Design of Inverted Nano-Cone Arrayed SERS Substrate for Rapid Detection of Pathogens
Abstract
:Featured Application
Abstract
1. Introduction
2. Photon Funneling Inverted Nanocone Design and Numerical Analysis
3. Results
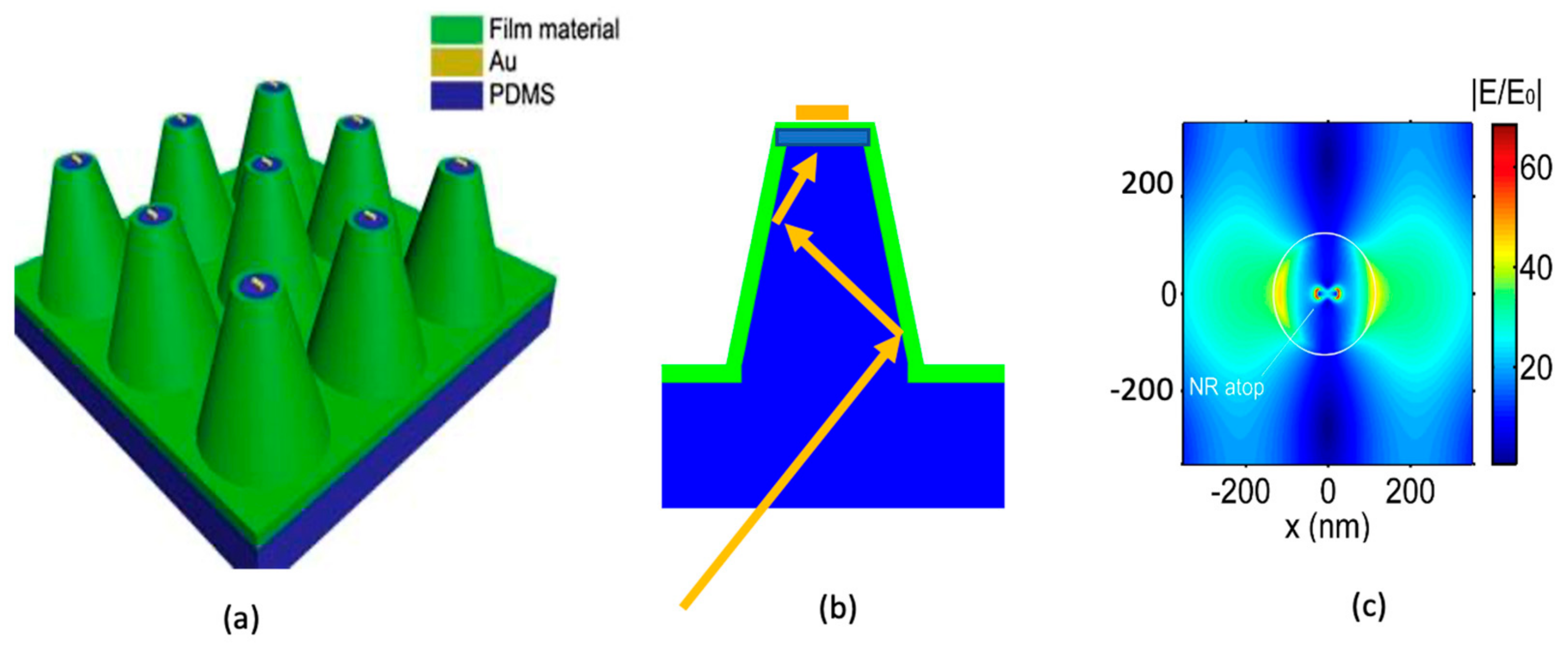
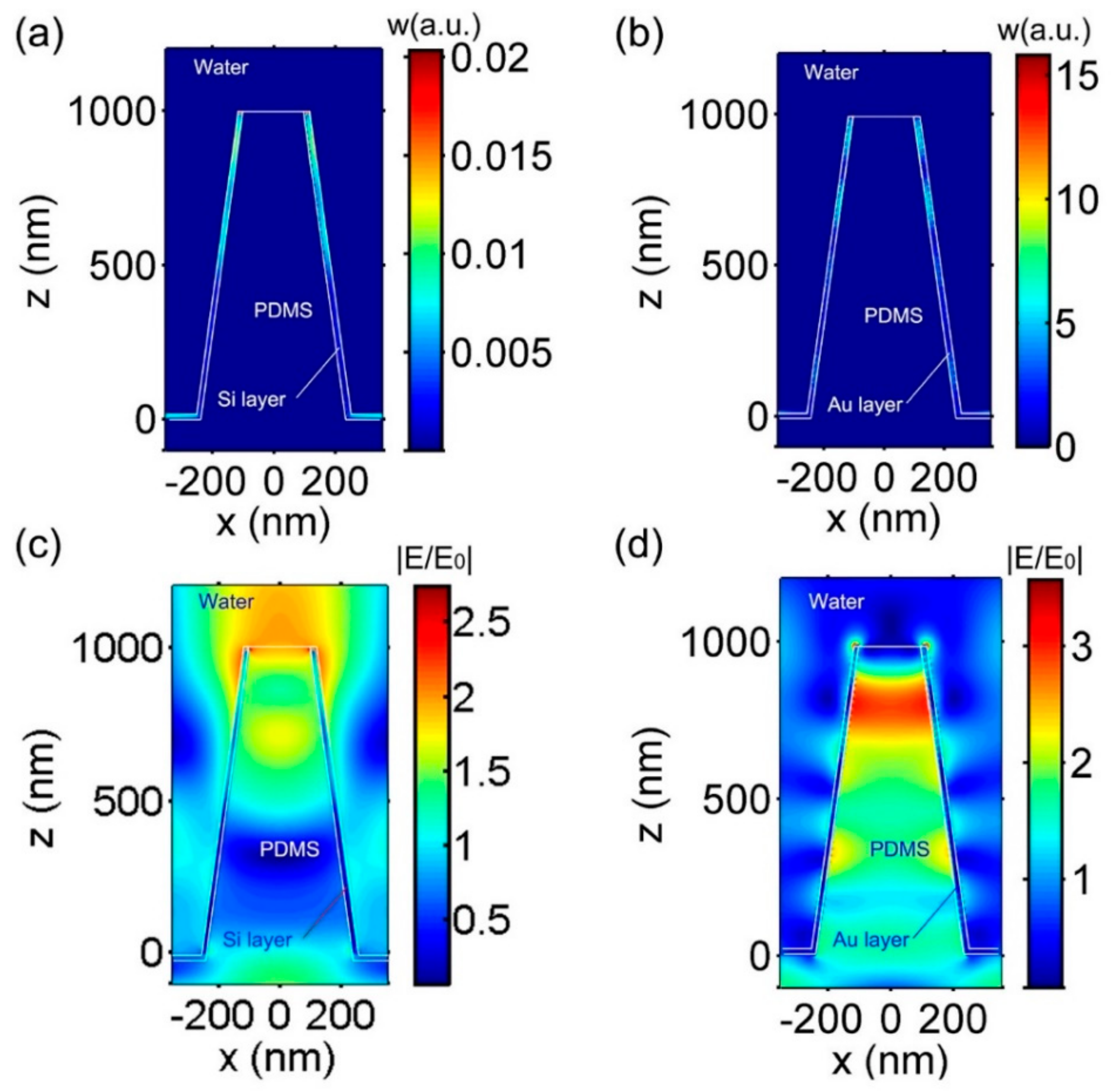
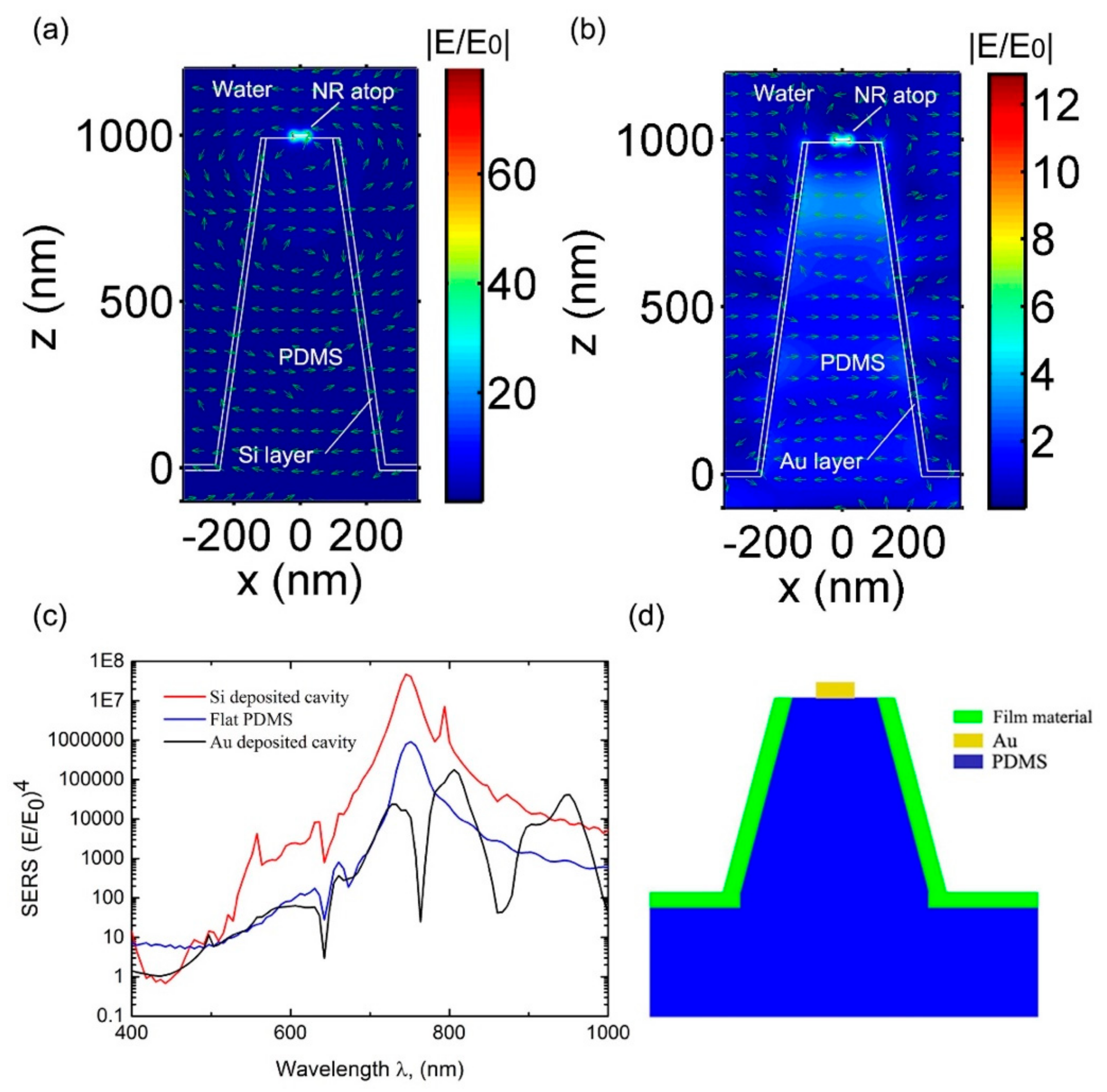
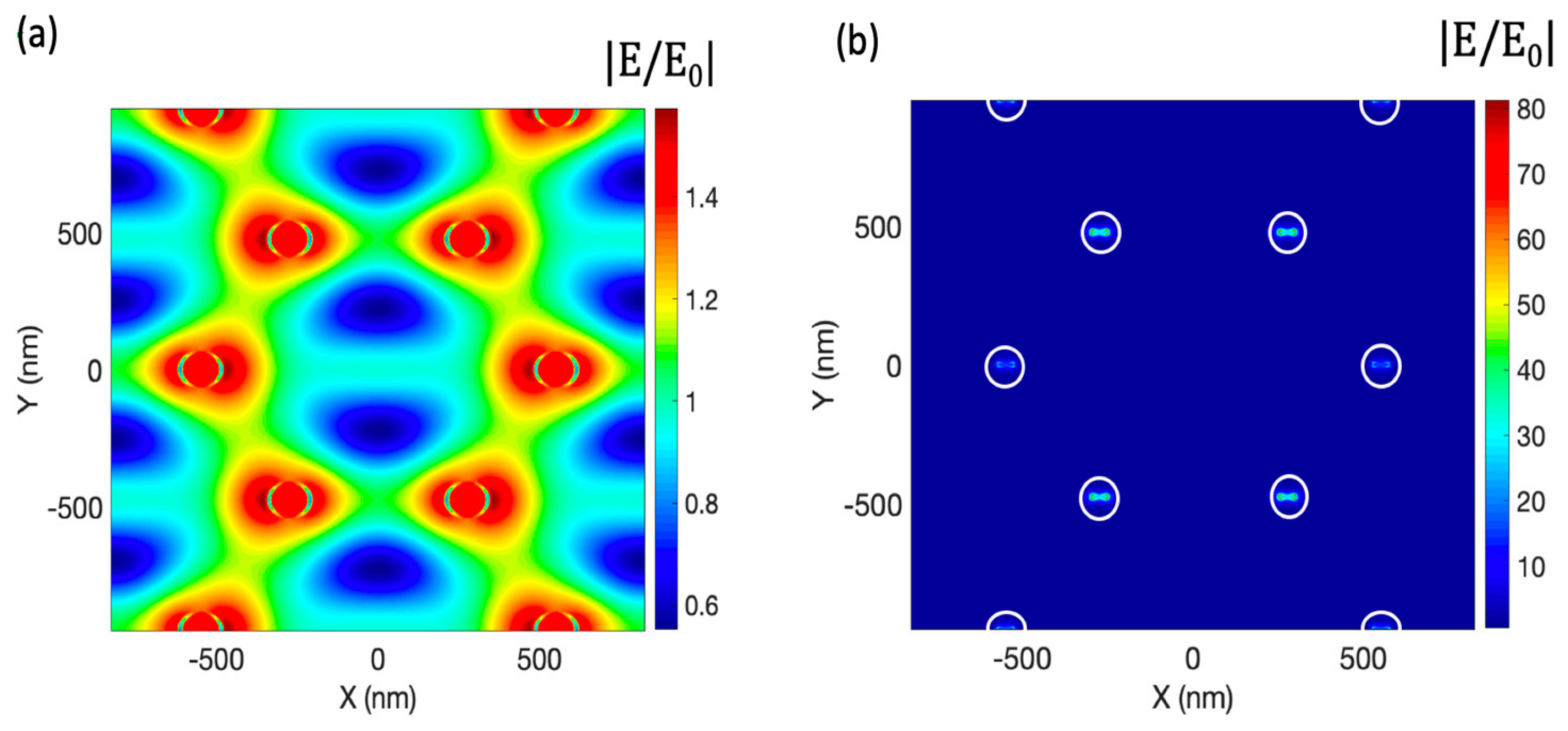
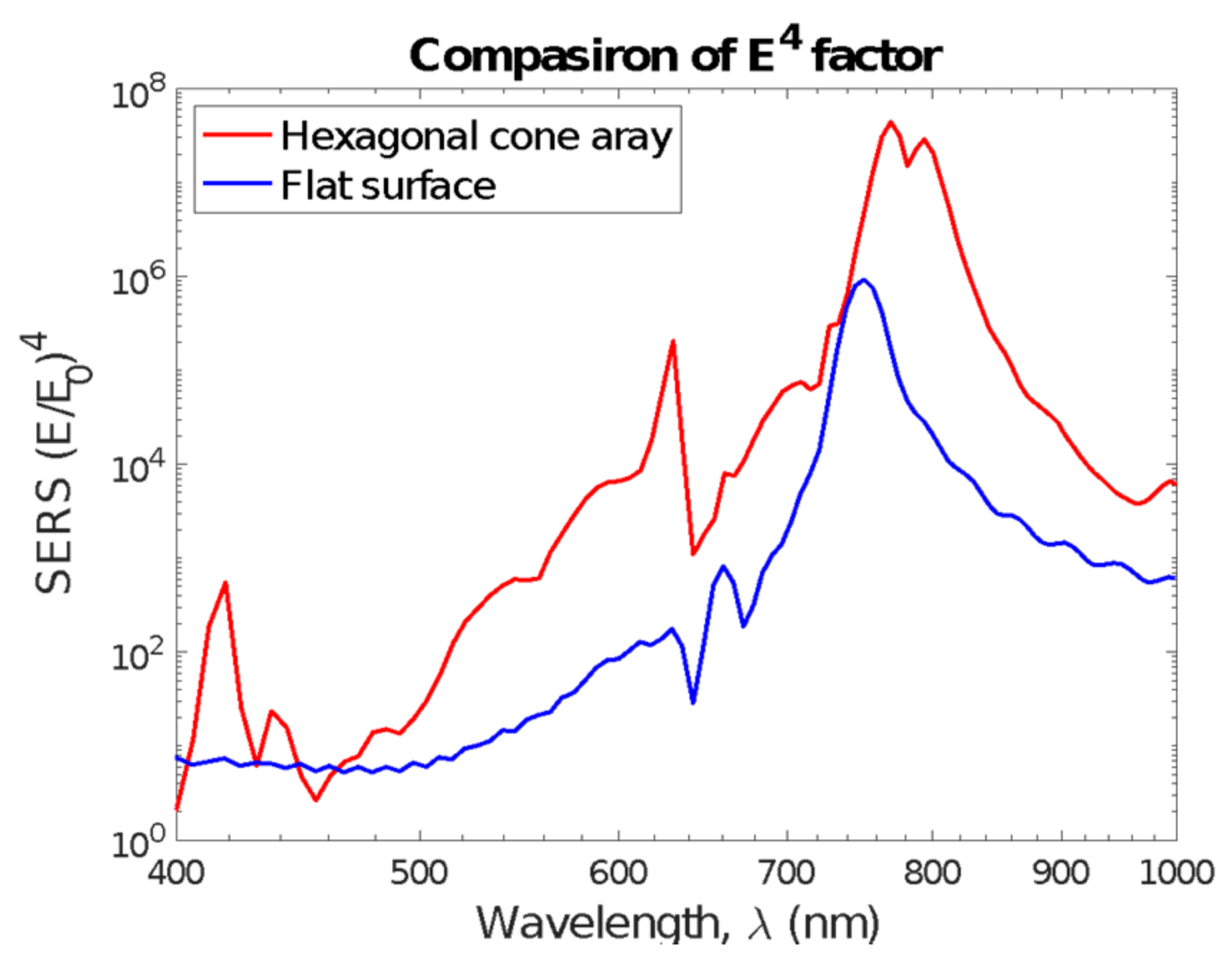
3.1. Nanocone Design and Material Selection for High Field Enhancemnt
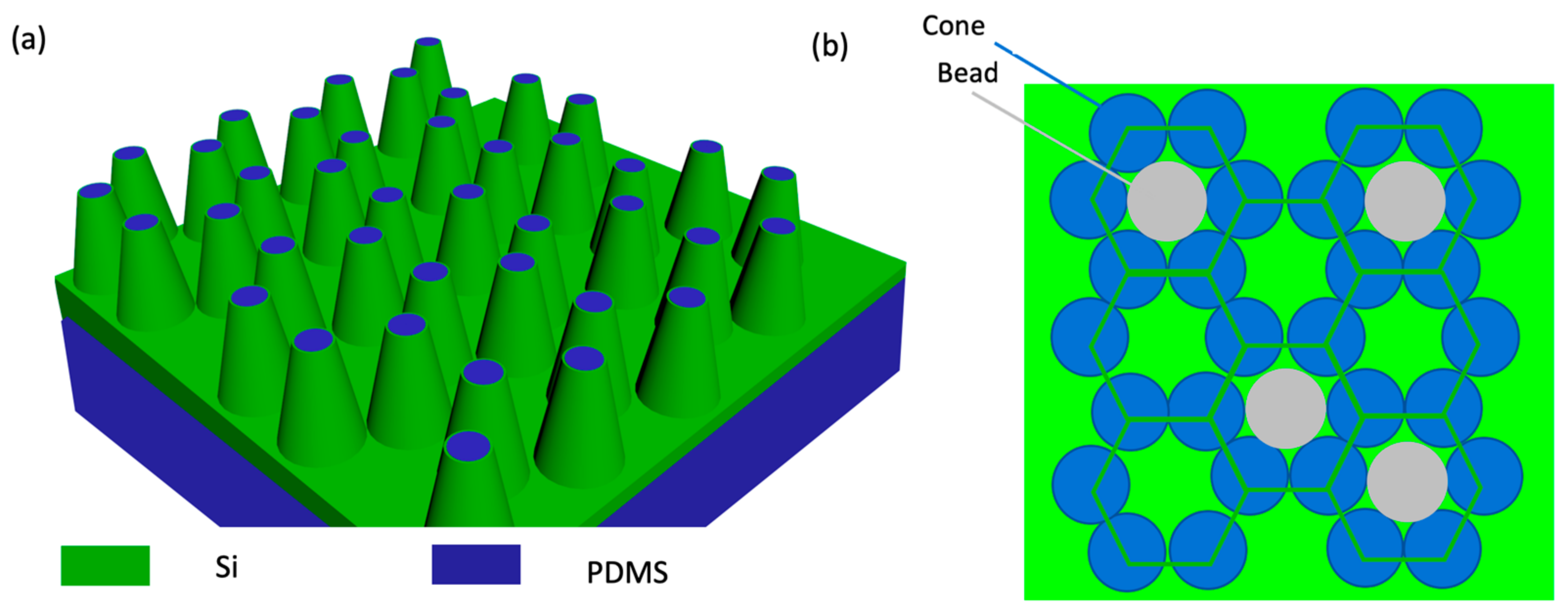
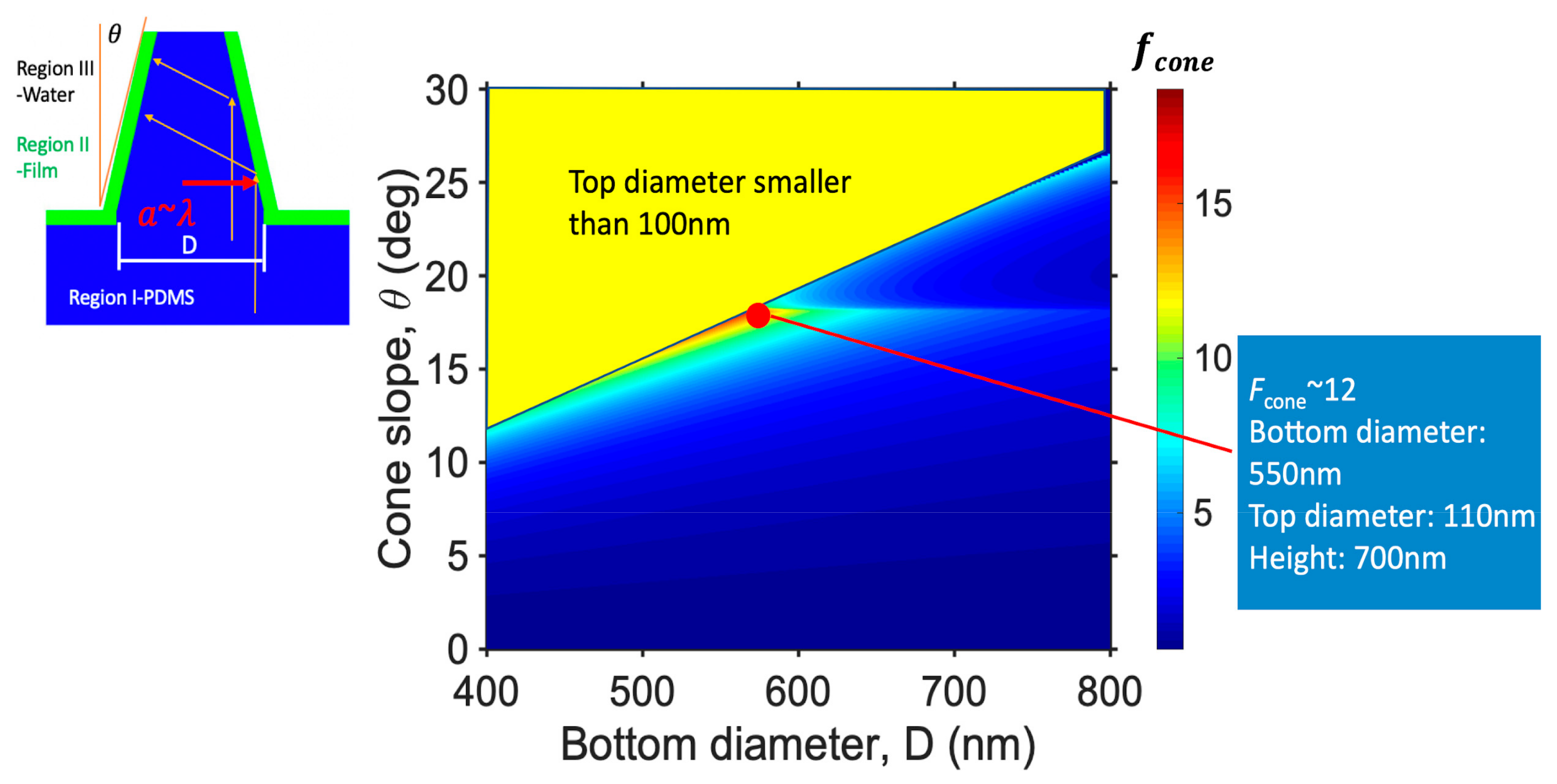
3.2. Nanocone Array Geometry Optimization for Tight Target Cell-Hot Spot Contact
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Jawad, I.; Lukšić, I.; Rafnsson, S.B. Assessing available information on the burden of sepsis: Global estimates of incidence, prevalence and mortality. J. Glob. Health 2012, 2, 10404. [Google Scholar] [CrossRef]
- Mancini, N.; Carletti, S.; Ghidoli, N.; Cichero, P.; Burioni, R.; Clementi, M. The Era of Molecular and Other Non-Culture-Based Methods in Diagnosis of Sepsis. Clin. Microbiol. Rev. 2010, 23, 235–251. [Google Scholar] [CrossRef] [Green Version]
- Kirpalani, H.; Huang, L.; Michenko, M.J.; Duffet, M. Manual of Pediatric Intensive Care. PMPH-USA. 2009. Available online: https://www.wolterskluwer.com/en/solutions/ovid/manual-of-pediatric-intensive-care-8132 (accessed on 25 August 2021).
- Assad, O.N.; Gilboa, T.; Spitzberg, J.; Juhasz, M.; Weinhold, E.; Meller, A. Light-enhancing plasmonic-nanopore biosubstrate for superior single-molecule detection. Adv. Mater. 2017, 29, 1605442. [Google Scholar] [CrossRef]
- Yanik, A.A.; Cetin, A.E.; Huang, M.; Artar, A.; Mousavi, S.H.; Khanikaev, A.; Connor, J.; Shvets, G.; Altug, H. Seeing protein monolayers with naked eye through plasmonic Fano resonances. Proc. Natl. Acad. Sci. USA 2011, 108, 11784–11789. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.L.; Huang, J.B.; Chang, J.W.; Wu, S.H.; Wei, P.K. Ultrasensitive biosubstrates using enhanced fano resonances in capped gold nanoslit arrays. Sci. Rep. 2015, 5, 8547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.K.; Li, C.T.; Yen, T.J.; Lai, Y.C.; Chang, Y.T. A multi-functional plasmonic biosubstrate. Opt. Express 2010, 18, 9561–9569. [Google Scholar]
- Kim, K.; Yoon, J.K. Raman Scattering of 4-Aminobenzenethiol Sandwiched between Ag/Au Nanoparticle and Macroscopically Smooth Au Substrate. J. Phys. Chem. B 2005, 109, 20731–20736. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ling, X.; Xiao, J.; Dresselhaus, M.S.; Kong, J.; Xu, H.; Liu, Z.; Zhang, J. Surface enhanced Raman spectroscopy on a flat graphene surface. Proc. Natl. Acad. Sci. USA 2012, 109, 9281–9286. [Google Scholar] [CrossRef] [Green Version]
- Tao, A.R.; Yang, P. Polarized Surface-Enhanced Raman Spectroscopy on Coupled Metallic Nanowires. J. Phys. Chem. B 2005, 109, 15687–15690. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.X.; Zou, W.J.; Yang, Z.L.; Yuan, Y.X.; Xu, M.M.; Yao, J.L.; Gu, R.A. Surface-enhanced Raman spectroscopy on single Fe2O3@Au spindle nanoparticle: Polarization dependence and FDTD simulation. J. Opt. 2015, 17, 114014. [Google Scholar] [CrossRef]
- Puttaswamy, S.; Lee, B.D.; Sengupta, S. Novel Electrical Method for Early Detection of Viable Bacteria in Blood Cultures. J. Clin. Microbiol. 2011, 49, 2286–2289. [Google Scholar] [CrossRef] [Green Version]
- Premasiri, W.R.; Sauer-Budge, A.F.; Lee, J.C.; Klapperich, C.M.; Ziegler, L.D. Rapid bacterial diagnostics via surface enhanced Raman microscopy. Spectroscopy 2012, 27, s8-31. [Google Scholar]
- Yue, W.; Wang, Z.; Whittaker, J.; Lopez-Royo, F.; Yang, Y.; Zayats, A.V. Amplification of surface-enhanced Raman scattering due to substrate-mediated localized surface plasmons in gold nanodimers. J. Mater. Chem. C 2017, 5, 4075–4084. [Google Scholar] [CrossRef] [Green Version]
- Shioi, M.; Jans, H.; Lodewijks, K.; Van Dorpe, P.; Lagae, L.; Kawamura, T. Tuning the interaction between propagating and localized surface plasmons for surface enhanced Raman scattering in water for biomedical and environmental applications. Appl. Phys. Lett. 2014, 104, 243102. [Google Scholar] [CrossRef]
- Shan, D.; Huang, L.; Li, X.; Zhang, W.; Wang, J.; Cheng, L.; Feng, X.; Liu, Y.; Zhu, J.; Zhang, Y. Surface Plasmon Resonance and Interference Coenhanced SERS Substrate of AAO/Al-Based Ag Nanostructure Arrays. J. Phys. Chem. C 2014, 118, 23930–23936. [Google Scholar] [CrossRef]
- Paniel, N.; Baudart, J. Colorimetric and electrochemical genosensors for the detection of Escherichia coli DNA without amplification in seawater. Talanta 2013, 115, 133–142. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Wang, C.; Shao, N.; Dong, P.; Xiao, R.; Wang, S. Magnetically Assisted Surface-Enhanced Raman Spectroscopy for the Detection of Staphylococcus aureus Based on Aptamer Recognition. ACS Appl. Mater. Interfaces 2015, 7, 20919–20929. [Google Scholar] [CrossRef]
- Sha, M.; Xu, H.; Natan, M.J.; Cromer, R. Surface-Enhanced Raman Scattering Tags for Rapid and Homogeneous Detection of Circulating Tumor Cells in the Presence of Human Whole Blood. J. Am. Chem. Soc. 2008, 130, 17214–17215. [Google Scholar] [CrossRef] [Green Version]
- Lesaicherre, M.L.; Paxon, T.L.; Mondello, F.J.; Burrell, M.C.; Linsebigler, A. Portable Raman instrument for rapid biological agent detection and identification. SPIE Def. Secur. Sens. 2009, 7319, 73190C. [Google Scholar] [CrossRef]
- Chou, J.B.; Yeng, Y.X.; Lee, Y.E.; Lenert, A.; Rinnerbauer, V.; Celanovic, I.; Soljačić, M.; Fang, N.X.; Wang, E.N.; Kim, S.-G. Enabling Ideal Selective Solar Absorption with 2D Metallic Dielectric Photonic Crystals. Adv. Mater. 2014, 26, 8041–8045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palik, E.D. Handbook of Optical Constants of Solids (Volume 3); Academic Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Zhang, Z.M. Nano/Microscale Heat Transfer; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Zhao, B.; Zhao, J.M.; Zhang, Z.M. Enhancement of near-infrared absorption in graphene with metal gratings. Appl. Phys. Lett. 2014, 105, 031905. [Google Scholar] [CrossRef]
- Hosseini, P.; Abidi, S.Z.; Du, E.; Papageorgiou, D.P.; Choi, Y.; Park, Y.; Higgins, J.M.; Kato, G.; Suresh, S.; Dao, M.; et al. Cellular normoxic biophysical markers of hydroxyurea treatment in sickle cell disease. Proc. Natl. Acad. Sci. USA 2016, 113, 9527–9532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimault, A.-S.; Vial, A.; De La Chapelle, M.L. Modeling of regular gold nanostructures arrays for SERS applications using a 3D FDTD method. Appl. Phys. A 2006, 84, 111–115. [Google Scholar] [CrossRef]
- Tira, C.; Daniela, T.; Timea, S.; Simion, A. Finite-difference time-domain (FDTD) design of gold nanoparticle chains with specific surface plasmon resonance. J. Mol. Struct. 2014, 1072, 137–143. [Google Scholar] [CrossRef]
- Vial, A.; Laroche, T. Description of dispersion properties of metals by means of the critical points model and application to the study of resonant structures using the FDTD method. J. Phys. D Appl. Phys. 2007, 40, 7152–7158. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Z.; Liao, F.; Cai, Q.; Li, Y.; Lee, S.; Shao, M. The effect of dielectric constants on noble metal/semiconductor SERS enhancement: FDTD simulation and experiment validation of Ag/Ge and Ag/Si substrates. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Lail, N.I.; Camesano, T.A. The effect of solvent polarity on the molecular surface properties and adhesion of Escherichia coli. Colloids Surf. B Biointerfaces 2006, 51, 62–70. [Google Scholar] [CrossRef]
- Lee, C.S.; Lee, S.-W.; Chuang, S.-L. Normal Modes in an Overmoded Circular Waveguide Coated with Lossy Material. IEEE Trans. Microw. Theory Tech. 1986, 34, 773–785. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Z.; Asiri, S.; Elsharif, A.; Alamoudi, W.; Al-Suhaimi, E.; Kim, S.-G. Design of Inverted Nano-Cone Arrayed SERS Substrate for Rapid Detection of Pathogens. Appl. Sci. 2021, 11, 8067. https://doi.org/10.3390/app11178067
Jia Z, Asiri S, Elsharif A, Alamoudi W, Al-Suhaimi E, Kim S-G. Design of Inverted Nano-Cone Arrayed SERS Substrate for Rapid Detection of Pathogens. Applied Sciences. 2021; 11(17):8067. https://doi.org/10.3390/app11178067
Chicago/Turabian StyleJia, Zixun, Sarah Asiri, Asma Elsharif, Widyan Alamoudi, Ebtesam Al-Suhaimi, and Sang-Gook Kim. 2021. "Design of Inverted Nano-Cone Arrayed SERS Substrate for Rapid Detection of Pathogens" Applied Sciences 11, no. 17: 8067. https://doi.org/10.3390/app11178067
APA StyleJia, Z., Asiri, S., Elsharif, A., Alamoudi, W., Al-Suhaimi, E., & Kim, S.-G. (2021). Design of Inverted Nano-Cone Arrayed SERS Substrate for Rapid Detection of Pathogens. Applied Sciences, 11(17), 8067. https://doi.org/10.3390/app11178067





