Effects of Electron Beam Irradiation on Acaricide-Resistant and Susceptible Strains of Tetranychusurticae (Acari: Tetranychidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Mites
2.2. Preparation and Electron Beam Irradiation of Experimental Mites
2.3. SDS-PAGE
2.4. Data Analysis
3. Results
3.1. Resistance Ratio of T. urticae to Acaricides
3.2. Effect of Electron Beam Irradiation on Eggs
3.3. Effect of Electron Beam Irradiation on Nymphs
3.4. Effect of Electron Beam Irradiation on Adults
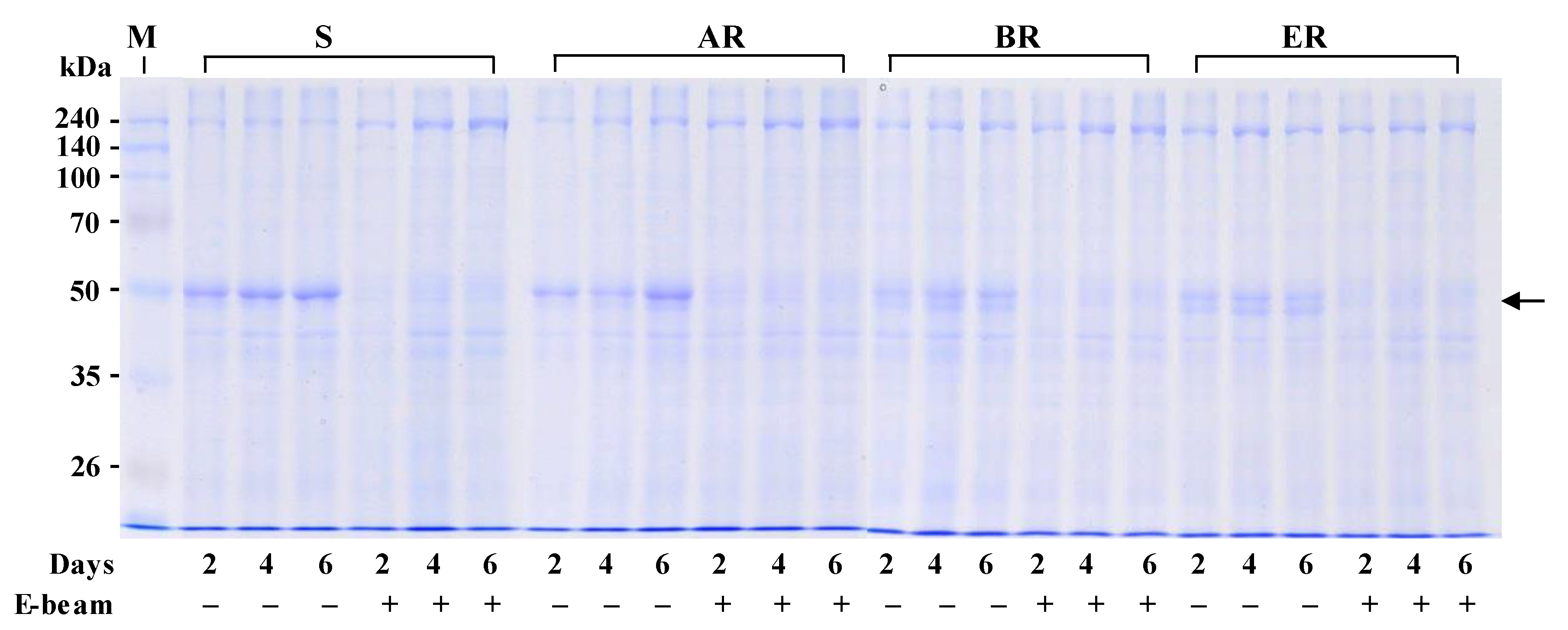
3.5. Changes in Protein Synthesis Caused by Electron Beam Irradiation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asada, M. Genetics and biochemical mechanisms of acaricide resistance in phytophagous mights. J. Pestic. Sci. 1978, 3, 61–68. [Google Scholar] [CrossRef][Green Version]
- Takafuji, A.; Ozawa, A.; Nemoto, H.; Gotoh, T. Spider mites of Japan: Their biology and control. Exp. Appl. Acarol. 2000, 24, 319–335. [Google Scholar] [CrossRef]
- Lee, Y.S.; Song, M.H.; Ahn, K.S.; Lee, K.Y.; Kim, J.W.; Kim, G.H. Monitoring of acaricide resistance in two-spotted spider mite (Tetranychus urticae) populations from Rose Greenhouses in Korea. J. Asia-Pac. Entomol. 2003, 6, 91–96. [Google Scholar] [CrossRef]
- Whalon, M.E.; Mota-Sanchez, D.; Hollingworth, R.M.; Duynslager, L. Arthropod Pesticide Resistance Database. Available online: http://www.pesticideresistance.org (accessed on 21 January 2009).
- Kim, G.H.; Song, C.; Park, N.J.; Cho, K.Y. Inheritance if resistance in dicofol-selected strain of the two-spotted spider mite, Tetranychus urticae Koch (Acarina: Tetranychidae), and its cross resostance. Korean J. Appl. Entomol. 1994, 33, 230–236. [Google Scholar]
- Follett, P.A. Irradiation to control insects in fruits and vegetables for export from Hawaii. Radiat. Phys. Chem. 2004, 71, 163–166. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.M.; Lee, S.C. Effect of electron-beam irradiation on the characteristics of green tea (Camellia sinensis L.). J. Korean Soc. Food Sci. Nutr. 2006, 35, 774–779. [Google Scholar] [CrossRef]
- Moon, S.R.; Son, B.K.; Yang, J.O.; Woo, J.S.; Yoon, C.M.; Kim, G.H. Effect of electron-beam irradiation on development and reproduction of Bemisia tabaci, Myzus persicae, Plutella xylostella and Tetranychus urticae. Korean J. Appl. Entomol. 2010, 49, 129–137. [Google Scholar] [CrossRef][Green Version]
- Barkai-Golan, R.; Follett, P.A. Irradiation for Quality Improvement, Microbial Safety and Phytosanitation of Fresh Produce; Academic Press: San Diego, CA, USA, 2017. [Google Scholar]
- Osouli, S.; Ziaie, F.; Irani Nejad, K.H.; Moghaddam, M. Application of gamma irradiation on eggs, active and quiescence stages of Tetranychus urticae Koch as a quarantine treatment of cut flowers. Radiat. Phys. Chem. 2013, 90, 111–119. [Google Scholar] [CrossRef]
- Hasan, M.M.; Todoriki, S.; Miyanoshita, A.; Imamura, T.; Hayashi, T. Soft-electron beam and gamma-radiation sensitivity and DNA damage in phosphine-resistant and -susceptible strains of Rhyzopertha dominica. J. Econ. Entomol. 2006, 99, 1912–1919. [Google Scholar] [CrossRef]
- Yun, S.H.; Koo, H.N.; Kim, H.K.; Cho, S.; Kim, G.H. Effects of electron beam irradiation on six insect pests in different sections of flower boxes for export. J. Asia-Pac. Entomol. 2015, 18, 629–636. [Google Scholar] [CrossRef]
- Koo, H.N.; Yun, S.H.; Yoon, C.; Kim, G.H. Electron beam irradiation induces abnormal development and the stabilization of p53 protein of American serpentine leafminer, Liriomyza trifolii (Burgess). Radiat. Phys. Chem. 2012, 81, 86–92. [Google Scholar] [CrossRef]
- Yun, S.H.; Lee, S.W.; Koo, H.N.; Kim, G.H. Assessment of electron beam-induced abnormal development and DNA damage in Spodoptera litura (F.) (Lepidoptera: Noctuidae). Radiat. Phys. Chem. 2014, 96, 44–49. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT User’s Guide: Statistics, Version 9.1; SAS Institute: Cary, NC, USA, 2003. [Google Scholar]
- Hayashi, T.; Todoriki, S.S. Low energy electron irradiation of food for microbial control. In Irradiation for Food Safety and Quality; Loaharanu, P., Thomas, P., Eds.; Technomic Publishing: Lancaster, PA, USA, 2001; pp. 118–128. [Google Scholar]
- Todoriki, S.; Kikuchi, O.K.; Nakaoka, M.; Miike, M.; Hayashi, T. Soft electron (low energy electron) processing of foods for microbial control. Radiat. Phys. Chem. 2002, 63, 349–351. [Google Scholar] [CrossRef]
- Nakakita, H.; Hayashi, T.; Aoki, S.; Kawashima, K. Radiosensitivity of phosphine-resistant and susceptible strains of the ßour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Jpn. J. Appl. Entomol. Zool. 1985, 29, 242–246. [Google Scholar] [CrossRef]
- Hsu, C.H.; Chi, B.C.; Liu, M.Y.; Li, J.H.; Chen, C.J.; Chen, R.Y. Phosphine-induced oxidative damage in rats: Role of glutathione. Toxicology 2002, 179, 1–8. [Google Scholar] [CrossRef]
- Yun, S.H.; Koo, H.N.; Lee, S.W.; Kim, H.K.; Kim, Y.; Han, B.; Kim, G.H. A comparative study on the effects of electron beam irradiation on imidacloprid-resistant and-susceptible Aphis gossypii (Hemiptera: Aphididae). Radiat. Phys. Chem. 2015, 112, 151–157. [Google Scholar] [CrossRef]
- Koo, H.N.; Yoon, S.H.; Shin, Y.H.; Yoon, C.; Woo, J.S.; Kim, G.H. Effect of electron beam irradiation on developmental stages of Plutella xylostella (Lepidoptera: Plutellidae). J. Asia-Pac. Entomol. 2011, 14, 243–247. [Google Scholar] [CrossRef]
- Koo, H.N.; Yun, S.H.; Kim, H.J.; Kim, H.K.; Kim, G.H. X-ray irradiation control of Frankliniella occidentalis and Frankliniella intonsa (Thysanoptera: Thripidae) in the exportation of freshly cut lily flowers. J. Econ. Entomol. 2017, 110, 416–420. [Google Scholar] [CrossRef]
- Koo, H.N.; Yun, S.H.; Kim, H.; Kim, G.H. Elucidation of molecular expression associated with abnormal development and sterility caused by electron beam irradiation in Spodoptera litura (F.) (Lepidoptera: Noctuidae). Int. J. Radiat. Biol. 2019, 95, 360–367. [Google Scholar] [CrossRef]
- Yun, S.H.; Koo, H.N.; Kim, H.K.; Yang, J.O.; Kim, G.H. X-ray irradiation as a quarantine treatment for the control of six insect pests in cut flower boxes. J. Asia-Pac. Entomol. 2016, 19, 31–38. [Google Scholar] [CrossRef]
- Cho, S.R.; Kim, M.; Shin, E.; Kim, H.K.; Koo, H.N.; Kim, G.H. X-ray irradiation-induced abnormal development and DNA damage in Phthorimaea operculella (Lepidoptera: Gelechiidae). Appl. Sci. 2021, 11, 5068. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.M.; Park, C.G. X-ray radiation and developmental inhibition of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae). Int. J. Radiat. Biol. 2016, 92, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, S.O.; Jang, S.A.; Kim, J.; Park, C.G. X-ray radiation and development inhibition of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). Radiat. Phys. Chem. 2015, 115, 148–152. [Google Scholar] [CrossRef]
- Kim, J.; Chung, S.O.; Jang, M.; Jang, S.A.; Park, C.G. Developmental inhibition and DNA damage of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) by gamma radiation. Int. J. Radiat. Biol. 2015, 91, 827–832. [Google Scholar] [CrossRef]
- Bakri, A.; Mehta, K.; Lance, D.R. Sterilizing insects with ionizing radiation. In Sterile Insect Technique. Principles and Practice in Areawide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 233–268. [Google Scholar]
- Bakri, A.; Heather, N.; Hendrichs, J.; Ferris, I. Fifty years of radiation biology in entomology: Lessons learned from IDIDAS. Ann. Entomol. Soc. Am. 2005, 98, 1–12. [Google Scholar] [CrossRef]
- Bloem, K.A.; Bloem, S.; Carpenter, J. Impact of moth suppression/eradication programmes using the sterile insect technique or inherited sterility. In Sterile Insect Technique. Principles and Practice in Areawide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 677–700. [Google Scholar]
| Acaricide | Strain | LC50 (ppm, 95% CL a) | RR b |
|---|---|---|---|
| Acequinocyl | AR | >2400 | >108.5 |
| S | 22.0 (19.1–25.5) | 1.0 | |
| Bifenazate | BR | >1340 | >705.3 |
| S | 1.9 (1.6–2.3) | 1.0 | |
| Etoxazole | ER | >5000 | >5,000,000 |
| S | 0.0011 (0.0008–0.0014) | 1.0 |
| Dose (Gy) | Strain | Hatchability (%) | Adult Longevity (Day) | No. of Eggs (♀/Total) | Hatchability (F1, %) | Emergence Rate (%) |
|---|---|---|---|---|---|---|
| 150 | AR | 0.0 ± 0.0 a a | - | - | - | - |
| BR | 0.0 ± 0.0 a | - | - | - | - | |
| ER | 0.0 ± 0.0 a | - | - | - | - | |
| S | 0.0 ± 0.0 a | - | - | - | - | |
| 100 | AR | 8.8 ± 11.1 a | 10.9 ± 3.9 cd | 29.3 ± 17.2 abc | 50.5 ± 20.0 a | 32.5 ± 4.6 a |
| BR | 14.7 ± 20.4 ab | 4.4 ± 0.8 abcd | 3.8 ± 7.1 a | 72.9 ± 19.2 b | - | |
| ER | 14.1 ± 24.3 ab | 6.0 ± 1.9 ab | 7.9 ± 11.1 ab | 91.2 ± 10.4 bc | - | |
| S | 0.0 ± 0.0 a | - | - | - | - | |
| 50 | AR | 62.2 ± 35.1 cd | 6.2 ± 3.4 abcd | 51.6 ± 22.5 bc | 88.7 ± 6.9 bc | 85.6 ± 20.4 b |
| BR | 47.0 ± 40.2 bc | 4.9 ± 3.0 a | 27.6 ± 26.2 abc | 92.0 ± 7.0 c | 85.7 ± 4.3 b | |
| ER | 82.6 ± 13.0 cd | 3.9 ± 2.3 abcd | 11.5 ± 17.6 ab | 93.4 ± 10.9 c | 74.4 ± 17.6 b | |
| S | 93.7 ± 2.1 d | 3.6 ± 1.8 abc | 2.3 ± 3.1 a | 86.5 ± 15.9 bc | 80.1 ± 7.9 b | |
| 0 | AR | 92.4 ± 9.1 d | 8.8 ± 4.0 bcd | 46.7 ± 28.8 c | 95.1 ± 4.1 c | 91.7 ± 7.0 b |
| BR | 94.6 ± 3.5 d | 7.0 ± 2.9 abcd | 37.8 ± 30.3 bc | 93.0 ± 8.8 c | 83.5 ± 4.7 b | |
| ER | 87.6 ± 8.4 d | 7.4 ± 2.9 d | 49.7 ± 19.4 c | 92.3 ± 11.3 c | 96.0 ± 4.6 b | |
| S | 93.1 ± 5.6 d | 7.8 ± 2.6 bcd | 44.6 ± 22.7 c | 92.6 ± 6.7 c | 89.1 ± 18.2 b |
| Dose (Gy) | Strain | Emergence Rate (%) | Adult Longevity (Day) | No. of Eggs (♀/Total) | Hatchability (F1, %) |
|---|---|---|---|---|---|
| 400 | AR | 71.4 ± 2.0 a a | 12.3 ± 3.8 abcde | 0.0 ± 0.0 a | - |
| BR | 72.6 ± 0.7 abc | 9.5 ± 3.1 abcd | 0.0 ± 0.0 a | - | |
| ER | 73.2 ± 2.5 abc | 9.6 ± 4.7 abcde | 0.0 ± 0.0 a | - | |
| S | 77.1 ± 1.6 abcde | 11.7 ± 5.2 abcde | 0.0 ± 0.0 a | - | |
| 300 | AR | 72.2 ± 1.0 ab | 11.5 ± 5.9 abcde | 0.1 ± 0.3 a | 0.0 ± 0.0 a |
| BR | 75.2 ± 4.2 abcd | 14.4 ± 7.8 bcde | 0.0 ± 0.0 a | - | |
| ER | 82.1 ± 3.8 abcdef | 10.1 ± 3.6 abcde | 0.1 ± 0.3 a | 0.0 ± 0.0 a | |
| S | 88.4 ± 2.0 def | 14.1 ± 6.5 cde | 1.3 ± 3.6 a | 0.0 ± 0.0 a | |
| 200 | AR | 84.5 ± 4.3 abcdef | 14.4 ± 6.6 de | 3.4 ± 5.6 ab | 6.4 ± 11.2 a |
| BR | 86.8 ± 0.7 cdef | 11.9 ± 7.2 abcde | 9.3 ± 9.0 abc | 0.7 ± 2.2 a | |
| ER | 83.3 ± 0.6 abcdef | 9.7 ± 4.7 abcd | 7.1 ± 7.6 ab | 0.0 ± 0.0 a | |
| S | 84.8 ± 3.9 abcdef | 15.5 ± 7.0 e | 5.5 ± 5.9 ab | 6.3 ± 10.8 a | |
| 100 | AR | 84.8 ± 2.7 abcdef | 8.8 ± 3.3 abcde | 25.1 ± 19.3 cd | 57.9 ± 17.2 bc |
| BR | 86.4 ± 3.1 bcdef | 8.9 ± 4.5 abcde | 5.3 ± 6.4 ab | 0.0 ± 0.0 a | |
| ER | 87.8 ± 4.0 def | 8.9 ± 4.1 abcde | 19.5 ± 10.2 bc | 44.8 ± 23.2 b | |
| S | 85.7 ± 6.2 abcdef | 7.0 ± 3.3 a | 5.1 ± 5.2 ab | 81.8 ± 22.0 cd | |
| 0 | AR | 89.9 ± 5.2 ef | 9.0 ± 3.1 abcde | 41.2 ± 17.2 d | 92.8 ± 3.5 d |
| BR | 85.9 ± 4.4 bcdef | 8.7 ± 3.1 abc | 41.1 ± 23.9 d | 94.5 ± 4.8 d | |
| ER | 93.9 ± 5.8 f | 9.7 ± 3.0 abcde | 37.4 ± 9.8 d | 96.9 ± 2.9 d | |
| S | 88.4 ± 4.2 def | 8.4 ± 1.8 ab | 41.4 ± 19.1 d | 91.3 ± 8.7 d |
| Dose (Gy) | Strain | Adult Longevity (Day) | No. of Eggs (♀/Total) | Hatchability (F1, %) |
|---|---|---|---|---|
| 400 | AR | 9.9 ± 4.2 ab a | 25.3 ± 7.6 abc | 0.0 ± 0.0 a |
| BR | 9.9 ± 4.6 ab | 23.7 ± 7.6 ab | 0.0 ± 0.0 a | |
| ER | 7.7 ± 3.4 ab | 26.0 ± 13.8 abc | 0.0 ± 0.0 a | |
| S | 9.3 ± 4.8 ab | 27.9 ± 3.4 abc | 0.0 ± 0.0 a | |
| 300 | AR | 40.3 ± 4.3 b | 25.6 ± 10.7 abc | 0.0 ± 0.0 a |
| BR | 8.3 ± 3.9 ab | 21.3 ± 5.8 a | 0.7 ± 2.6 a | |
| ER | 7.5 ± 2.3 ab | 23.2 ± 7.6 abc | 0.0 ± 0.0 a | |
| S | 7.3 ± 1.8 ab | 33.5 ± 10.6 abc | 7.8 ± 4.0 a | |
| 200 | AR | 9.5 ± 4.8 ab | 27.8 ± 12 abc | 1.5 ± 3.1 a |
| BR | 9.4 ± 4.4 ab | 35.8 ± 11.8 abc | 7.5 ± 11.3 a | |
| ER | 7.5 ± 1.5 ab | 23.8 ± 8.9 ab | 1.5 ± 2.2 a | |
| S | 6.7 ± 2.0 a | 28.4 ± 7.9 abc | 0.2 ± 0.7 a | |
| 100 | AR | 8.3 ± 3.7 ab | 41.5 ± 25.4 bc | 50.3 ± 32.8 b |
| BR | 9.1 ± 4.4 ab | 39.0 ± 16.4 abc | 49.4 ± 18.7 b | |
| ER | 6.9 ± 1.3 a | 27.0 ± 13.9 abc | 54.6 ± 28.5 b | |
| S | 6.8 ± 2.0 a | 29.2 ± 8.0 abc | 73.1 ± 7.7 c | |
| 0 | AR | 8.2 ± 1.8 ab | 36.6 ± 14.9 abc | 88.0 ± 10.9 cd |
| BR | 8.5 ± 2.8 ab | 43.8 ± 17.3 c | 91.9 ± 4.9 d | |
| ER | 8.5 ± 3.4 ab | 30.5 ± 18.0 abc | 86.9 ± 11 cd | |
| S | 8.2 ± 3.5 ab | 32.1 ± 9.6 abc | 97.9 ± 2.3 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, H.-N.; Oh, J.-H.; Jeon, J.-C.; Kang, W.-J.; Cho, S.-R.; Kim, Y.; Kim, G.-H. Effects of Electron Beam Irradiation on Acaricide-Resistant and Susceptible Strains of Tetranychusurticae (Acari: Tetranychidae). Appl. Sci. 2021, 11, 8116. https://doi.org/10.3390/app11178116
Koo H-N, Oh J-H, Jeon J-C, Kang W-J, Cho S-R, Kim Y, Kim G-H. Effects of Electron Beam Irradiation on Acaricide-Resistant and Susceptible Strains of Tetranychusurticae (Acari: Tetranychidae). Applied Sciences. 2021; 11(17):8116. https://doi.org/10.3390/app11178116
Chicago/Turabian StyleKoo, Hyun-Na, Jin-Hyun Oh, Jong-Chan Jeon, Won-Jin Kang, Sun-Ran Cho, Yuri Kim, and Gil-Hah Kim. 2021. "Effects of Electron Beam Irradiation on Acaricide-Resistant and Susceptible Strains of Tetranychusurticae (Acari: Tetranychidae)" Applied Sciences 11, no. 17: 8116. https://doi.org/10.3390/app11178116
APA StyleKoo, H.-N., Oh, J.-H., Jeon, J.-C., Kang, W.-J., Cho, S.-R., Kim, Y., & Kim, G.-H. (2021). Effects of Electron Beam Irradiation on Acaricide-Resistant and Susceptible Strains of Tetranychusurticae (Acari: Tetranychidae). Applied Sciences, 11(17), 8116. https://doi.org/10.3390/app11178116





