Chemical and Energy Recovery Alternatives in SWRO Desalination through Electro-Membrane Technologies
Abstract
:1. Introduction
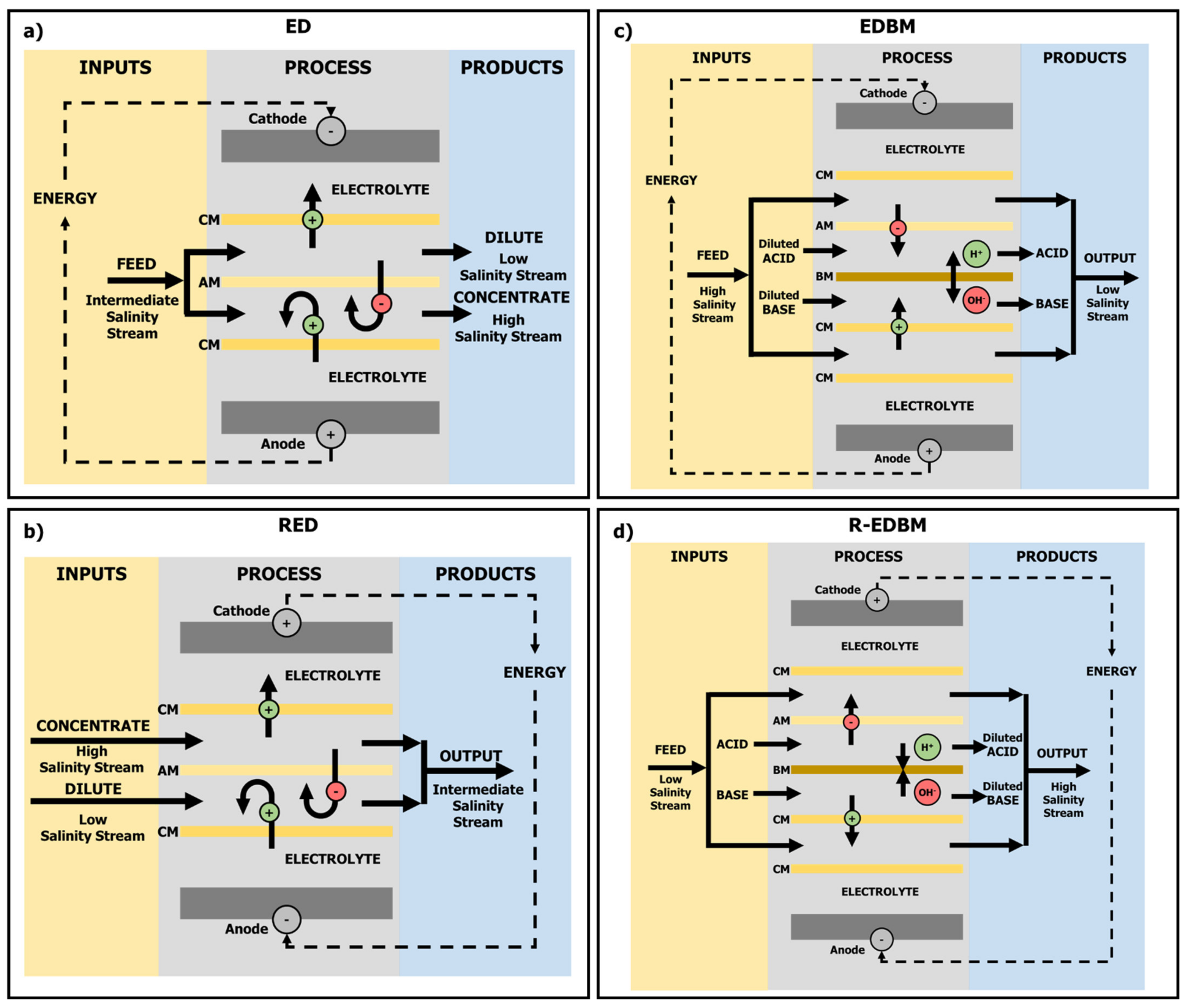
2. Electro-Membrane Technologies: Current Status and Challenges
| Electro-Membrane Technology | Membranes | Average Energy Consumption (C)/Production (P) | Feed Requirements | TRL |
|---|---|---|---|---|
| ED | Anionic Cationic | (C) 3–7 kWh·m−3 for BW 17 kWh·m−3 for SW [2] | Intermediate Salinity | 9 [13] |
| EDBM | Anionic Cationic Bipolar | (C) 1.6–9.0 kWh·kg−1 acid (<1.8 M acid) [33] | High Salinity | 5/6 [32,34] |
| RED | Anionic Cationic | (P) 0.09–1.86 W·m−2 [19] | Low vs. High Salinity | 8 [27] |
| R-EDBM | Anionic Cationic Bipolar | (P) 2.9–17.0 W·m−2 [32] | Acid vs. Base | 5/6 [32,34] |
3. Alternatives for Electro-Membrane Technologies Integration for Brine Management in SWRO Facilities
3.1. Alternative A0—Direct Brine Disposal
3.2. Alternative A1—Chemicals towards Self-Supply
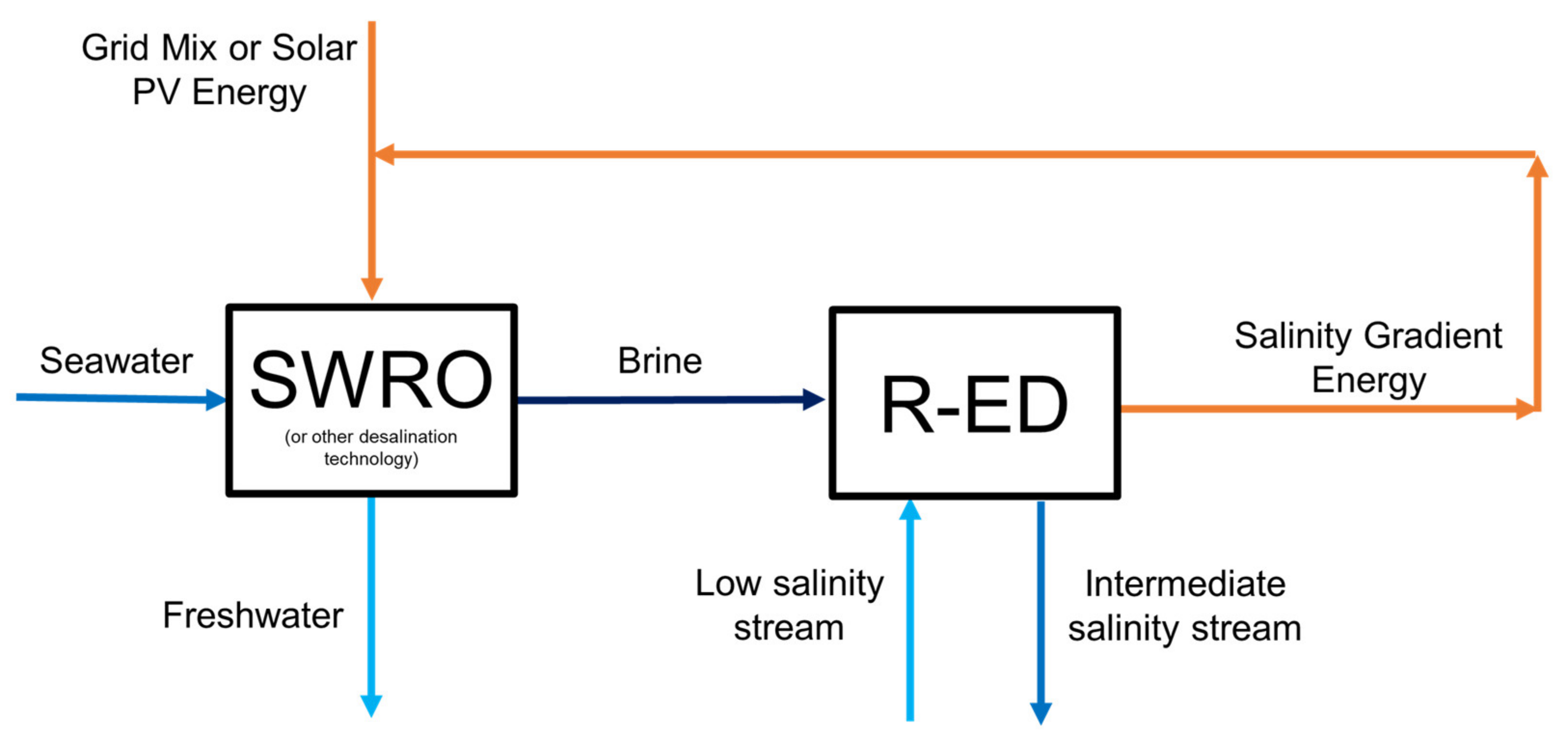
3.3. Alternative A2—Salinity Gradient Energy Harvesting
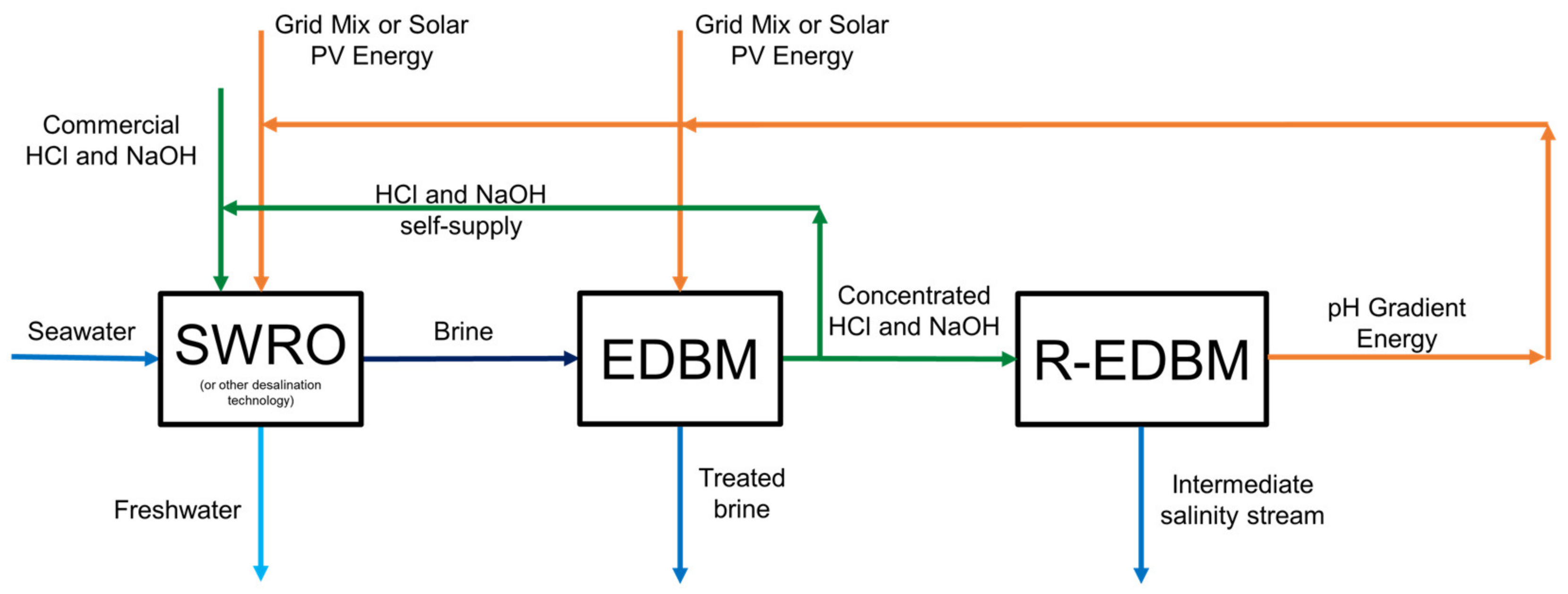
3.4. Alternative A3—Chemicals towards Self-Supply and pH Gradient Energy Harvesting
3.5. Alternative A4—Chemicals towards Self-Supply and Salinity Gradient Energy Harvesting
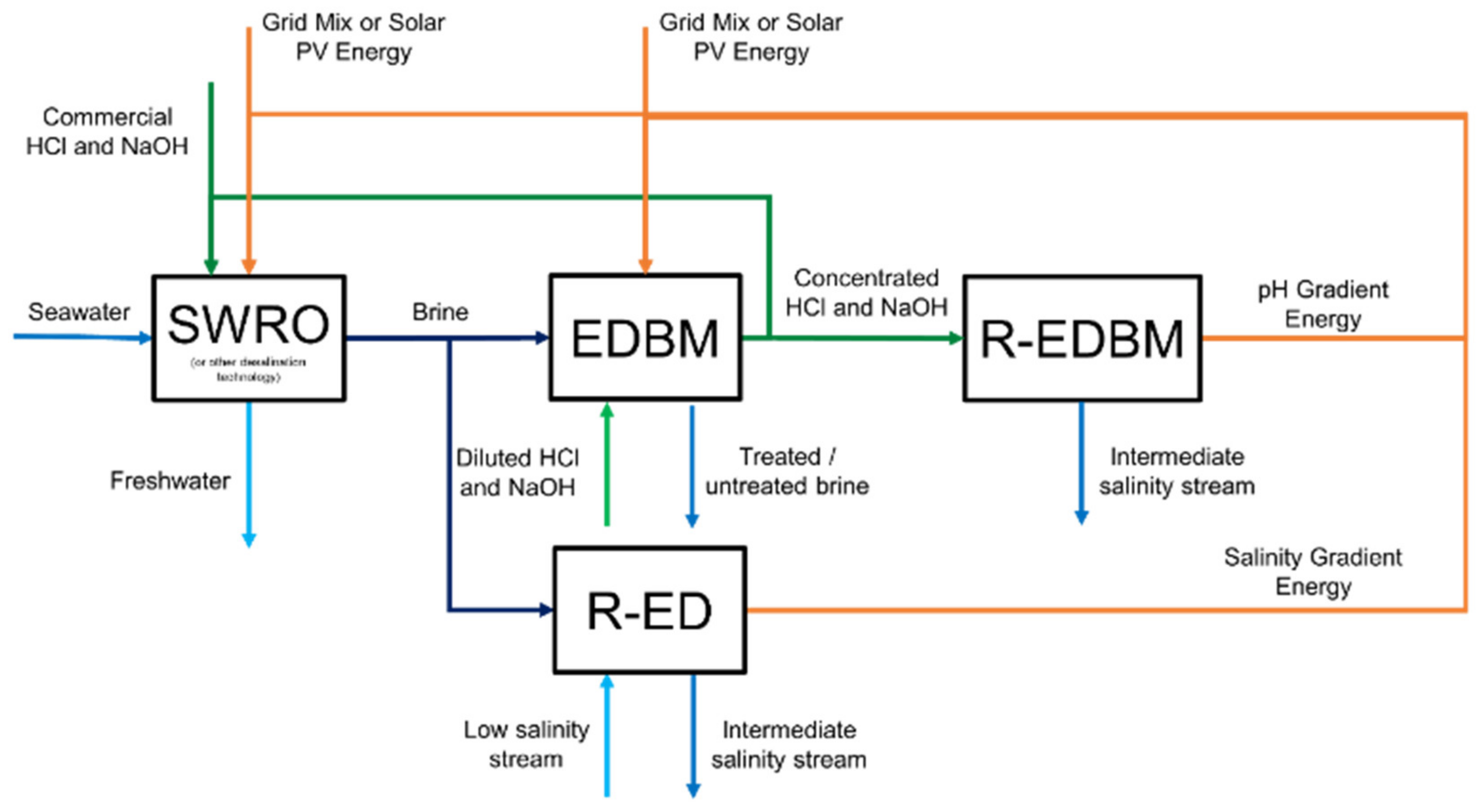
3.6. Alternative A5—Chemicals towards Self-Supply, pH Gradient Energy and Salinity Gradient Energy Harvesting
4. Benefits and Challenges of the Proposed Alternatives for Electro-Membrane Technologies Integration for Brine Management in SWRO Facilities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jones, E.; Qadir, M.; Van Vliet, M.T.H.; Smakhtin, V.; Kang, S. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Kress, N. Desalination Technologies. In Marine Impacts of Seawater Desalination; Elsevier: Amsterdam, The Netherlands, 2019; pp. 11–34. [Google Scholar]
- Meneses, M.; Pasqualino, J.C.; Céspedes-Sánchez, R.; Castells, F. Alternatives for reducing the environmental impact of the main residue from a desalination plant. J. Ind. Ecol. 2010, 14, 512–527. [Google Scholar] [CrossRef]
- Pérez-González, A.; Urtiaga, A.M.; Ibáñez, R.; Ortiz, I. State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res. 2012, 46, 267–283. [Google Scholar] [CrossRef]
- Reig, M.; Casas, S.; Aladjem, C.; Valderrama, C.; Gibert, O.; Valero, F.; Centeno, C.M.; Larrotcha, E.; Cortina, J.L. Concentration of NaCl from seawater reverse osmosis brines for the chlor-alkali industry by electrodialysis. Desalination 2014, 342, 107–117. [Google Scholar] [CrossRef]
- Casas, S.; Aladjem, C.; Cortina, J.L.; Larrotcha, E.; Cremades, L.V. Seawater Reverse Osmosis Brines as a New Salt Source for the Chlor-Alkali Industry: Integration of NaCl Concentration by Electrodialysis. Solvent Extr. Ion Exch. 2012, 30, 322–332. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, V.W.C.; Fane, A.G. An improved life cycle impact assessment (LCIA) approach for assessing aquatic eco-toxic impact of brine disposal from seawater desalination plants. Desalination 2013, 308, 233–241. [Google Scholar] [CrossRef]
- Bindels, M.; Carvalho, J.; Gonzalez, C.B.; Brand, N.; Nelemans, B. Techno-economic assessment of seawater reverse osmosis (SWRO) brine treatment with air gap membrane distillation (AGMD). Desalination 2020, 489, 114532. [Google Scholar] [CrossRef]
- Ortiz-Albo, P.; Torres-Ortega, S.; González Prieto, M.; Urtiaga, A.; Ibañez, R. Techno-Economic Feasibility Analysis for Minor Elements Valorization from Desalination Concentrates. Sep. Purif. Rev. 2019, 48, 220–241. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; El Haj Assad, M.; Sayed, E.T.; Soudan, B. Recent progress in the use of renewable energy sources to power water desalination plants. Desalination 2018, 435, 97–113. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development|Department of Economic and Social Affairs. Available online: https://sdgs.un.org/2030agenda (accessed on 15 June 2021).
- European Comision. A European Green Deal | European Commission. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 6 July 2021).
- Fernandez-Gonzalez, C.; Dominguez-Ramos, A.; Ibañez, R.; Irabien, A. Sustainability assessment of electrodialysis powered by photovoltaic solar energy for freshwater production. Renew. Sustain. Energy Rev. 2015, 47, 604–615. [Google Scholar] [CrossRef]
- Esmaeilion, F. Hybrid Renewable Energy Systems for Desalination; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; Volume 10, ISBN 0123456789. [Google Scholar]
- Campione, A.; Cipollina, A.; Calise, F.; Tamburini, A.; Galluzzo, M.; Micale, G. Coupling electrodialysis desalination with photovoltaic and wind energy systems for energy storage: Dynamic simulations and control strategy. Energy Convers. Manag. 2020, 216, 112940. [Google Scholar] [CrossRef]
- Das, B.K.; Al-Abdeli, Y.M.; Woolridge, M. Effects of battery technology and load scalability on stand-alone PV/ICE hybrid micro-grid system performance. Energy 2019, 168, 57–69. [Google Scholar] [CrossRef]
- Arévalo, P.; Benavides, D.; Lata-García, J.; Jurado, F. Energy control and size optimization of a hybrid system (photovoltaic-hidrokinetic) using various storage technologies. Sustain. Cities Soc. 2020, 52, 101773. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, H.; Wang, H.; Ladewig, B.P.; Yao, Z. A comprehensive review on the synthesis and applications of ion exchange membranes. Chemosphere 2021, 282, 130817. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis applications in wastewater treatment for environmental protection and resources recovery: A systematic review on progress and perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef]
- San Román, M.F.; Ortiz-Gándara, I.; Bringas, E.; Ibañez, R.; Ortiz, I. Membrane selective recovery of HCl, zinc and iron from simulated mining effluents. Desalination 2018, 440, 78–87. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, C.; Bazinet, L.; Xu, T. Electrodialysis-Based Separation Technologies in the Food Industry. In Separation of Functional Molecules in Food by Membrane Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 349–381. [Google Scholar]
- Ganiyu, S.O.; Martínez-Huitle, C.A. The use of renewable energies driving electrochemical technologies for environmental applications. Curr. Opin. Electrochem. 2020, 22, 211–220. [Google Scholar] [CrossRef]
- Mir, N.; Bicer, Y. Integration of electrodialysis with renewable energy sources for sustainable freshwater production: A review. J. Environ. Manag. 2021, 289, 112496. [Google Scholar] [CrossRef] [PubMed]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Tristán, C.; Fallanza, M.; Ibáñez, R.; Ortiz, I. Reverse electrodialysis: Potential reduction in energy and emissions of desalination. Appl. Sci. 2020, 10, 7317. [Google Scholar] [CrossRef]
- Zoungrana, A.; Çakmakci, M. From non-renewable energy to renewable by harvesting salinity gradient power by reverse electrodialysis: A review. Int. J. Energy Res. 2021, 45, 3495–3522. [Google Scholar] [CrossRef]
- Cipollina, A.; Micale, G.; Tamburini, A.; Tedesco, M.; Gurreri, L.; Veerman, J.; Grasman, S. Reverse electrodialysis. In Sustainable Energy from Salinity Gradients; Elsevier: Amsterdam, The Netherlands, 2016; pp. 135–180. [Google Scholar]
- Mier, M.; Ibañez, R.; Otiz, I. Influence of ion concentration on the kinetics of electrodialysis with bipolar membranes. Sep. Purif. Technol. 2008, 59, 197–205. [Google Scholar] [CrossRef]
- Reig, M.; Casas, S.; Valderrama, C.; Gibert, O.; Cortina, J.L. Integration of monopolar and bipolar electrodialysis for valorization of seawater reverse osmosis desalination brines: Production of strong acid and base. Desalination 2016, 398, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Reig, M.; Casas, S.; Gibert, O.; Valderrama, C.; Cortina, J.L. Integration of nanofiltration and bipolar electrodialysis for valorization of seawater desalination brines: Production of drinking and waste water treatment chemicals. Desalination 2016, 382, 13–20. [Google Scholar] [CrossRef]
- Giesbrecht, P.K.; Freund, M.S. Recent Advances in Bipolar Membrane Design and Applications. Chem. Mater. 2020, 32, 8060–8090. [Google Scholar] [CrossRef]
- Pärnamäe, R.; Gurreri, L.; Post, J.; van Egmond, W.J.; Culcasi, A.; Saakes, M.; Cen, J.; Goosen, E.; Tamburini, A.; Vermaas, D.A.; et al. The acid–base flow battery: Sustainable energy storage via reversible water dissociation with bipolar membranes. Membranes 2020, 10, 409. [Google Scholar] [CrossRef]
- Herrero-Gonzalez, M.; Diaz-Guridi, P.; Dominguez-Ramos, A.; Irabien, A.; Ibañez, R. Highly concentrated HCl and NaOH from brines using electrodialysis with bipolar membranes. Sep. Purif. Technol. 2020, 242, 116785. [Google Scholar] [CrossRef]
- Muñoz-Cruzado-Alba, J.; Musca, R.; Ballestín-Fuertes, J.; Sanz-Osorio, J.F.; Rivas-Ascaso, D.M.; Jones, M.P.; Catania, A.; Goosen, E. Power Grid Integration and Use-Case Study of Acid-Base Flow Battery Technology. Sustainability 2021, 13, 6089. [Google Scholar] [CrossRef]
- Morales-Mora, M.A.; Pijpers, J.J.H.; Antonio, A.C.; de la Soto, J.C.; Calderón, A.M.A. Life cycle assessment of a novel bipolar electrodialysis-based flow battery concept and its potential use to mitigate the intermittency of renewable energy generation. J. Energy Storage 2021, 35, 102339. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.; Yang, D.R.; Hong, S. A comprehensive review of energy consumption of seawater reverse osmosis desalination plants. Appl. Energy 2019, 254, 113652. [Google Scholar] [CrossRef]
- Palomar, P.; Losada, I.J. Impacts of Brine Discharge on the Marine Environment. Modelling as a Predictive Tool. In Desalination, Trends and Technologies; Books on Demand: Norderstedt, Germany, 2011; ISBN 978-953-307-311-8. [Google Scholar]
- Fernandez-Gonzalez, C.; Dominguez-Ramos, A.; Ibañez, R.; Irabien, A. Electrodialysis with Bipolar Membranes for Valorization of Brines. Sep. Purif. Rev. 2016, 45, 275–287. [Google Scholar] [CrossRef]
- Panagopoulos, A. Water-energy nexus: Desalination technologies and renewable energy sources. Environ. Sci. Pollut. Res. 2021, 28, 21009–21022. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.-J. Environmental impacts of desalination and brine treatment—Challenges and mitigation measures. Mar. Pollut. Bull. 2020, 161, 111773. [Google Scholar] [CrossRef]
- Soliman, M.N.; Guen, F.Z.; Ahmed, S.A.; Saleem, H.; Khalil, M.J.; Zaidi, S.J. Energy consumption and environmental impact assessment of desalination plants and brine disposal strategies. Process Saf. Environ. Prot. 2021, 147, 589–608. [Google Scholar] [CrossRef]
- Bello, A.S.; Zouari, N.; Da’ana, D.A.; Hahladakis, J.N.; Al-Ghouti, M.A. An overview of brine management: Emerging desalination technologies, life cycle assessment, and metal recovery methodologies. J. Environ. Manag. 2021, 288, 112358. [Google Scholar] [CrossRef]
- Fernández-Torquemada, Y.; Sánchez-Lizaso, J.L. Effects of salinity on leaf growth and survival of the Mediterranean seagrass Posidonia oceanica (L.) Delile. J. Exp. Mar. Biol. Ecol. 2005, 320, 57–63. [Google Scholar] [CrossRef]
- Gacia, E.; Invers, O.; Manzanera, M.; Ballesteros, E.; Romero, J. Impact of the brine from a desalination plant on a shallow seagrass (Posidonia oceanica) meadow. Estuar. Coast. Shelf Sci. 2007, 72, 579–590. [Google Scholar] [CrossRef]
- Del-Pilar-Ruso, Y.; De-la-Ossa-Carretero, J.A.; Giménez-Casalduero, F.; Sánchez-Lizaso, J.L. Effects of a brine discharge over soft bottom Polychaeta assemblage. Environ. Pollut. 2008, 156, 240–250. [Google Scholar] [CrossRef]
- Sánchez-Lizaso, J.L.; Romero, J.; Ruiz, J.; Gacia, E.; Buceta, J.L.; Invers, O.; Fernández Torquemada, Y.; Mas, J.; Ruiz-Mateo, A.; Manzanera, M. Salinity tolerance of the Mediterranean seagrass Posidonia oceanica: Recommendations to minimize the impact of brine discharges from desalination plants. Desalination 2008, 221, 602–607. [Google Scholar] [CrossRef]
- Roberts, D.A.; Johnston, E.L.; Knott, N.A. Impacts of desalination plant discharges on the marine environment: A critical review of published studies. Water Res. 2010, 44, 5117–5128. [Google Scholar] [CrossRef]
- Yoon, S.J.; Park, G.S. Ecotoxicological effects of brine discharge on marine community by seawater desalination. Desalin. Water Treat. 2011, 33, 240–247. [Google Scholar] [CrossRef]
- Belkin, N.; Rahav, E.; Elifantz, H.; Kress, N.; Berman-Frank, I. Enhanced salinities, as a proxy of seawater desalination discharges, impact coastal microbial communities of the eastern Mediterranean Sea. Environ. Microbiol. 2015, 17, 4105–4120. [Google Scholar] [CrossRef] [PubMed]
- Belkin, N.; Rahav, E.; Elifantz, H.; Kress, N.; Berman-Frank, I. The effect of coagulants and antiscalants discharged with seawater desalination brines on coastal microbial communities: A laboratory and in situ study from the southeastern Mediterranean. Water Res. 2017, 110, 321–331. [Google Scholar] [CrossRef]
- De-la-Ossa-Carretero, J.A.; Del-Pilar-Ruso, Y.; Loya-Fernández, A.; Ferrero-Vicente, L.M.; Marco-Méndez, C.; Martinez-Garcia, E.; Giménez-Casalduero, F.; Sánchez-Lizaso, J.L. Bioindicators as metrics for environmental monitoring of desalination plant discharges. Mar. Pollut. Bull. 2016, 103, 313–318. [Google Scholar] [CrossRef]
- Röthig, T.; Ochsenkühn, M.A.; Roik, A.; Van Der Merwe, R.; Voolstra, C.R. Long-term salinity tolerance is accompanied by major restructuring of the coral bacterial microbiome. Mol. Ecol. 2016, 25, 1308–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro Barrio, R.; Sola, I.; Blanco-Murillo, F.; Del-Pilar-Ruso, Y.; Fernández-Torquemada, Y.; Sánchez-Lizaso, J.L. Application of salinity thresholds in Spanish brine discharge regulations: Energetic and environmental implications. Desalination 2021, 501, 114901. [Google Scholar] [CrossRef]
- Missimer, T.M.; Maliva, R.G. Environmental issues in seawater reverse osmosis desalination: Intakes and outfalls. Desalination 2018, 434, 198–215. [Google Scholar] [CrossRef]
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Ellen MacArthur Foundation. What Is a Circular Economy?|Ellen MacArthur Foundation. Available online: https://www.ellenmacarthurfoundation.org/circular-economy/concept (accessed on 28 June 2019).
- Ahmed, M.; Arakel, A.; Hoey, D.; Thumarukudy, M.R.; Goosen, M.F.A.; Al-Haddabi, M.; Al-Belushi, A. Feasibility of salt production from inland RO desalination plant reject brine: A case study. Desalination 2003, 158, 109–117. [Google Scholar] [CrossRef]
- Sanmartino, J.A.; Khayet, M.; García-Payo, M.C.; El-Bakouri, H.; Riaza, A. Treatment of reverse osmosis brine by direct contact membrane distillation: Chemical pretreatment approach. Desalination 2017, 420, 79–90. [Google Scholar] [CrossRef]
- Kumar, A.; Naidu, G.; Fukuda, H.; Du, F.; Vigneswaran, S.; Drioli, E.; Lienhard, J.H. Metals Recovery from Seawater Desalination Brines: Technologies, Opportunities, and Challenges. ACS Sustain. Chem. Eng. 2021, 9, 7704–7712. [Google Scholar] [CrossRef]
- Sea4value—Mining Value from Brines—Sea4value. Available online: https://sea4value.eu/ (accessed on 21 July 2021).
- SEArcular Mine. Available online: https://searcularmine.eu/ (accessed on 18 July 2021).
- Tate, J. Industrial Reverse Osmosis System Design. Water Cond. Purif. Mag. 2008, 7, 3. [Google Scholar]
- Ras, C.; von Blottnitz, H. A comparative life cycle assessment of process water treatment technologies at the Secunda industrial complex, South Africa. Water SA 2012, 38, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Gonzalez, M.; Diaz-Guridi, P.; Dominguez-Ramos, A.; Ibañez, R.; Irabien, A. Photovoltaic solar electrodialysis with bipolar membranes. Desalination 2018, 433, 155–163. [Google Scholar] [CrossRef]
- Herrero-Gonzalez, M.; Admon, N.; Dominguez-Ramos, A.; Ibañez, R.; Wolfson, A.; Irabien, A. Environmental sustainability assessment of seawater reverse osmosis brine valorization by means of electrodialysis with bipolar membranes. Environ. Sci. Pollut. Res. 2020, 27, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Gonzalez, M.; Wolfson, A.; Dominguez-Ramos, A.; Ibañez, R.; Irabien, A. Monetizing Environmental Footprints: Index Development and Application to a Solar-Powered Chemicals Self-Supplied Desalination Plant. ACS Sustain. Chem. Eng. 2018, 6, 14533–14541. [Google Scholar] [CrossRef] [Green Version]
- Tristán, C.; Rumayor, M.; Dominguez-Ramos, A.; Fallanza, M.; Ibáñez, R.; Ortiz, I. Life cycle assessment of salinity gradient energy recovery by reverse electrodialysis in a seawater reverse osmosis desalination plant. Sustain. Energy Fuels 2020, 4, 4273–7284. [Google Scholar] [CrossRef]
- Tufa, R.A.; Curcio, E.; van Baak, W.; Veerman, J.; Grasman, S.; Fontananova, E.; Di Profio, G. Potential of brackish water and brine for energy generation by salinity gradient power-reverse electrodialysis (SGP-RE). RSC Adv. 2014, 4, 42617–42623. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Pawlowski, S.; Crespo, J.G.; Velizarov, S. Pressure drop in reverse electrodialysis: Experimental and modeling studies for stacks with variable number of cell pairs. J. Membr. Sci. 2014, 462, 96–111. [Google Scholar] [CrossRef]
- Tristán, C.; Fallanza, M.; Ibáñez, R.; Ortiz, I. Recovery of salinity gradient energy in desalination plants by reverse electrodialysis. Desalination 2020, 496, 114699. [Google Scholar] [CrossRef]
- Culcasi, A.; Gurreri, L.; Micale, G.; Tamburini, A. Bipolar membrane reverse electrodialysis for the sustainable recovery of energy from pH gradients of industrial wastewater: Performance prediction by a validated process model. J. Environ. Manag. 2021, 287, 112319. [Google Scholar] [CrossRef]
- Mei, Y.; Liu, L.; Lu, Y.-C.; Tang, C.Y. Reverse Electrodialysis Chemical Cell for Energy Harvesting from Controlled Acid–Base Neutralization. Environ. Sci. Technol. 2019, 53, 4640–4647. [Google Scholar] [CrossRef] [PubMed]
- Culcasi, A.; Gurreri, L.; Zaffora, A.; Cosenza, A.; Tamburini, A.; Micale, G. On the modelling of an Acid/Base Flow Battery: An innovative electrical energy storage device based on pH and salinity gradients. Appl. Energy 2020, 277, 115576. [Google Scholar] [CrossRef]
- Xia, J.; Eigenberger, G.; Strathmann, H.; Nieken, U. Flow battery based on reverse electrodialysis with bipolar membranes: Single cell experiments. J. Membr. Sci. 2018, 565, 157–168. [Google Scholar] [CrossRef]
- Xia, J.; Eigenberger, G.; Strathmann, H.; Nieken, U. Acid-Base Flow Battery, Based on Reverse Electrodialysis with Bi-Polar Membranes: Stack Experiments. Processes 2020, 8, 99. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, J.H.; Maurya, S.; Shin, S.H.; Lee, J.Y.; Chang, I.S.; Moon, S.H. Proof-of-concept experiments of an acid-base junction flow battery by reverse bipolar electrodialysis for an energy conversion system. Electrochem. Commun. 2016, 72, 157–161. [Google Scholar] [CrossRef]
- Zholkovskij, E.K.; Müller, M.C.; Staude, E. The storage battery with bipolar membranes. J. Membr. Sci. 1998, 141, 231–243. [Google Scholar] [CrossRef]
- Zaffora, A.; Culcasi, A.; Gurreri, L.; Cosenza, A.; Tamburini, A.; Santamaria, M.; Micale, G. Energy harvesting by waste acid/base neutralization via bipolar membrane reverse electrodialysis. Energies 2020, 13, 5510. [Google Scholar] [CrossRef]
- Van Egmond, W.J.; Saakes, M.; Noor, I.; Porada, S.; Buisman, C.J.N.; Hamelers, H.V.M. Performance of an environmentally benign acid base flow battery at high energy density. Int. J. Energy Res. 2018, 42, 1524–1535. [Google Scholar] [CrossRef] [Green Version]
- Xevgenos, D.; Marcou, M.; Louca, V.; Avramidi, E.; Ioannou, G.; Argyrou, M.; Stavrou, P.; Mortou, M.; Küpper, F.C. Aspects of environmental impacts of seawater desalination: Cyprus as a case study. Desalin. Water Treat. 2021, 211, 15–30. [Google Scholar] [CrossRef]
- Lee, K.; Jepson, W. Environmental impact of desalination: A systematic review of Life Cycle Assessment. Desalination 2021, 509, 115066. [Google Scholar] [CrossRef]
- Giacalone, F.; Papapetrou, M.; Kosmadakis, G.; Tamburini, A.; Micale, G.; Cipollina, A. Application of reverse electrodialysis to site-specific types of saline solutions: A techno-economic assessment. Energy 2019, 181, 532–547. [Google Scholar] [CrossRef]
- Jalili, Z.; Krakhella, K.W.; Einarsrud, K.E.; Burheim, O.S. Energy generation and storage by salinity gradient power: A model-based assessment. J. Energy Storage 2019, 24, 100755. [Google Scholar] [CrossRef]
- Noack, J.; Wietschel, L.; Roznyatovskaya, N.; Pinkwart, K.; Tübke, J. Techno-Economic Modeling and Analysis of Redox Flow Battery Systems. Energies 2016, 9, 627. [Google Scholar] [CrossRef]



| Element | Concentrations in SWRO Brines (g·L−1) | ||||
|---|---|---|---|---|---|
| [5,6] | [7] | [8] | [9] | Average | |
| Cl | 38.8 | 43.67 | 38.49 | 41.83 | 40.70 |
| Na | 20.8 | 24.65 | 20.08 | 25.24 | 22.69 |
| SO4 | 5.41 | 6.75 | 5.42 | 6.05 | 5.91 |
| Mg | 2.64 | 2.88 | 2.60 | 2.87 | 2.75 |
| Ca | 0.83 | 0.89 | 0.79 | 0.96 | 0.87 |
| K | 0.75 | 0.89 | 0.84 | 0.78 | 0.82 |
| Alternative Code | RED | EDBM | R-EDBM |
|---|---|---|---|
| A0 | 🗶 | 🗶 | 🗶 |
| A1 | 🗶 | ✓ | 🗶 |
| A2 | ✓ | 🗶 | 🗶 |
| A3 | 🗶 | ✓ | ✓ |
| A4 | ✓ | ✓ | 🗶 |
| A5 | ✓ | ✓ | ✓ |
| Alternative Code | Impacts Reduction | Challenges | ||||
|---|---|---|---|---|---|---|
| Climate Change | Water Bodies | Raw Material Consumption | TRL | OPEX | CAPEX | |
| A0 | - - | - - | - - | + + | 0 | 0 |
| A1 | - | - | - | + | + | - |
| A2 | + | + + | + | 0 | + | - |
| A3 | 0 | 0 | 0 | - | + | - |
| A4 | + | + + | + + | - | + + | - - |
| A5 | + + | + + | + + | - | + + | - - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero-Gonzalez, M.; Ibañez, R. Chemical and Energy Recovery Alternatives in SWRO Desalination through Electro-Membrane Technologies. Appl. Sci. 2021, 11, 8100. https://doi.org/10.3390/app11178100
Herrero-Gonzalez M, Ibañez R. Chemical and Energy Recovery Alternatives in SWRO Desalination through Electro-Membrane Technologies. Applied Sciences. 2021; 11(17):8100. https://doi.org/10.3390/app11178100
Chicago/Turabian StyleHerrero-Gonzalez, Marta, and Raquel Ibañez. 2021. "Chemical and Energy Recovery Alternatives in SWRO Desalination through Electro-Membrane Technologies" Applied Sciences 11, no. 17: 8100. https://doi.org/10.3390/app11178100
APA StyleHerrero-Gonzalez, M., & Ibañez, R. (2021). Chemical and Energy Recovery Alternatives in SWRO Desalination through Electro-Membrane Technologies. Applied Sciences, 11(17), 8100. https://doi.org/10.3390/app11178100







