA. In patients with a burn area over 15% TSBA, enzymatic debridement is done in two sessions. For the treatment of every 1% TSBA burn, we used NexoBrid™ 2 g of sterile powder in 20 g of sterile gel, which are mixed till homogenization just prior to administration, no more than 15 min. Considering that the substance does not act on dry wounds, the pre-soaking phase is very important and, according to the instructions, must take at least 2 h. We used a modified internal protocol in which the enzymatic debridement process was divided into three stages, with an extension of the pre-soaking period to >6 h (Figure 2).
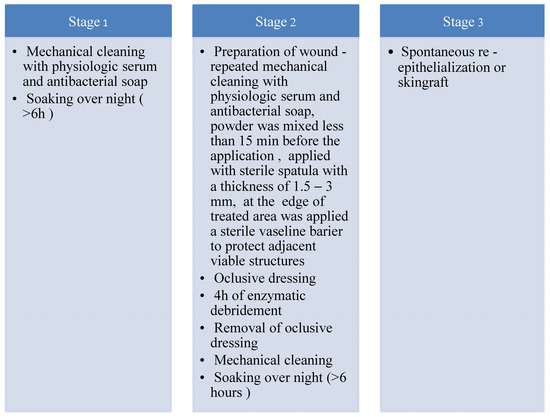
Figure 2.
Modified intern protocol of enzymatic debridement.
In the second stage, after enzymatic debridement, burn depth was reassessed (Figure 3). In the case of full-thickness burns, we used split-thickness skin autografts harvested from an anatomical area with intact skin and as little exposure as possible to ensure minimum morbidity. Excision and grafting were early or delayed, depending on the bleeding pattern. If the pattern of bleeding after enzymatic debridement shows pinpoint capillary bleeding, it is the sign of a possible spontaneous re-epithelialization within 3 weeks.
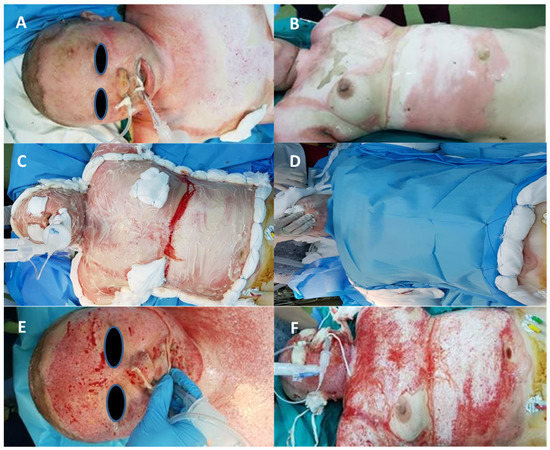
Figure 3.
Bromelain application. (A,B)—deep burn before bromelain application, (C)—bromelain application, (D)—oclusive dressing, (E,F)—after bromelain application.
If the pattern of bleeding showed large-caliber vessels or a large amount of subcutaneous fat, an immediate split-thickness skin grafting was indicated (Table 2).

Table 2.
Localization of burns and treatment choice after enzymatic debridement.
Patients who underwent surgery (split-thickness skin grafting) after NexoBrid™ received firm dressing for 3–5 days postoperatively, followed by daily wound cleaning with antibacterial soap and sterile dressings until complete healing.
None of the patients from the first group experienced complications such as extensive blood loss, severe anemia, or need for blood transfusion.
All patients were mobilized early by the physiotherapist, except for those who underwent surgery, in whom extensive movements were initiated after 7–10 postoperative days. All patients in the study group were recommended the use of compression bandages or silicone fixation dressing tape for 6 months after wound healing and continued physiotherapy.
The patients from the second group underwent early surgical debridement with an electric dermatome in the first 72 h. In 12 cases, the surgical debridement was followed by STSG, and 3 cases were left for spontaneous re-epithelialization, the burns being located on the posterior thorax; 6 patients from the second group experienced extensive bleeding, and 3 of them required blood transfusion.
3. Results
Enzymatic debridement was effective in 86% of patients, with 2 out of 15 patients requiring classic surgical debridement following enzymatic debridement. In the 10 patients in whom enzyme debridement proved to be effective, wound healing by spontaneous re-epithelialization occurred within approximately 3 weeks, and, in 3 of them, a STSG was applied in first 3 days post-debridement. In the two cases that required secondary surgical debridement, STSG was indicated. Thus, the indication for STSG in the enzymatic debridement group was established in 33% of the patients and in 80% of the patients from the surgical debridement group. That means STSG was avoided in 47% of the patients by using enzymatic debridement.
In the course of the study, it was shown that for pain management, intravenous algosedation was sufficient in most cases. All 30 patients participated in the follow-up program 6 months after treatment. The evolution of post-burn scars was assessed using the Vancouver Scar Scale (Table 3).

Table 3.
Vancouver Scar Scale.
Most followed-up patients had hyperpigmented, hyperemic, supple, and flat scars. According to the Vancouver Scar Scale, the mean pigmentation values did not differ significantly between the two groups, being 1.7 in the first studied group and 1.6 in the second one. The vascularity mean value was 1.6 in both groups. There was a difference of 0.3 in the mean scores of pliability. The enzymatic debridement group gained a better score in terms of pliability, probably thanks to better preservation of the unburned dermis compared to the surgical debridement group. The height of the eschar was slightly higher in the second group, with a mean value of 0.6, compared to the first group, with a mean value of 0.5. In two cases, patients did not follow the medical recommendation to wear elastic bands and abandoned the physiotherapy sessions and, as a consequence, showed poorer results in terms of scar quality (hyperpigmentation, hypertrophic), with progression to contracture within one year.
4. Discussion
Excision of burn eschar within the first 48 h after burn injury is a key moment in the treatment of partial- or full-thickness burns, thus significantly reducing the rate of bacterial colonization, infections, sepsis, and, of course, length of hospital stay [12]. The need to preserve as much viable tissue as possible has resulted in alternative methods of surgical debridement, especially for areas with complex anatomy, which include muscles, tendons, vessels and well-compacted nerves, and a lack of subcutaneous tissue that would provide additional protection to the hands and face. These structures are at risk of injury during surgical debridement [13].
The need for non-surgical burn debridement resulted in the development of bromelain-based agents. In 1957, Heinicke and Gortner described a new protease from pineapple fruit [14]. In 1971, Levine demonstrated the therapeutic properties of bromelain [15]. Levenson proved the efficiency of bromelain in enzymatic debridement using a pig model [16]. Bromelain is a major protease of Ananas Comosus. Bromelain is usually found in the fruit and stem of pineapple, with multiple differences and similarities. Fruit and stem bromelain belong to the group of cysteine proteases (fruit bromelain—BAA21848 and stem bromelain—CAA08861). Bromelain is a defense protein that protects the pineapple fruit during its development [17]. It contains a mixture of proteolytic enzymes and non-enzymatic substances, being used in the treatment of edema, inflammation, burns, and pain. Bromelain preparations are used for non-surgical debridement but require adequate pain treatment [18]. During application, the healthy surrounding skin has to be protected.
Early eschar debridement and removal remains the cornerstone of modern burn therapy [19]. Rosenberg et al., who conducted a comparative study between enzymatic debridement (75 burns) and surgical debridement (81 burns), found that mean times to complete debridement were 2.2 ± 1.4 days for bromelain and 8.7 ± 5.7 days for surgical debridement [20]. Similar results have been reported by Shultz et al. in 20 patients with hand burns [21]. These results are significant because the prolonged time to complete eschar debridement and failure to completely remove the eschar could lead to hypertrophic scarring. Our study highlights the characteristics, differences, and needs of enzymatic debridement in burn lesions. The need for proper pain management, the efficient use of bromelain, and the resulting shortening of burn debridement time have been emphasized. It was possible to assess the correctness of the preprocedural burn lesion depth assessment as well as the time and characteristics of healing and post-healing rehabilitation. Our study also highlights the higher aesthetic quality of the post-burn scars treated with enzymatic debridement compared to those treated with surgical excision, according to the values obtained from the Vacouver Scar Scale.
Since 2012, NexoBrid™ has been approved as a minimally invasive treatment [14]. The instructions for bromelain use recommend for it to be applied to less than 15% TBSA in one session. At the 2017 European Consensus, it was agreed that burns up to 30% TBSA and those exceeding 72 h of management can be enzymatically debrided in a single session, but this is currently off-label use [22]. In this study, we applied bromelain within the first 72 to no more than 15% TBSA of the burned area.
Moti Harats et al. have reported promising results from the use of bromelain 72 h to 5 days post-burn [23]. The effectiveness of bromelain has also been demonstrated in chemical and electrical burns and even in children. It is also argued that the efficacy of enzymatic debridement is reduced when used after the 5th post-burn day, probably due to the decreased eschar permeability.
In our study, we obtained complete debridement in 86% of the cases, with similar results (90%) being reported by Rosenberg [24]. The reduced need for autografts is demonstrated by Shultz et al., who reported that only 15% of bromelain-debrided burn wounds required autografts compared to 77% in the surgical debridement group. Thus, 85% of enzymatically debridement burns were found to have good healing via re-epithelialization [25].
In 2016, Cordts et al. reported a study on 16 patients with deep burns in which they used NexoBrid™, having recorded no case of infection or other complications to this product. In 2018, Edmondson et al. claimed that early excision and grafting would not be the ”gold standard” treatment for deep burns for long; it could be potentially replaced by enzymatic debridement [26]. The use of bromelain is safe, but the occurrence of side effects such as allergic reactions, vomiting, nausea, diarrhea, and heavy menstrual bleeding must be taken into account [27,28]. It is recommended that both oral and topical administration to patients with coagulation disorders, patients on chronic anticoagulants or antiplatelet drugs, patients with hypertension, liver, or kidney disease, or pregnant women [29,30] be avoided.
5. Conclusions
Enzymatic debridement is an alternative to surgical debridement that provides speed, tissue selectivity, and safety. Bromelain enzymatic debridement appears to be superior to surgical debridement in terms of its ability to preserve healthy tissues (avoiding blood loss during surgical burn excision) and graft donor sites, with associated morbidity, better outcomes, and early mobilization of patients. The use of NexoBrid™ shows a better aesthetic appearance of the scars compared to those obtained after surgical debridement. At the same time, the use of bromelain requires further larger sample size studies to be sure of the safety and efficacy of this method and to clearly define the indications, limitations, and contraindications of this procedure. Meanwhile, surgical debridement followed by autografting remains the gold standard in burn surgery. The association of these two types of treatment can be considered in extensive burns of variable depth. The efficiency of enzymatic debridement in case of electrical or chemical burns, as well as the safety of using this treatment option in burns of greater TBSA, is yet to be determined.
We also observed that the early use of NexoBrid™ in deep, circular burns of the limbs could prevent the development of compartment syndrome; however, further studies are necessary for the confirmation of this hypothesis.
Author Contributions
Conceptualization, M.P. and P.C.; methodology, V.P., N.V. and S.L. (Sorinel Lunca); software, P.C. and A.F.; validation, M.B.-A., A.P. and B.V.; formal analysis, S.L. (Stefana Luca); investigation, M.P. and P.C.; resources, M.P.; data curation, S.L. (Sorinel Lunca) and N.V.; writing—original draft preparation, M.P. and P.C.; writing—review and editing, M.P. and B.V.; visualization, A.F.; supervision, M.P.; project administration, B.V.; funding acquisition, V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Emergency Clinical Hospital “Sf. Spiridon” (Nr 139/12.07.21).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data supporting reported results can be found in patient medical files located in the archive of “Sf. Spiridon” Emergency Clinical Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hirche, C.; Almeland, S.K.; Dheansa, B.; Fuchs, P.; Governa, M.; Hoeksema, H.; Korzeniowski, T.; Lumenta, D.B.; Marinescu, S.; Martinez-Mendez, J.R.; et al. Eschar removal by bromelain based enzymatic debridement (Nexobrid®) in burns: European consensus guidelines update. Burns 2020, 46, 782–796. [Google Scholar] [CrossRef]
- Krieger, Y.; Rosenberg, L.; Lapid, O.; Glesinger, R.; Bogdanov-Berezovsky, A.; Silberstein, E.; Sagi, A.; Judkins, K. Escharotomy using an enzymatic debridement agent for treating experimental burninduced compartment syndrome in an animal model. J. Trauma 2005, 58, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; McClain, S.A.; Taira, B.R.; Rooney, J.; Steinhauff, N.; Rosenberg, L. Rapid and Selective Enzymatic Debridement of Porcine Comb Burns With Bromelain-Derived Debrase®: Acute-Phase Preservation of Noninjured Tissue and Zone of Stasis. J. Burn. Care Res. 2010, 31, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.M.; Herman, C.T.; Bailey, R.C. Bromelain Decreases Neutrophil Interactions with P-Selectin, but Not E-Selectin, In Vitro by Proteolytic Cleavage of P-Selectin Glycoprotein Ligand-1. PLoS ONE 2013, 8, e78988. [Google Scholar] [CrossRef] [Green Version]
- Hale, L.P.; Greer, P.K.; Sempowski, G.D. Bromelain treatment alters leukocyte expression of cell surface molecules involved in cellular adhesion and activation. Clin. Immunol. 2002, 104, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Marz, R.; Schmolz, M.; Drewelow, B.; Eschmann, K.; Meiser, P. Placebo-controlled randomized clinical trial on the immune modulating activities of low- and high-dose bromelain after oral administration—New evidence on the antiinflam-matory mode of action of bromelain. Phytother. Res. 2013, 27, 199–204. [Google Scholar] [CrossRef]
- Howat, R.C.L.; Lewis, G.D. The effect of bromelain therapy on episiotomy wounds-a double blind controlled clinical trial. BJOG Int. J. Obstet. Gynaecol. 1972, 79, 951–953. [Google Scholar] [CrossRef]
- Spaeth, G.L. The effect of bromelains on the inflammatory response caused by cataract extraction: A double-blind study. Eye Ear Nose Throat Mon. 1968, 47, 634–639. [Google Scholar]
- Cordts, T.; Horter, J.; Vogelpohl, J.; Kremer, T.; Kneser, U.; Hernekamp, J.-F. Enzymatic debridement for the treatment of severely burned upper extremities—Early single center experiences. BMC Dermatol. 2016, 16, 8. [Google Scholar] [CrossRef] [Green Version]
- Krieger, Y.; Bogdanov-Berezovsky, A.; Gurfinkel, R.; Silberstein, E.; Sagi, A.; Rosenberg, L. Efficacy of enzymatic debridement of deeply burned hands. Burns 2012, 38, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Koller, J.; Bukovcan, P.; Orság, M.; Kvalténi, R.; Gräffinger, I. Enzymatic necrolysis of acute deep burns--report of preliminary results with 22 patients. Acta Chir. Plast. 2008, 50, 109–114. [Google Scholar]
- Xiao-Wu, W.; Herndon, D.N.; Spies, M.; Sanford, A.P.; Wolf, S.E. Effects of Delayed Wound Excision and Grafting in Severely Burned Children. Arch. Surg. 2002, 137, 1049–1054. [Google Scholar] [CrossRef] [Green Version]
- van Zuijlen, P.; Kreis, R.; Vloemans, A.; Groenevelt, F.; Mackie, D. The prognostic factors regarding long-term functional outcome of full-thickness hand burns. Burns 1999, 25, 709–714. [Google Scholar] [CrossRef]
- Heitzmann, W.; Fuchs, P.C.; Schiefer, J.L. Historical Perspectives on the Development of Current Standards of Care for En-zymatic Debridement. Medicina 2020, 56, 706. [Google Scholar] [CrossRef] [PubMed]
- Levine, N.; Seifter, E.; Levenson, S.M. Enzymatic debridement of burns. Surg. Forum 1971, 22, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Levenson, S.M.; Gruber, D.K.; Gruber, C.; Lent, R.; Seifter, E. Chemical debridement of burns: Mercaptans. J. Trauma 1981, 21, 632–644. [Google Scholar] [CrossRef]
- Ramli, A.N.M.; Abdul Manas, N.H.; Hamid, A.A.; Hamid, H.A.; Illias, R.M. Comparative structural analysis of fruit and stem bromelain from Ananascomosus. Food Chem. 2018, 266, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Perbix, W.; Shoham, Y.; Daali, S.; Charalampaki, C.; Fuchs, P.C.; Schiefer, J. Our initial learning curve in the enzy-matic debridement of severely burned hands -management and pit falls of initial treatments and our development of apost debridement wound treatment algorithm. Burns 2017, 43, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Krigier, Y.; Shoham, Y.; Bogdanov-Berezovsky, A.; Silberstein, E.; Sagi, A.; Levy, A.; Rosenberg, N.; Rubin, G.; Egozi, D.; Ullman, Y.; et al. Review of 30 years of research and development of enzymatic debridement agent for burns. Harefuah 2016, 155, 281–285. [Google Scholar]
- Rosenberg, L.; Krieger, Y.; Bogdanov-Berezovski, A.; Silberstein, E.; Shoham, Y.; Singer, A.J. A novel rapid and selective enzymatic debridement agent for burn wound management: A multi-center RCT. Burns 2014, 40, 466–474. [Google Scholar] [CrossRef]
- Schulz, A.; Shoham, Y.; Rosenberg, L.; Rothermund, I.; Perbix, W.; Christian Fuchs, P.; Lipensky, A.; Schiefer, J.L. Enzymatic Versus Traditional Surgical Debridement of Severely Burned Hands: A Comparison of Selectivity, Efficacy, Healing Time, and Three-Month Scar Quality. J. Burns Care Res. 2017, 38, e745–e755. [Google Scholar] [CrossRef]
- Hirche, C.; Citterio, A.; Hoeksema, H.; Koller, J.; Lehner, M.; Martinez, J.R.; Monstrey, S.; Murray, A.; Plock, J.A.; Sander, F.; et al. Eschar removal by bromelain based enzymatic debridement (Nexobrid®) in burns: An European consensus. Burns 2017, 43, 1640–1653. [Google Scholar] [CrossRef]
- Harats, M.; Haik, J.; Cleary, M.; Vashurin, I.; Aviv, U.; Kornhaber, R. A Retrospective Review of an Off-label Brome-lain-based Selective Enzymatic Debridement (Nexobrid®) in the Treatment of Deep, Partial, and Full Thickness Burns and Hard to Heal Wounds. Isr. Med. Assoc. J. 2020, 22, 83–88. [Google Scholar]
- Rosenberg, L.; Lapid, O.; Bogdanov-Berezovsky, A.; Glesinger, R.; Krieger, Y.; Silberstein, E.; Sagi, A.; Judkins, K.; Singer, A.J. Safety and efficacy of a proteolytic enzyme for enzymatic burn debridement: A preliminary report. Burns 2004, 30, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Fuchs, P.C.; Rothermundt, I.; Hoffmann, A.; Rosenberg, L.; Shoham, Y.; Oberländer, H.; Schiefer, J. Enzymatic debridement of deeply burned faces: Healing and early scarring based on tissue preservation compared to traditional surgical debridement. Burns 2017, 43, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, S.-J.; Jumabhoy, I.A.; Murray, A. Time to start putting down the knife: A systematic review of burns excision tools of randomised and non-randomised trials. Burns 2018, 44, 1721–1737. [Google Scholar] [CrossRef] [PubMed]
- Brien, S.; Lewith, G.; Walker, A.F.; Middleton, R.; Prescott, P.; Bundy, R. Bromelain as an adjunctive treatment for moder-ate-to-severe osteoarthritis of the knee: A randomized placebo-controlled pilot study. QJM 2006, 99, 841–850. [Google Scholar] [CrossRef] [Green Version]
- Pertea, M.; Grosu, O.M.; Veliceasa, B.; Velenciuc, N.; Ciobanu, P.; Tudor, R.; Poroch, V.; Lunca, S. Effectiveness and Safety of Wide Awake Local Anesthesia no Tourniquet (WALANT) Technique in Hand Surgery. Rev. Chim. 2019, 70, 3587–3591. [Google Scholar] [CrossRef]
- Gutfreund, A.E.; Taussig, S.J.; Morris, A.K. Effect of oral bromelain on blood pressure and heart rate of hypertensive patients. Hawaii Med. J. 1978, 37, 143–146. [Google Scholar] [PubMed]
- Pertea, M.; Velenciuc, N.; Poroch, V.; Ciobanu, P.; Boanca, M.; Grosu, O.M.; Lunca, S. Efficacy of Negative Pressure Therapy (NPWT) in the Management of Wounds of Different Etiologies. Rev. Chim. 2018, 69, 1980–1986. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).