1. Introduction
Rare-earth (RE)-based magnets have received worldwide attention as they are the strongest magnets known to date, with energy outputs as high as 430 KJ/m
3. Their high productivity, affordability, energy efficiency and excellent magnetic properties make NdFeB magnets an ideal choice for high-technology applications, such as in wind turbines, hybrid and electric vehicles, air conditioning compressors, fans, mobile phones, hard disk drive, and speakers [
1,
2,
3]. Nd is the essential component of NdFeB magnets, while Dy is added up to 6wt% in order to enhance a magnet’s ability to operate at elevated temperatures and to increase the coercive force [
4,
5,
6]. Both Nd and Dy belong to a class of metals named ‘critical metals’. Critical metals can be defined in terms of their high global demand, susceptibility to future scarcity, unavailability of substitutes, and low recycling rates [
7]. They can also be described as metals that are prone to global access and supply risks due to their high demand for use in green energy technologies [
8,
9]. The supply risks can result from political, geological, or technological constraints. Almost all leading agencies around the world, including the European Commission, U.S. Department of Energy, American Physical Society, Materials Research Society, and UN International Resource Panel have declared Nd and Dy to be critical, which means that the chances of disruption of their supply is high, with associated impacts on the linked technological growth [
10]. Similarly, In Korea, REEs are on a list of around 35 rare metals that are expected to be depleted in the future with the development of Korean industry in the 21st century.
In short, green energy is at the helm of the circular economy transformation, and NdFeB magnets are essential for the transition into a cleaner energy matrix. Their use in key technologies make them pivotal in supporting several Sustainable Development Goals (SDGs), such as SDG 7: Ensure Access to Affordable, Reliable, Sustainable, and Modern Energy for All; SDG 13: Climate Action; and SDG 11: Sustainable Cities and Communities.
China is abundant in REE resources and controls the global REE (rare earth element) industry, having established a dominant position across the entire value chain. This control extends all the way from mining to the production of key intermediate products such as magnets. China supplied roughly 85% of global total refined rare earth production in 2020; for Dy alone, this was around 95% of the global production [
11].
The mining of raw materials for REE magnets results in significantly adverse environmental and social impacts [
12,
13], as harmful chemicals such as hydrochloric and sulfuric acid are used [
14]. Moreover, radioactive products such as uranium and thorium can be generated during the extraction process [
13]. Magnets produced from secondary resources have significantly less environmental impact than those produced from primary resources [
15,
16]. Furthermore, magnets produced from secondary resources have a great advantage of not generating any radioactive waste, as compared to primary production, where these elements are normally present in significant amounts, depending on the geological settings. Although the recycling of magnets requires solid collection schemes and transportation infrastructure, previous studies investigating the material flow in REE magnets in Denmark and Korea indicated that it is absolutely essential to develop a second material flow for REEs through recycling or re-use to achieve sustainability and a circular economy [
17,
18,
19]. The processes that have been studied for recycling magnet scrap to recover REEs can be classified into hydrometallurgy and pyrometallurgy. Hydrometallurgical approaches that aim to completely dissolve the material to regain elements or oxides are energy- and time-consuming; thus, they are costly and come with a large environmental footprint, as they require extensive use of chemicals [
20,
21]. On the contrary, pyrometallurgical methods can be alternatives to hydrometallurgy methods as extractive-type process. This process can be applied for heavily oxidized REE magnets to recover REEs. Even though hydrometallurgical recycling should be developed for technical competitiveness, pyrometallurgy is more suitable for use in recycling technology in Korea because the usage of chemicals is strictly regulated rather than the electrical consumption because of the robust electricity infrastructure. As such, liquid metal extraction (LME), one of the pyrometallurgical processes, is based on a material being selectively diffused into a liquid metal agent to form a metallic alloy. When separating REEs and agents, this method is not only able to extract pure REEs in metallic form, but the agents can also be re-applied for materialization processes, without producing any waste. Because Nd and Dy, which comprise up to 50% of electronic waste and scrap, are still being exported or disposed of in Korea [
22], the crisis of the circular economy in REEs can be overcome using the recycling and materialization (ReMaT) method based on pyrometallurgical processes. In this study, we share the roadmap and results for Korea’s strategy towards ensuring secure secondary resources for REEs through the implementation of the ReMaT process. Since REE recycling technology is a major hurdle in efficient recycling, we also share the progress related to the ReMaT process in terms of technological development. Lastly, we share the efforts made by the Republic of Korea to promote REE recycling through the International Standardization Organization.
2. Material Flow of REEs in Korea
REEs are essential components for production in high-tech manufacturing, such as in transportation, optics, defense, renewable energy, and agriculture applications, due to their exotic physical and chemical properties.
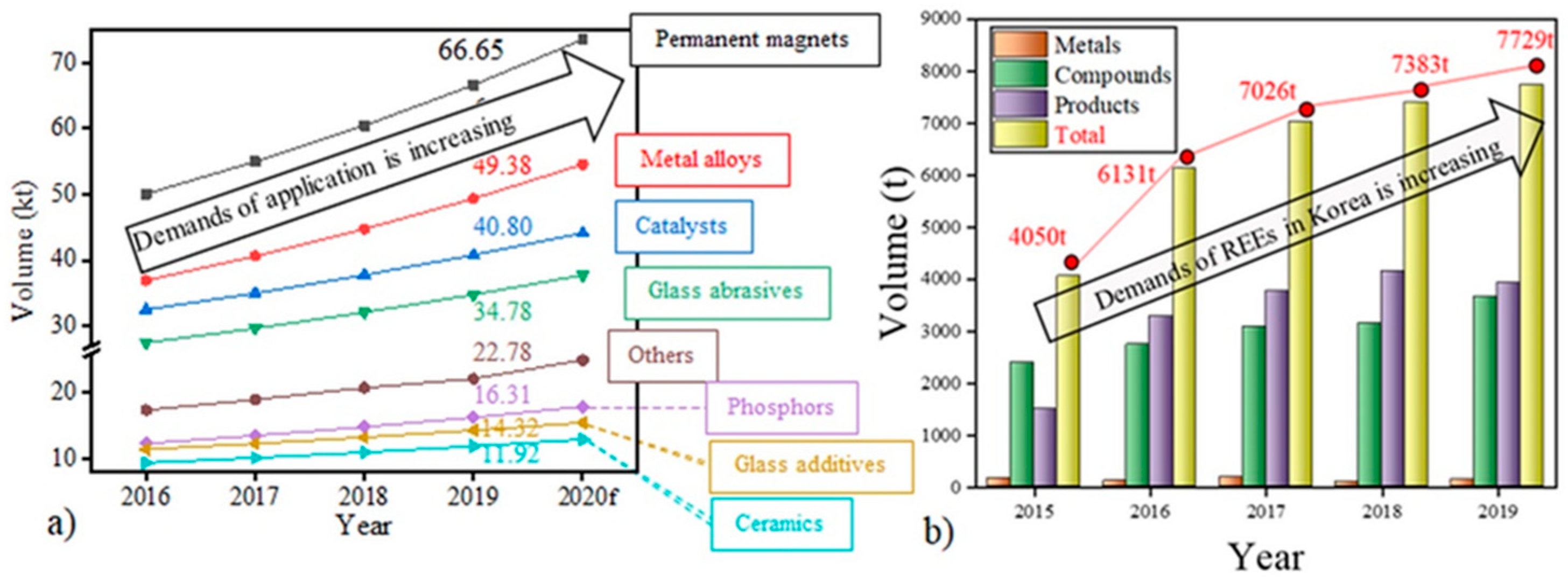
Figure 1a shows the global demand for REEs by application, showing that the compound annual growth rate (CAGR) of demand for REEs is increasing at 8.9%. Among them, the demand for REE magnets is the highest, reaching a volume of 66.65 kt in 2019. Similarly,
Figure 1b shows the demand for REEs in the Republic of Korea in the form of metal (RE metals), compounds (REE oxides and REE compounds), and products (RE magnets or products) from 2015 to 2019. The demand reached as high as 7.7 kt in 2019, up from 4.0 kt in 2015. Because most REE magnets are imported in finished or semi-manufactured form, it can be observed that the overall amount of REE products has increased due to the development of the magnet industry.
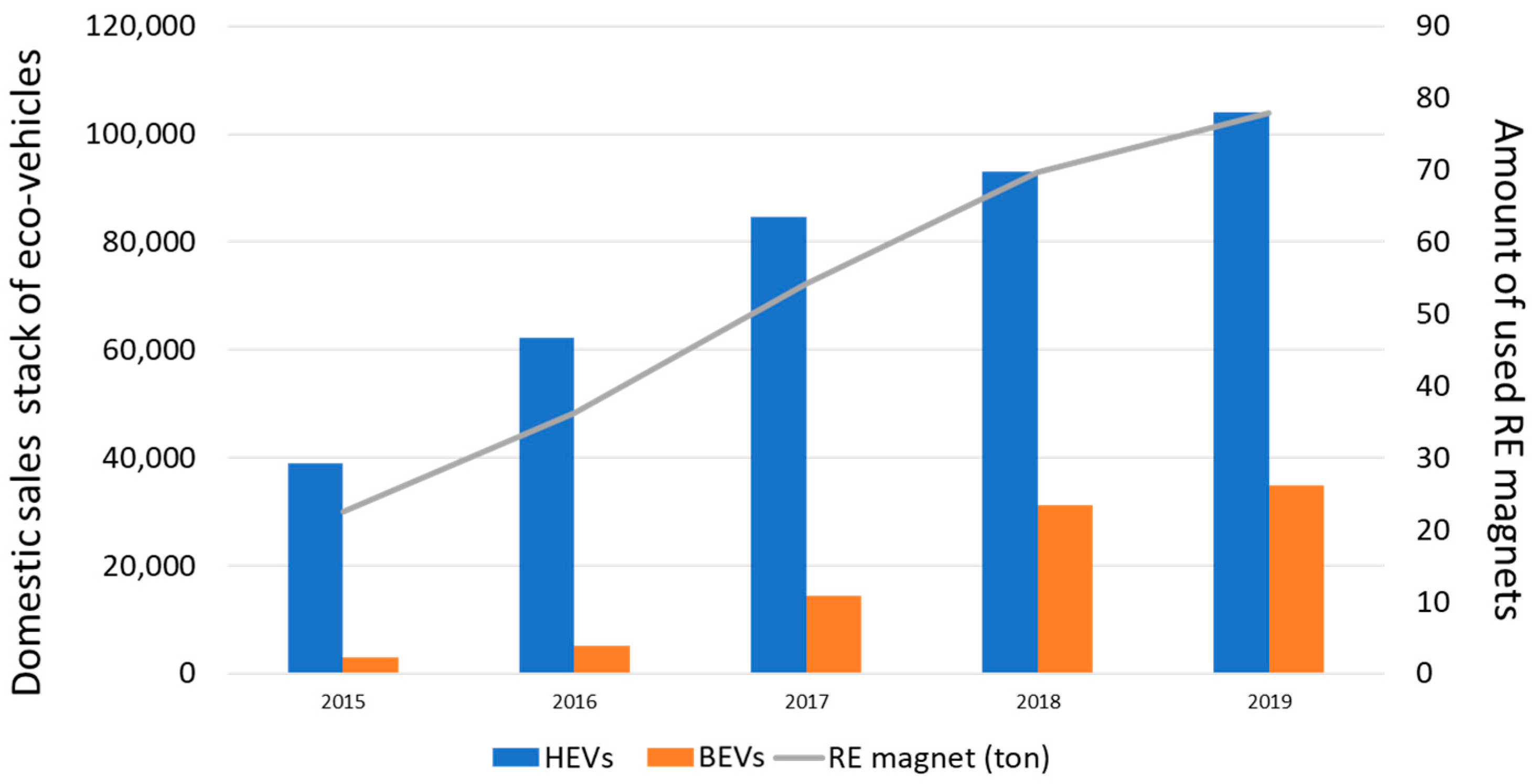
The increased demand for REE magnets from 2015 onwards can be largely linked to the growth of the automobile sector in Korea. Kia and Hyundai are two of the major world players in the automotive sector, who are set to make a mark in the transformation to eco-friendly vehicles. RE elements are applied to various parts in eco-friendly automobiles, including hybrid electrical vehicles (HEVs) and battery electrical vehicles (BEVs), which use about 1 and 2 kg of Nd per vehicle, respectively [
25].
Figure 2 show the domestic sales of eco-friendly vehicle from 2015 to 2019. Around 80 ton of rare earth elements were used in the manufacturing of the two types of vehicles in 2019. BEV sales are steeply increasing, while the HEV market is gradually becoming saturated because the transition from the traditional engines to electronic motors has been accelerated by environmental policy. Even if the sales of BEVs decrease, it can be expected that the number of REE magnets used will steadily increase due to sales of BEVs. The role of REE magnets in the development of the industrial economy of the Republic of Korea is indispensable.
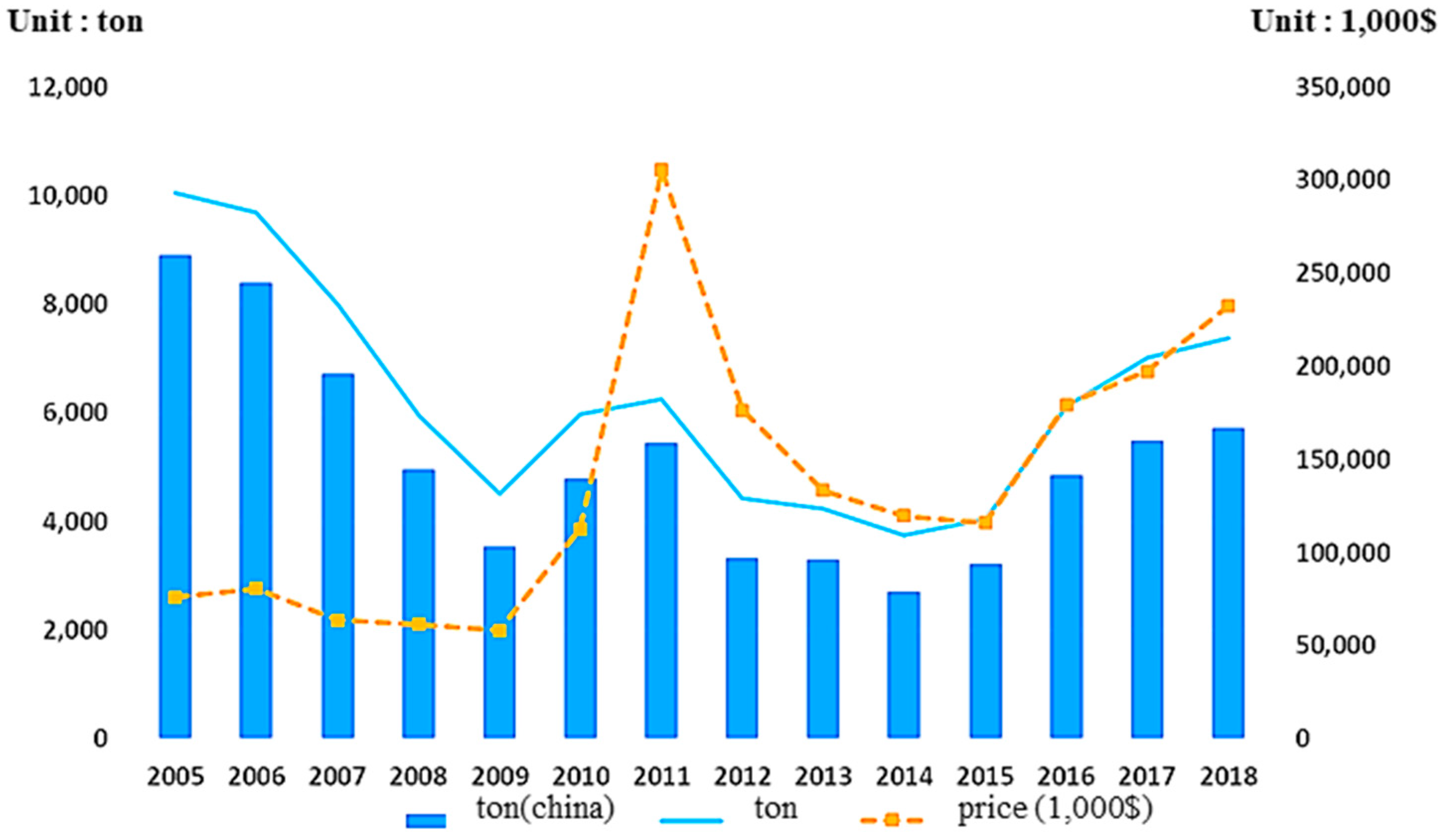
Figure 3 shows the total weight and price for REEs supplied to Korea from 2005 to 2018. In terms of price, it can be seen that the market never fully recovered from the price hike that resulted due to Chinese export restrictions in 2010 and 2011, which created an REE supply deficit. Although the global production rate has increased since 2013 due to China’s removal of export quotas, along with increased mining outside of China, the expected shortfall of certain REEs, including Nd and Dy, resulted in bullish market behavior [
26]. REE prices have seen an increasing trend since 2015, which is expected to continue. Moreover, the share of the Korean REE supply from China was as high as 89% in 2005, which decreased to 78% in 2018. This shows that the supply of REE resources in Korea is sensitive to the influence of REE restrictions in China; therefore, instability in the REE supply in Korea can be caused by China, as mentioned above. In addition, these figures pose an imminent threat to Korea, given the political stance Korea has in the trade negotiations between the US and China.
In order to deal with an unstable supply chain, a critical resource management analysis was conducted for the material flow of 63 materials in Korea, including REEs.
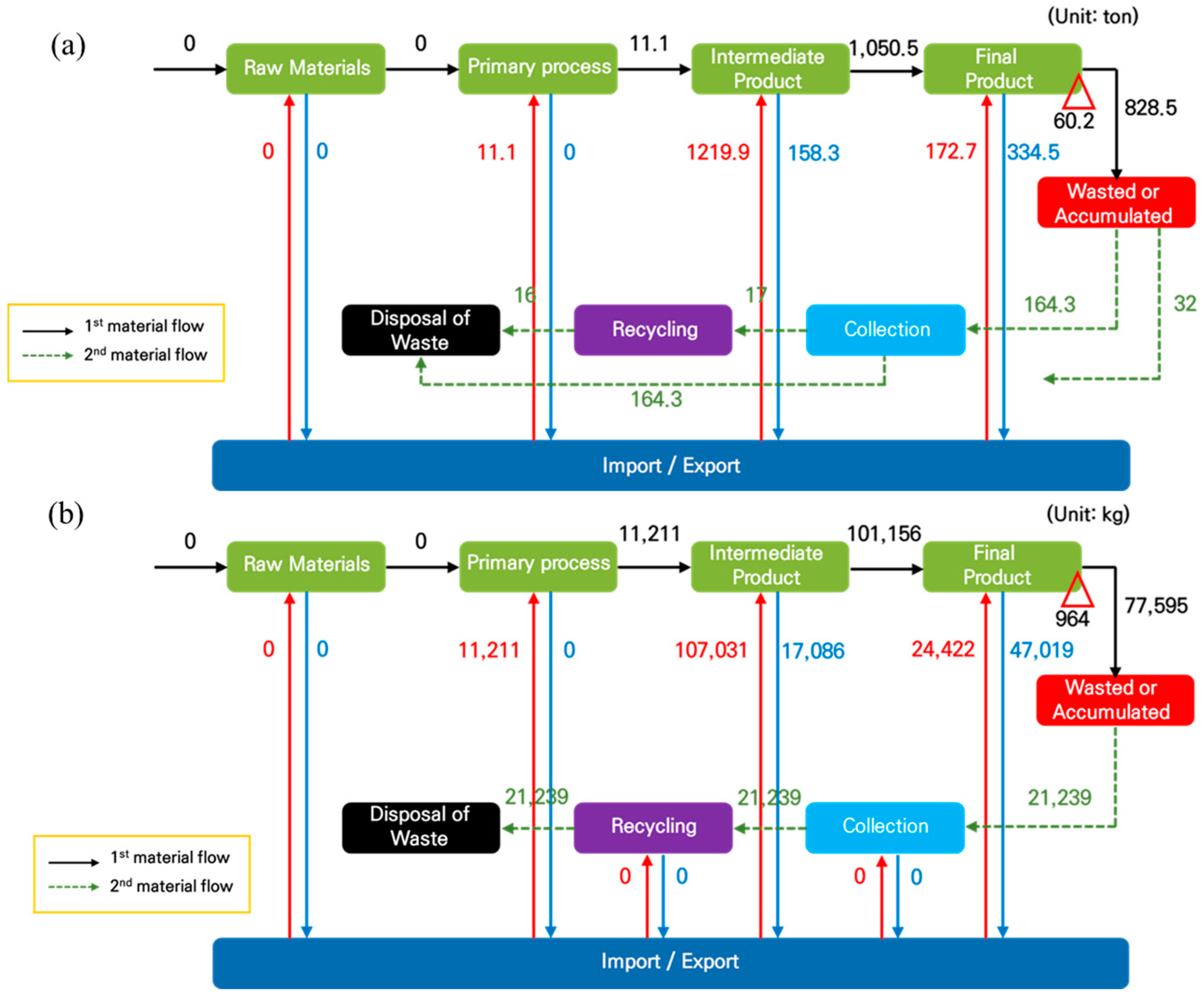
Figure 4a,b show the qualitative results of the materials flow analysis for Nd and Dy in 2016, respectively [
28]. In the first material flow, although there is a rare earth mine in Korea, it has no commercial value due to its low output; thus, the ores used for REEs has been investigated, as they are noy imported or exported in the raw materials step because of a lack of skills and facilities related to refining and processing. The REE magnets are totally imported with in final or semi-manufactured form, involving an intermediate process but no primary process, whereby oxides or fluorides are used directly as the catalyst, resistor, and condenser. After the materials are wasted or accumulated, the second materials flow involves collection and recycling; the total amounts of Nd and Dy are measured as the wasted amounts without recycling. Because the Republic of Korea is not only totally dependent on imports for NdFeB magnets but also because the economy heavily relies on the export of value-added products manufactured from imported REE magnets, a weak circular economy system can result from crises in the REE value chain in Korea; thus, Korea needs to find alternatives to strengthen the weak supply chains and prepare for resource crises.
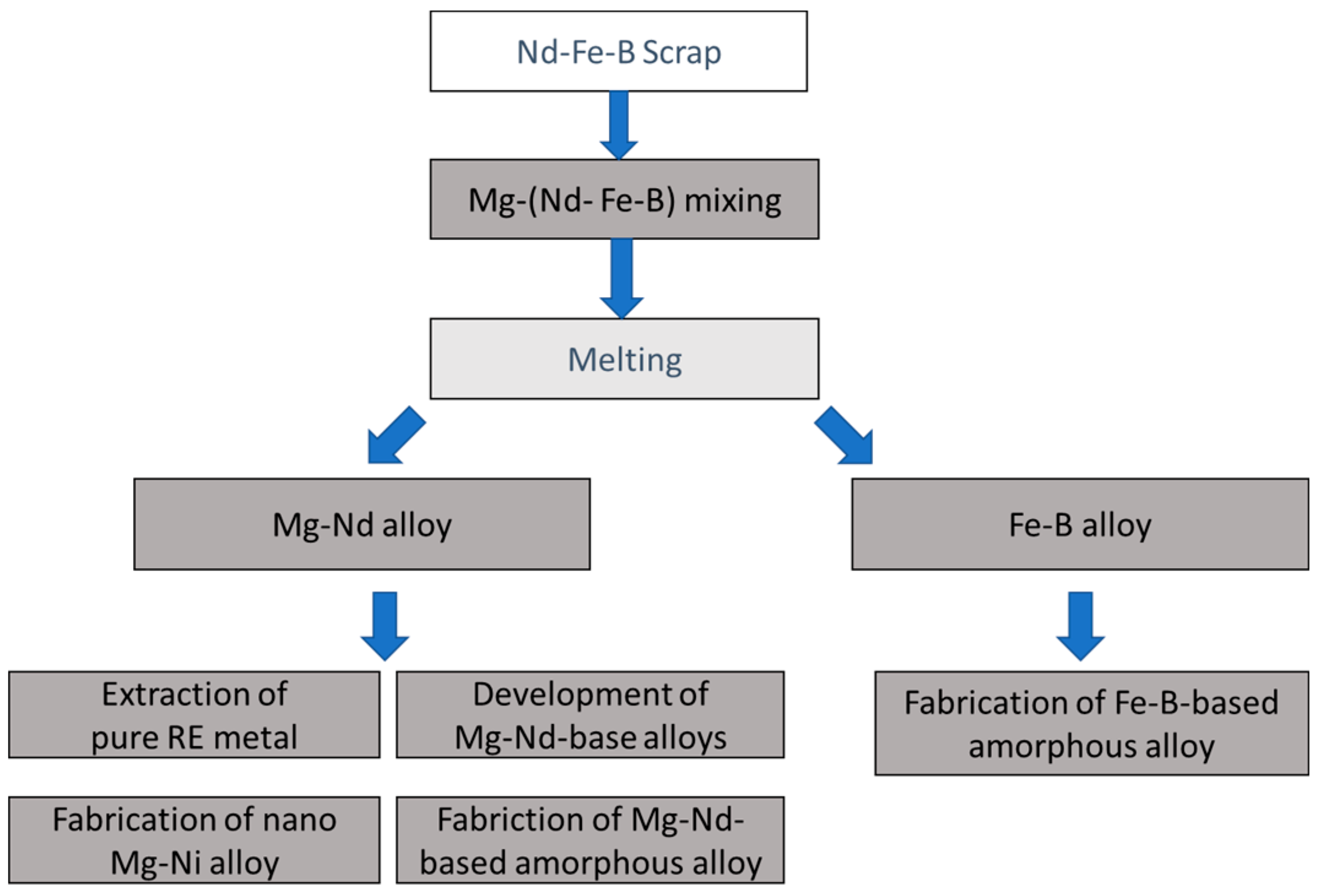
4. Liquid Metal Extraction (LME)
Mg was chosen as an extraction agent as it selectively reacts with REEs in REE magnets, leaving behind Fe-B. This was first demonstrated by Xu et al. [
35], whereby Mg infiltrated the magnet to pave way for the REEs to diffuse into Mg to form intermetallic compounds. Mg was used as an extraction agent in the ReMat process because: (1) it has a strong chemical affinity with Nd; (2) it hardly reacts with iron; (3) it shows high vapor pressures over 800 °C (0.045 atm@ 800 °C), meaning the elimination and transportation of Mg through the gas phase is easy; (4) its melting point is 922 K (649 °C) and it can be recovered and re-used via condensation into a pure solid [
36]. Additionally, Mg is a superior metallic material for structural components in the growing aerospace and automobile industries due to its advanced physiomechanical properties [
37]. Combined with the reported abundant resources of Mg in the earth’s crust, this implies that Mg will be readily available for REE recycling in the near future.
Xu et al. proved the viability of using Mg as an extraction agent. The diffusion behavior and diffusion coefficient were investigated in detail by Chae et al. [
38]. It was assumed that Dy exhibits a similar behavior as Nd, although the behavior of Dy had not substantiated by research studies; therefore, Kim et al. carried out research on the extraction behavior of Dy, whereby it was established that the extraction of Dy is difficult as compared to Nd because of the formation of the Dy-oxide phase. Oxygen has a greater affinity with oxygen, leading to the reaction of Dy with O, which is difficult to reduce.
An experimental demonstration of the high affinity of Mg for Nd resulting in the Mg-Nd intermetallic compounds and studies on detailed diffusion behavior had previously been carried out, although the possibility of the further separation of Nd from Mg-Nd alloy remained unexplored. Okabe et al. [
39] presented a two-step process for the direct extraction and recovery of Nd from magnet scraps. Mg-Nd alloys with varied compositions in the range of 22–63 mass% were achieved from LME reaction. These high concentrations of Nd in Mg using LME were unprecedented, which were achieved by introducing the concept of the recirculation of Mg. In the proposed apparatus, Mg reacted with magnet scrap material inside the iron crucible, taking the Nd away from it. The Nd-Mg liquid fell into the tantalum crucible, from where only Mg evaporated to condense on the top of the apparatus. The condensed Mg dropped into the iron crucible to react with the magnet scrap material. The recirculation of Mg is a novel concept, which was executed by maintaining a temperature difference between the top and bottom of the equipment. Through this recirculation process, the amount of Mg required was substantially decreased because of the recirculation principle, which was an innovative development from an environmental perspective. Takeda et al. [
40] further optimized this process in view of the major process parameters, such as the magnet-to-scrap ratio and temperature.
Although these attempts were successful, they were only performed at the laboratory scale, meaning the feasibility at the commercial level needed to be checked. The Hitachi Corporation utilized this technology for magnet recycling [
40]. They utilized a disassembly machine, which is crucial to obtain magnets from the recycling process. The machine could dissemble and remove coatings from magnets found in compressors in air conditioners and from HDDs. It takes one hour for the disassembly of 12 HDDs manually, whereas this machine can take apart 100 HDDs in the same time. Overall, a capacity to process 40 kg of magnets per day was reported. The extraction efficiencies in the semi-lab-scale process for Nd and Dy were reported to be 100% and 60%, respectively.
RE magnets available for recycling are present in different shapes and sizes in EoL products; however, no study had been performed to assess the effect of scrap size on the extraction efficiency. Na et al. [
41] compared the extraction efficiencies of three different scrap sizes with varying shapes i.e., 0.5, 1–5, and 5 mm. Oxidation and extraction were reported to have a direct relation. The smaller scrap size particles were observed to have high amounts of Nd
2O
3 due to the high surface-to-volume ratio compared to larger particle sizes. The maximum LME reaction time was 50 min, which is insufficient to reduce Nd
2O
3. The incomplete decomposition of Nd
2O
3 under the conditions employed in this experiment was explained thermodynamically using a binary phase diagram of Mg-Nd
2O
3 obtained from Factsage software.
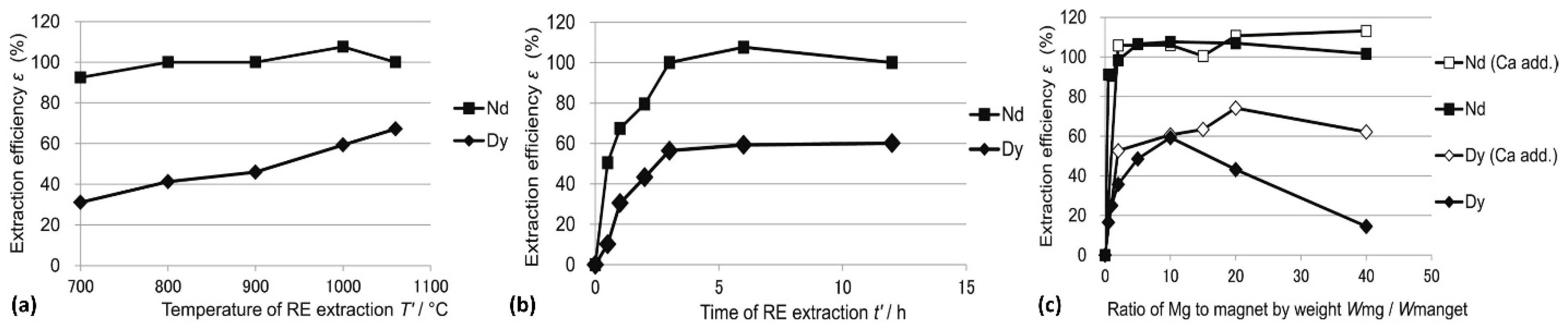
Dy had not been the focus of most of the prior research studies, even though Dy is a critical component in rare earth element magnets, especially in high-temperature applications. Akahori et al. [
42] investigated the optimum conditions of extraction by LME reaction for both Nd and Dy in terms of the temperature, time, and Mg-to-magnet ratio, which are shown in
Figure 6a–c, respectively. A maximum extraction efficiency of 67% was reported for Dy after an extraction time of 6 h at 1000 °C, with a Mg/magnet mass ratio of 10:1. Dy
2O
3 was reported as the major hurdle for incomplete decomposition. The extraction of Nd is favorable compared to Dy, as 100% extraction of Nd was observed after 3 h, with a Mg/magnet mass ratio of 2:1, even at 800 °C. The reason why the extraction rate of Nd is relatively much faster than Dy is the reduction behaviors of Nd
2O
3 and Dy
2O
3, which affect the extraction efficiency. Nd
2O
3 and Dy
2O
3 are obstacles to extraction in their metallic form; hence, Gibbs energy changes were calculated to observe the possibility of each reduction behavior. It was shown thermodynamically that Mg can reduce Nd, irrespective of whether in the form of Nd
2O
3 or Nd, under the aforementioned conditions. Additionally, Dy
2O
3 can be thermodynamically reduced by Mg under the aforementioned conditions, although the activity of Mg is more required to reduce Dy
2O
3 than Nd
2O
3; therefore, the relatively incomplete reduction of Dy
2O
3 causes the slower extraction rate of Dy than Nd.
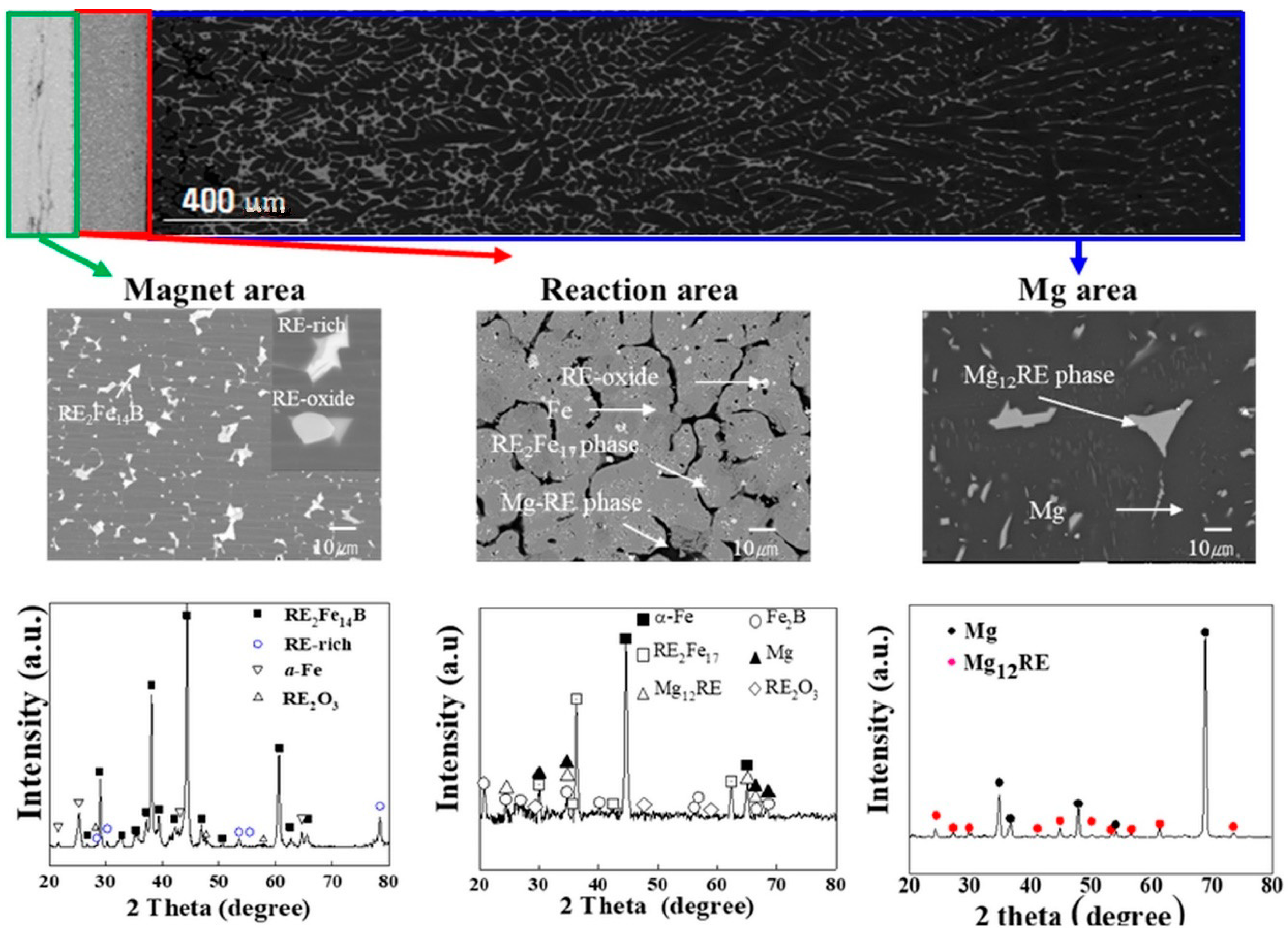
In order to describe the diffusion behavior of rare earth elements to Mg,
Figure 7 shows an SEM image and corresponding XRD patterns of the experimental results for a reaction time of 3 h at a temperature of 900 °C, with a magnet-to-Mg ratio of 1:10. The upper part of
Figure 7 shows the overall microstructure of the specimen, which can be divided into three distinct zones. These three distinct zones can be seen in the figure, i.e., the magnet area (unreacted raw material—magnet only), reaction area (reaction between magnet and Mg), and Mg area, where rare earth elements are diffused from the magnet. The NdFeB magnet was composed of RE
2Fe
14B (gray matrix), RE-oxide (white circular phases in grain boundary), and RE-rich (white rectangular phases in grain boundary) phases, as shown in the magnet area. Mg penetrated the magnet along the grain boundaries to decompose the RE-rich phase and RE2Fe14B matrix phase to Fe (gray matrix) and RE
2Fe
17 (white circular phases on the Fe matrix) in the reaction zone; however, RE-oxide in the magnet cannot be reduced easily due to its affinity with oxygen, meaning it remained in grain boundary, as shown in the reaction zone. Finally, rare earth elements diffused into the Mg area through the path provided by the Mg (black phases in the reaction zone) inside the magnet. It can be seen from the XRD patterns that Mg successfully decomposed the inherent phases of the NdFeB magnet (RE
2Fe
14B, RE-rich, and RE-oxide), which can be seen in the XRD patterns of the magnet area, while rare earth elements diffused into Mg to form intermetallic compounds, i.e., Mg
12RE (gray phases in the Mg area). The solid–liquid reaction mechanism can be explained by the diffusion interaction between Mg and the magnet, in which Mg exists in a liquid state at 900 °C, while the magnet remains as a solid. Mg liquid metal atoms diffuse into the magnet to form a diffusion layer on the surface, which in turn alters the chemical composition of the surface layer. This change of composition of the surface layer decreases the surface energy and melting point of the solid, allowing it to transform into liquid state by melting. At a low solubility limit in the solid phase, the diffusion from the melt into the solid proceeds mainly along structure defects (grain and sub-grain boundaries, etc.). The change of the chemical composition, as a result of such selective diffusion, causes the formation of liquid phases along grain boundaries [
43,
44].
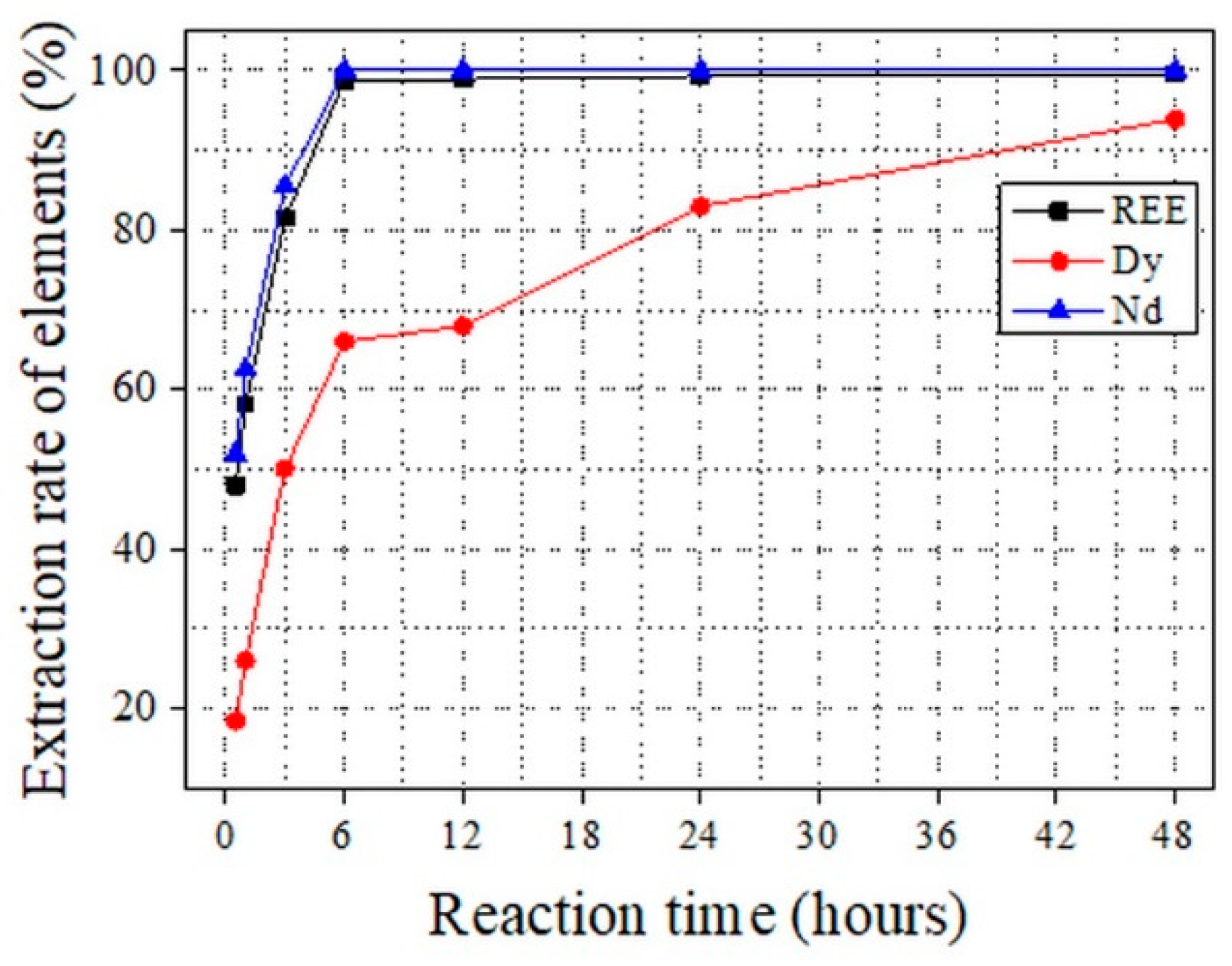
The LME reaction parameters were optimized as part of the ReMaT process.
Figure 8 shows the extraction efficiency results from the LME experiments for Nd and Dy at 900 °C for a magnet/Mg ratio of 1:10. An extraction efficiency of 100% was observed for Nd at an extraction time of 6 h, while an extraction efficiency of 94% was achieved for Dy at an extraction time of 48 h. Previously, the highest reported extraction efficiency for Dy in LME experiments was 74%, which was enhanced to 94% in our process. The explanation for this improvement is provided elsewhere [
45]. An overall extraction efficiency for REE of 97% was achieved as part of the ReMaT process.
5. Vacuum Distillation
The relationship between the magnesium vapor pressure and temperature can be calculated from Equations (1) and (2), where P is the magnesium vapor pressure in Pa and T is the temperature in K [
46]:
Only one research study has so far reported the use of vacuum distillation for the separation of REEs from Mg alloy in recycling processes [
36]. Okabe et al. performed VD on several 8 g samples of Mg-Nd alloy. The vacuum distillation experiments were performed in two steps to enhance the Mg removal efficiency; Mg-Nd alloy was heated to a temperature of 1123 K for two to six hours, then subsequently it was heated at 1313 K in the second step. Nd of 97.7 mass% was achieved as a result of this two-step vacuum distillation process.
As mentioned earlier, the Mg-Nd master alloy obtained after the LME experiment was cut into several pieces of 10 g each for the next step in the VD process. The distillation temperature must be above the melting point and below the boiling point of the metal with the higher vapor pressure (in this case Mg) so that it can melt and subsequently evaporate. The vapor pressure of Nd is extremely low compared to Mg, even at 1000 °C, i.e., approximately 0.1 Pa compared to 73,967.3 Pa for 0.73 Mg at the same temperature. This implies that liquid Mg has weak intermolecular forces and evaporates easily as compared to Nd [
47]. It was not necessary for the temperature to be much greater than the melting point of magnesium (650 °C); therefore, a temperature of 800 °C was set as the temperature for the first batch of VD experiments and distillation experiments were carried out for periods of 10 min to 6 h.
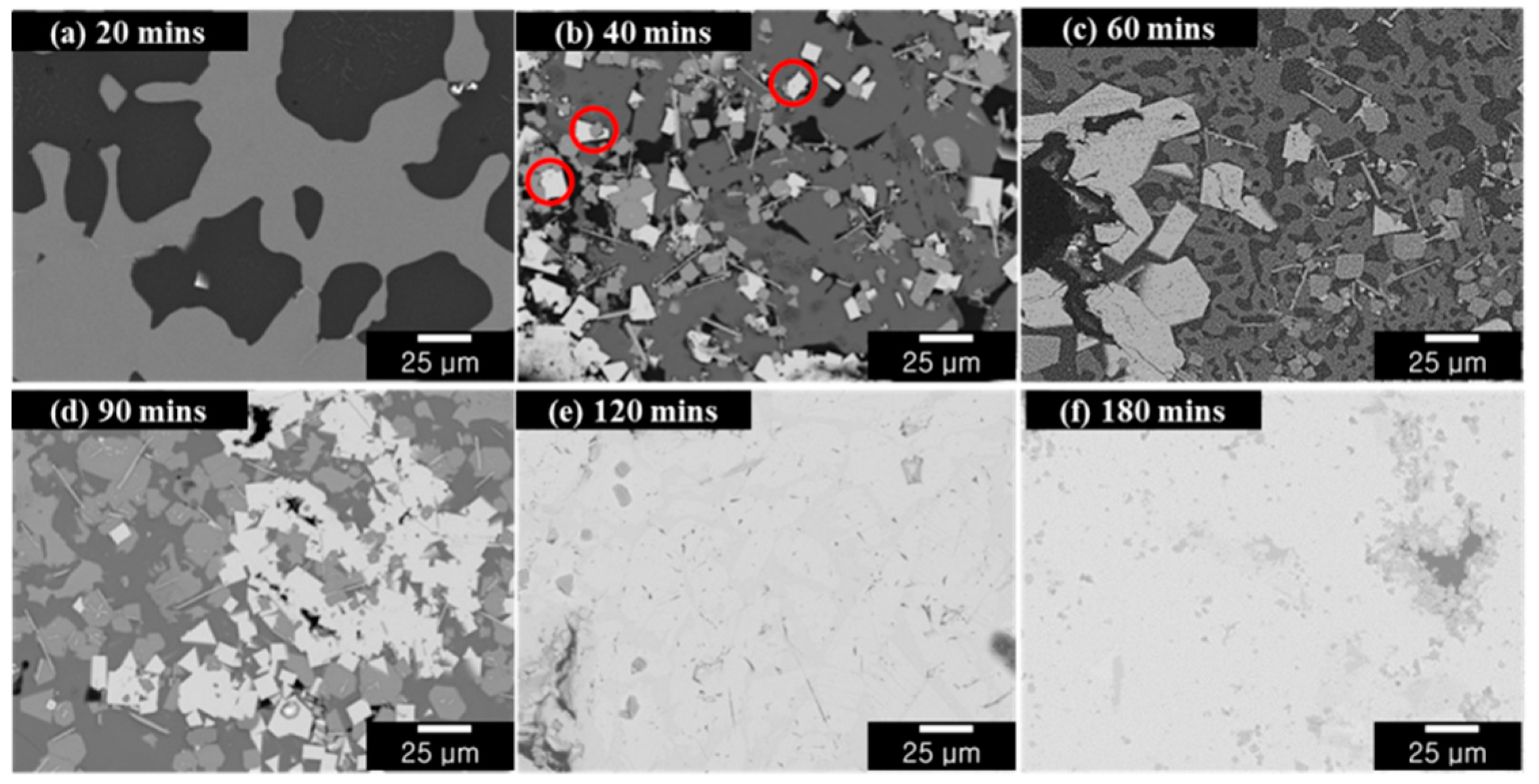
Although Mg evaporation did take place even after 10 min of distillation, there was no change in the morphology as compared to that of the LME sample, and Mg remained the dominant phase. Mg evaporation increased from 5 min onwards, although no change in the microstructure was observed until 20 min. The microstructure of the resultant samples after distillation times of 20, 40, 60, 90, 120, and 180 min are shown in
Figure 9a–f, respectively.
During the vaporization of Mg, Mg-Nd phases can be evolved into other phases. Because Mg
12Nd is the most stable phase in this system, Mg–Nd phases exist only as Mg
12Nd, as shown in
Figure 9. For the 20 min specimen, only dark Mg and light gray Mg
12Nd coexisted, as shown
Figure 9. As mentioned above, Mg is easily vaporized under pressure, hence Mg-related phases are converted to other phases very quickly. In addition, Mg
12Nd is the most stable phase in this system, meaning Mg–Nd phases exist only as Mg
12Nd; therefore, after only 40 min of distillation time, a white region containing Nd metal started to appear in the resultant Mg-Nd alloy, which has been highlighted. The dark region that was previously identified as α-Mg phase and the light grey region that was previously identified as Mg
12Nd decreased with increasing time. This was because the phase transformation of Mg to Mg
12Nd and then to Nd occurred. Finally, the white region increased with increasing distillation time until it became the dominant phase at distillation times of 120 min and above.
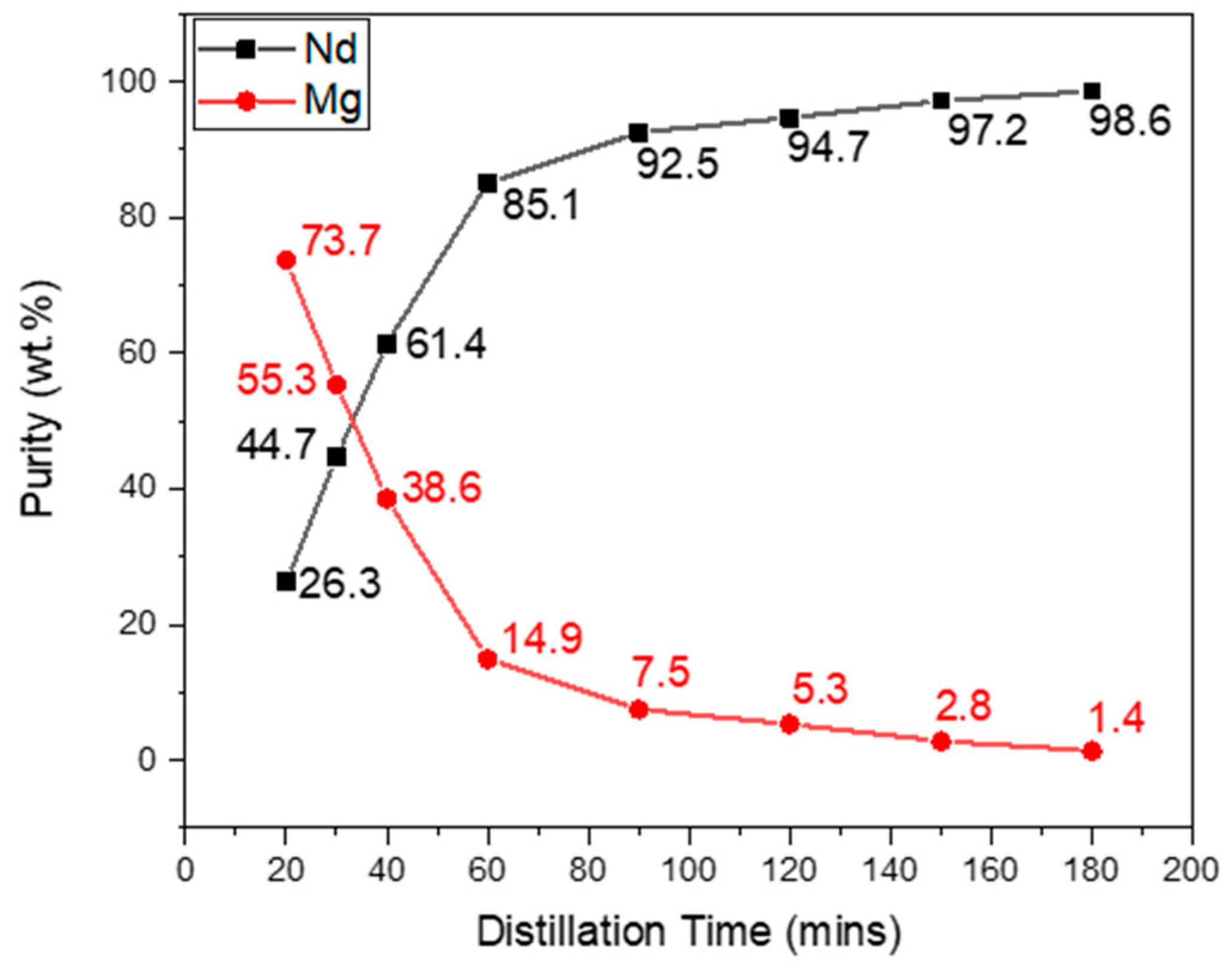
In order to confirm these findings, XRF measurements of the samples at the six mentioned distillation times were carried out to check the Nd purity levels in wt.% with increasing distillation time. The results are shown in
Figure 10.
As expected, the Nd concentration increased with increasing distillation time and the Mg concentration decreased. Between evaporation times of 20 and 40 min, Nd was found to be in higher concentrations than Mg. Nd of about 99% purity was obtained after distillation times of 120 min and above. The same extraction rate was achieved at 900 °C at 30 min, whereas it took 240 min at 700 °C to reach a similar level, i.e., 98% extraction efficiency.
6. Materialization
Due to the wide range of applications of Mg in the automobile and aerospace industries, it is crucial to enhance the properties of Mg alloys, especially for high-temperature applications. Several studies have investigated the replacement of conventional Mg-based alloys such as Mg-Al with Mg-RE, as the latter has significantly improved properties. The addition of REEs to Mg results in improvements in the mechanical strength, biocompatibility, corrosion resistance, bioactivity, and other properties [
48]. Similarly, the addition of Nd to Mg improves the hardening and strength of Mg after thermal treatment [
49].
For example, in biodegradable biomedical magnesium alloy (Mg-Nd-Zn-Zr), Nd is used as the main alloying element. Nd is preferred because of its high strength and because when it is added it forms an intermetallic phase (Mg
12Nd) with Mg, which acts as an obstacle to dislocation movement at elevated temperatures and causes precipitation strengthening. It has also been reported that Nd does not exhibit any toxicity. As Mg-Nd already has a significant strengthening effect, the further addition of a small amount of Zn can increase the ductility and deformability of the alloy [
50].
Addtionally, Mg-Nd from LME can be directly used as a raw material in making QE22 (Mg-2Ag-2Nd-0.5Zr; Ag = silver and Zr = zirconium) alloy. The same is true for WE54 (Mg-5Y-3RE-(1.5-2Nd)-0.5Zr; Y = yttrium) and NZ30K (Mg-3.0Nd-0.2Zn-0.5Zr; Zn = zinc) Mg alloys. These two alloys show improved tensile properties and creep resistance at both normal and high temperatures [
51].
Similarly, in the making of the Mg-2.0Zn-1.0Mn (ZM21) alloy, Nd was added in the form of the master alloy Mg-Nd (20 wt. %), which can be achieved from the LME process. Certain amounts of Ce and Nd were added to enhance the mechanical and other properties of the alloy. In this ZM21 alloy, the average grain sizes with the addition of 0.4 wt.% Nd and Ce reached 6 ± 3 mm and 13 ± 2 mm, respectively. The resulting changes of mechanical properties were mainly attributed to a reduction in the basal texture intensity and refinement of the grain size, dispersion density, and distribution position of fine second-phase particles [
51]. Moreover, Mg-Nd from our recycling process has been used as a master alloy to make other alloys, e.g., the addition of 2.5% yttrium to Mg-3%Nd showed significant improvements in the tensile strength, fatigue strength, and creep resistance. This alloy is utilized in automobiles and helicopters [
52,
53,
54]. Furthermore, the addition of 0.2 wt.% Zn and 0.4 wt.% Zr to Mg-3wt.%Nd alloy (Mg-Nd-Zn-Zr alloy) resulted in an alloy with increased hardening, as Zr worked as a grain refiner [
54].
It can be seen that Mg-Nd alloy is employed for various applications, while research into additional applications is also underway. The Mg-Nd alloy achieved from the LME process can either be used directly as an input for the materialization of the abovementioned alloys, or Nd can be separated by VD and applied for magnet manufacturing. This process can be extended to Mg-Dy alloy as well, which is one of the future prospects for the ReMaT process.
7. International Standards
The International Organization for Standards (ISO) established a technical committee (ISO/TC 298) in 2015 for rare earth elements, with the initial scope defined as “standardization in the field of rare earth mining, concentration, extraction, separation, and conversion to useful rare earth compounds/materials (including oxides, salts, metals, master alloys, etc.), which are key inputs to manufacturing, and further production processes in a safe and environmentally sustainable manner” [
55]. This technical committee consists of 34 countries including Korea, has five working groups (WGs) to date, and aims to establish universal standards for various industrial activities and standards for environmentally friendly technology. Korea is highly active in working group 2 (WG2; recycling), while the expert committee led by the Korean Agency for Technology and Standards (KATS) and the Korea Institute for Rare Metals (KIRAM) was established to keep up with global trends. Korean delegates proposed three documents starting from 2017 to aid recycling in view of Korea’s ReMaT process.
The documents are as follows:
ISO 22450:2020: Recycling of Rare Earth Elements—Requirements for Providing Information on Industrial Waste and End-of-Life Products;
ISO/FDIS 22453: Exchange of Information on Rare Earth Elements in Industrial Wastes and End-of-Life Products;
ISO/PRF TS 22,451: Recycling of Rare Earth Elements—Methods for the Measurement of Rare Earth Elements in Industrial Waste and End-of-Life Products.
These three documents comprehensively cover the downstream value chain of rare earth magnets. ISO 22,540 deals with the information that should be provided to the recycler by the waste handler, while 22,451 provides guidelines for conducting the quantitative analysis necessary for collecting this required information and 22,453 specifies the method of information exchange by which the desired information can reach the recycler in a systematic manner.
ISO 22450:2020 has been published, while ISO TS 22,453 and ISO FDIS 22,453 are one stage away from being published. These standards will streamline the recycling process and promote recycling by aiding the recycling industry, so that recycling can be carried out in a standardized and sustainable manner. This will allow for industrial waste and end-of-life products to efficiently reach recycling facilities from where Korean recyclers can execute the ReMaT strategy.