Synthesis and Formation Process of a Typical Doped Solid-Solution Ye’elimite (Ca3.8Na0.2Al5.6Fe0.2Si0.2SO16): Experiments and Kinetic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Sintering Conditions for Clinkers
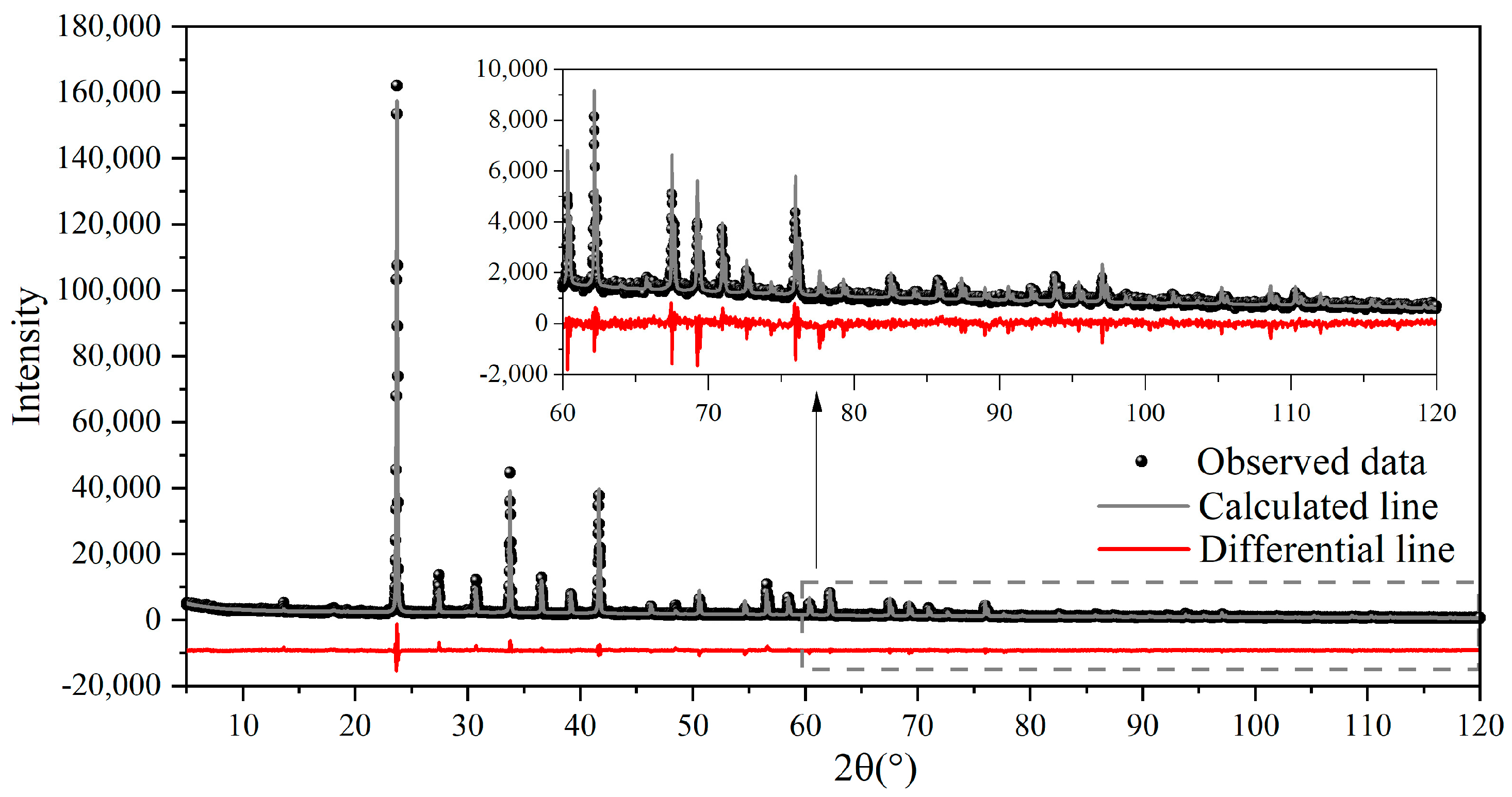
2.2. Test Methods
2.3. Kinetic Theory
3. Results
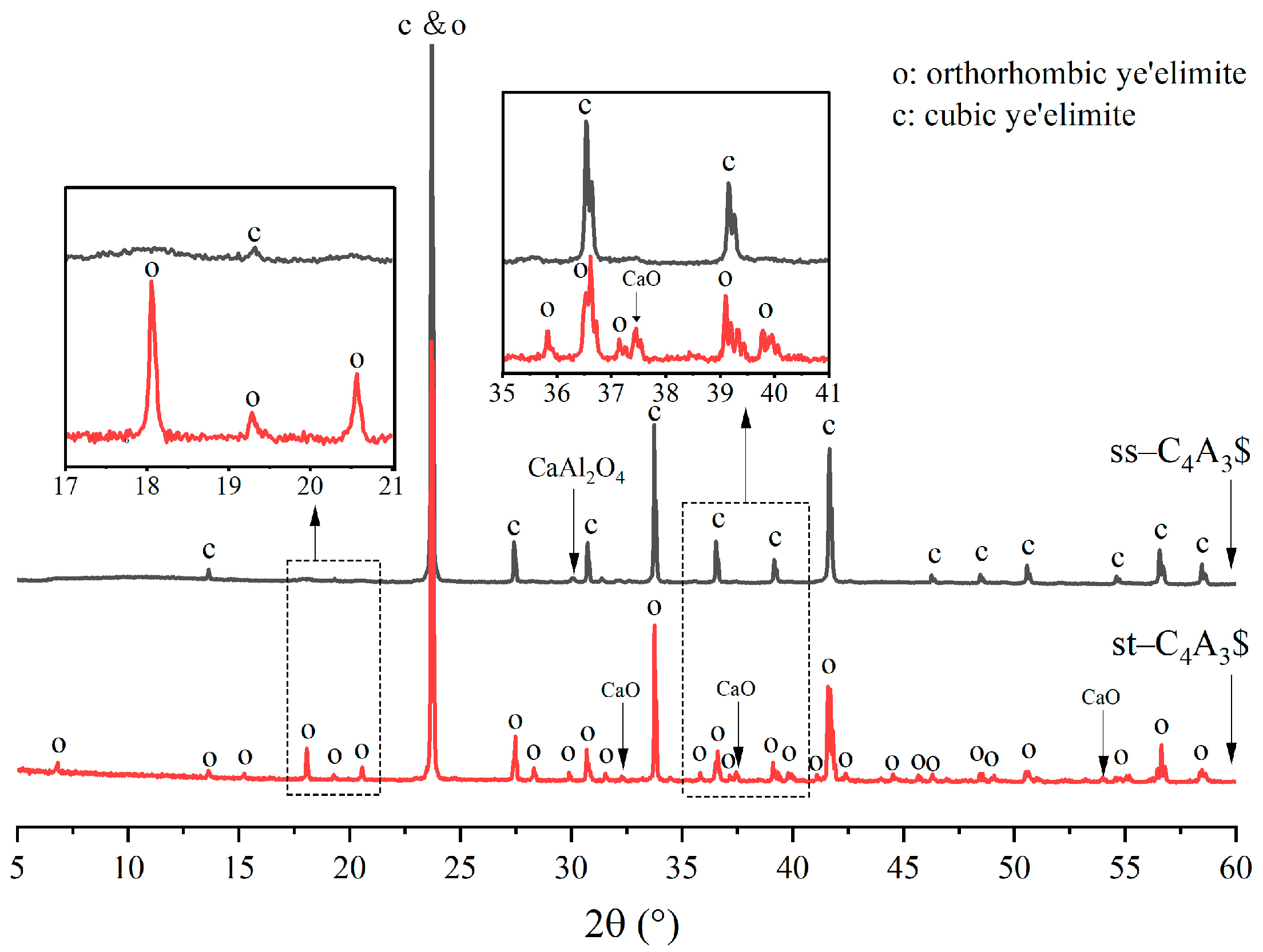
3.1. Crystallographic Distinctions between st-C4A3$ and ss-C4A3$
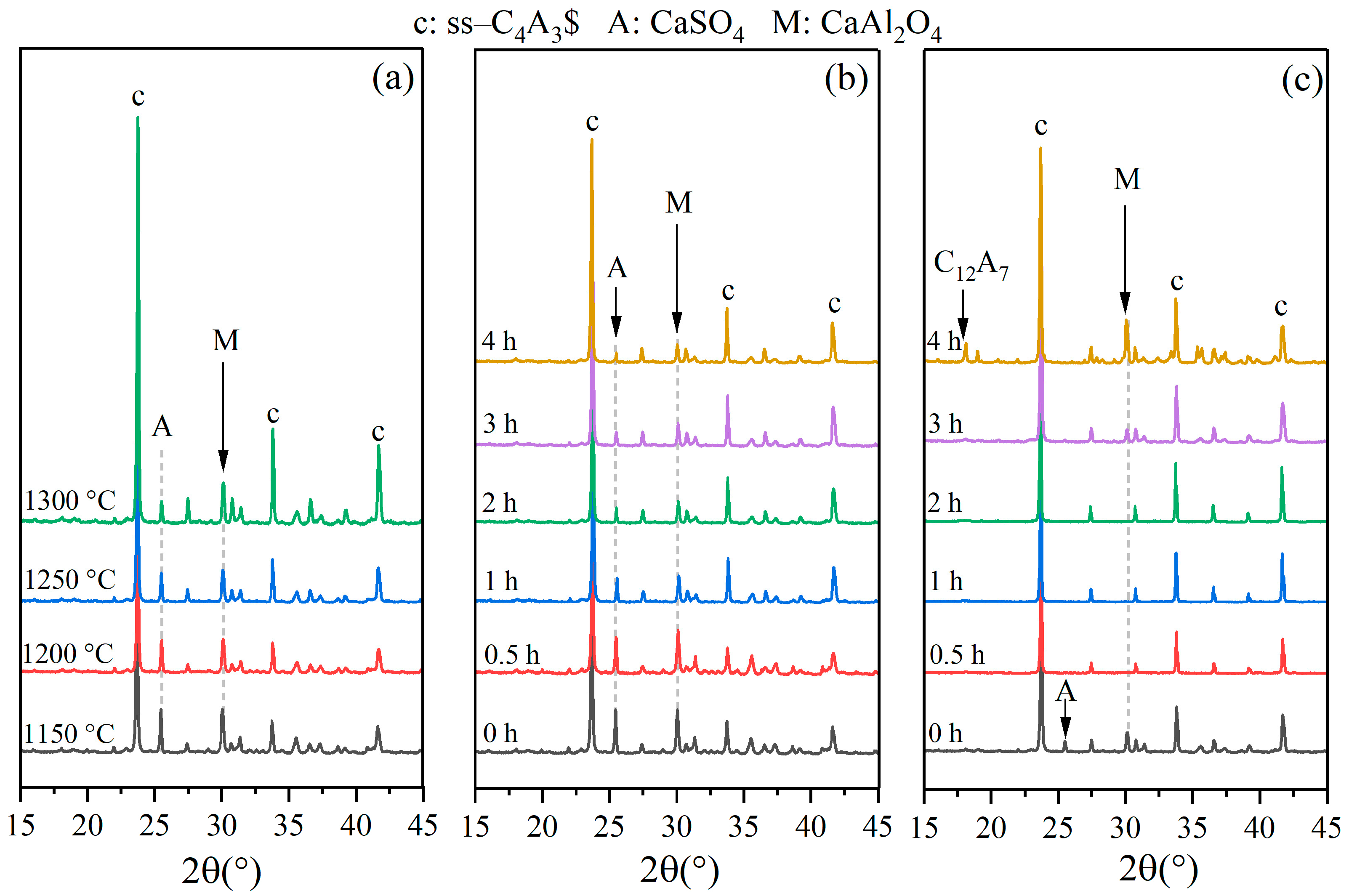
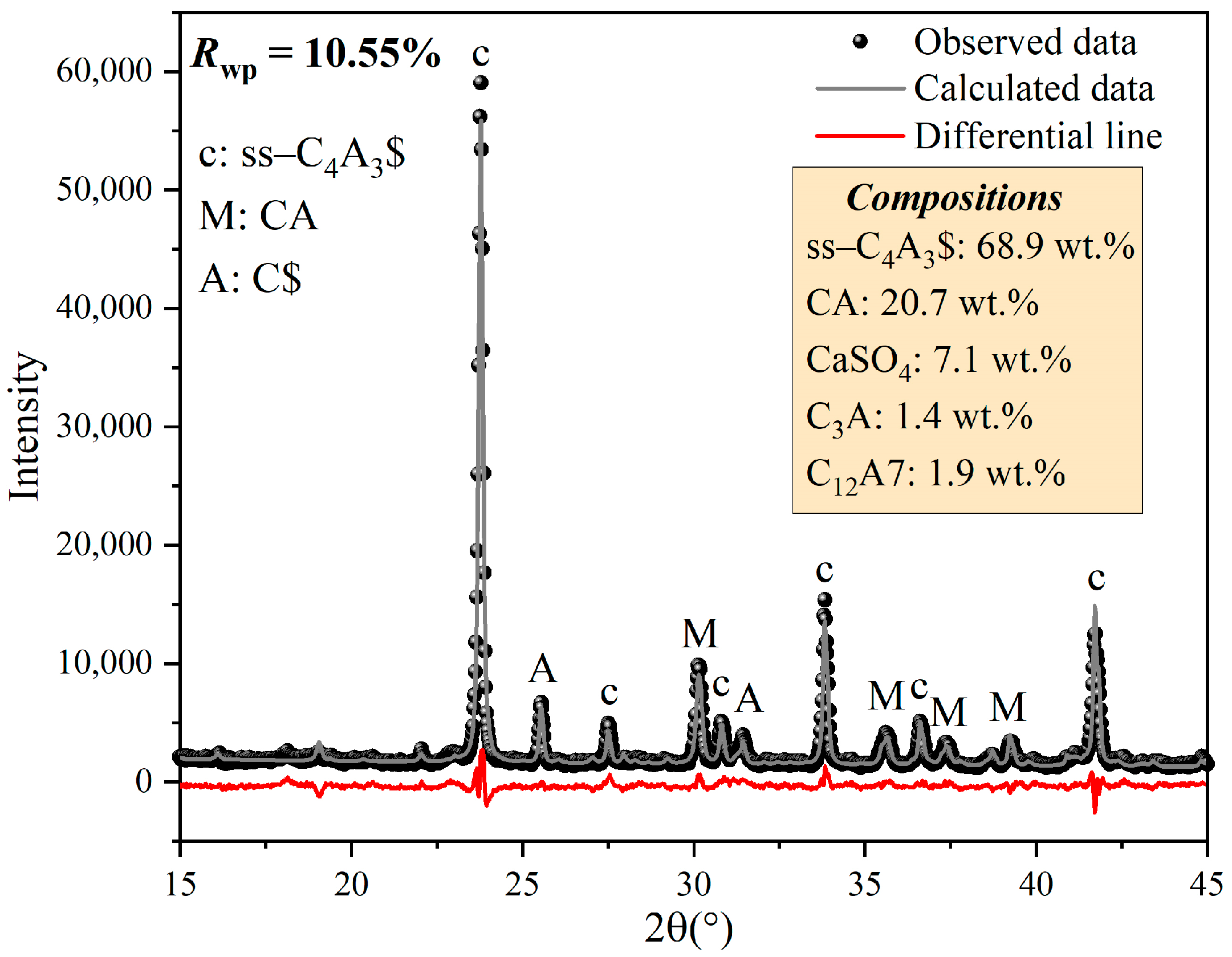
3.2. Formation Process of ss-C4A3$
3.3. Kinetic Analysis for the Formation Process of ss-C4A3$
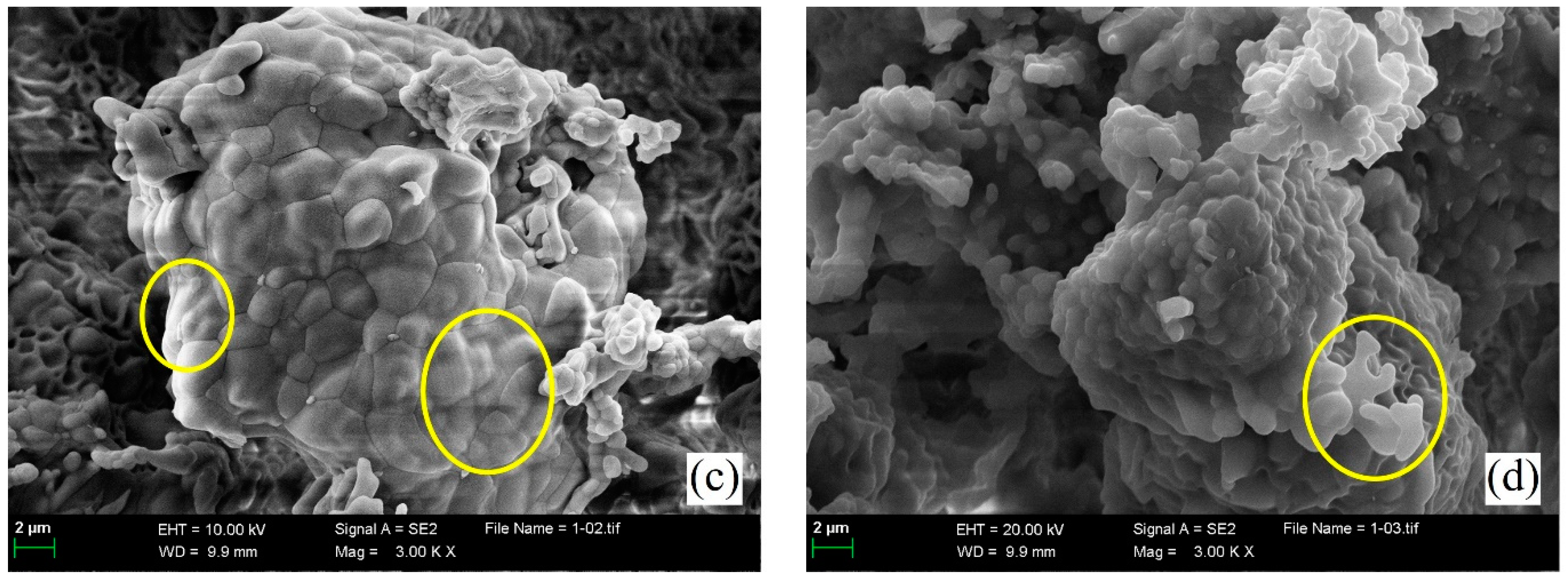
3.4. Influence of Sintering Temperature on Microstructural Morphologies of ss-C4A3$
4. Discussion
5. Conclusions
- (1)
- XRD patterns of st-C4A3$ and ss-C4A3$ tended to be similar, but st-C4A3$ was distinguished from ss-C4A3$ by some additional characteristic peaks. The formation process of ss-C4A3$ mainly resulted from solid reactions between the intermediate phases (calcium aluminate phases) and anhydrite, which did not involve the formation of st-C4A3$. In the conditions of 1150–1250 °C, ss-C4A3$ tended to be formed and stable for 4 h. However, when the sintering temperature increased to 1300 °C, ss-C4A3$ decomposed to generate calcium aluminate phases after 2 h.
- (2)
- Compared to other kinetic models, the three-dimensional diffusion model mostly conformed with the formation process of ss-C4A3$, and the fitting results obtained by the Jander model exhibited the highest correlation coefficients. The activation energy of ss-C4A3$ formation equaled 285.6 kJ/mol, which was lower than that of stoichiometric and strontium/barium-bearing ye’elimite.
- (3)
- The morphology features of ss-C4A3$ sintered at 1200 °C for 240 min were identified as approximately 1–2 μm polyhedral granules with clear boundaries; higher sintering temperatures or longer holding times would lead to granules fusing together.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Glasser, F.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate-belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Zhou, Q.; Milestone, N.; Hayes, M. An alternative to Portland Cement for waste encapsulation—The calcium sulfoaluminate cement system. J. Hazard. Mater. 2006, 136, 120–129. [Google Scholar] [CrossRef]
- Meng, K.; Cui, C.; Liang, Z.; Li, H.; Pei, H. A new approach for longitudinal vibration of a large-diameter floating pipe pile in visco-elastic soil considering the three-dimensional wave effects. Comput. Geotech. 2020, 128, 103840. [Google Scholar] [CrossRef]
- Khalil, N.; Aouad, G.; El Cheikh, K.; Rémond, S. Use of calcium sulfoaluminate cements for setting control of 3D-printing mortars. Constr. Build. Mater. 2017, 157, 382–391. [Google Scholar] [CrossRef]
- Cui, C.; Meng, K.; Xu, C.; Liang, Z.; Li, H.; Pei, H. Analytical solution for longitudinal vibration of a floating pile in saturated porous media based on a fictitious saturated soil pile model. Comput. Geotech. 2020, 131, 103942. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Zajac, M.; Ben Haha, M. CSA raw mix design: Effect on clinker formation and reactivity. Mater. Struc. 2015, 12, 3895–3911. [Google Scholar] [CrossRef]
- Alvarez-Pinazo, G.; Cuesta, A.; Garcia-Mate, M.; Santacruz, I.; Losilla, E.R.; Torre, A.G.; Leon-Reina, L.; Aranda, M.A.G. Rietveld quantitative phase analysis of Yeelimite-containing cements. Cem. Concr. Res. 2012, 42, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 12, 1232–1243. [Google Scholar] [CrossRef]
- Shi, C.; Fernandez, A.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 7, 750–763. [Google Scholar] [CrossRef]
- Hargis, C.W.; Telesca, A.; Monteiro, P.J.M. Calcium sulfoaluminate (Ye’elimite) hydration in the presence of gypsum, calcite, and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
- Winnefeld, F.; Martin, L.H.J.; Muller, C.J.B. Lothenbach, using gypsum to control hydration kinetics of CSA cements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
- Bizzozero, J.; Gosselin, C.; Scrivener, K.L. Expansion mechanisms in calcium aluminate and sulfoaluminate systems with calcium sulfate. Cem. Concr. Res. 2014, 56, 190–202. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Zajac, M.; Ben Haha, M.; Scrivener, K.L. Factors influencing the hydration kinetics of Ye’elimite; effect of mayenite. Cem. Concr. Res. 2018, 116, 113–119. [Google Scholar] [CrossRef]
- El, Y.; El, Y.; Smith, A. Examination of ye’elimite formation mechanisms. J. Eur. Ceram. Soc. 2019, 39, 5086–5095. [Google Scholar]
- Cuesta, A.; De la Torre, A.G.; Losilla, E.R.; Peterson, V.K.; Rejmak, P.; Ayuela, A.; Frontera, C.; Aranda, M.A.G. Structure, Atomistic Simulations, and Phase Transition of Stoichiometric Yeelimite. Chem. Mater. 2013, 25, 1680–1687. [Google Scholar] [CrossRef] [Green Version]
- Zea-Garcia, J.; Santacruz, I.; Aranda, M.; De, G. Alite-belite-ye’elimite cements: Effect of dopants on the clinker phase com-position and properties. Cem. Concr. Res. 2019, 115, 192–202. [Google Scholar] [CrossRef]
- Li, C.; Wu, M.; Yao, W. Effect of coupled B/Na and B/Ba doping on hydraulic properties of belite-ye’elimite-ferrite cement. Constr. Build. Mater. 2019, 208, 23–35. [Google Scholar] [CrossRef]
- Andaç, O.; Glasser, F.P. Polymorphism of calcium sulphoaluminate (Ca4Al6O16·SO3) and its solid solutions. Adv. Cem. Res. 1994, 6, 57–60. [Google Scholar] [CrossRef]
- Chang, J.; Cheng, X.; Liu, F.; Lu, L.; Teng, B. Influence of fluorite on the Ba-bearing sulphoaluminate cement. Cem. Concr. Res. 2001, 31, 213–216. [Google Scholar] [CrossRef]
- Zhao, J.; Chang, J. Crystallographic Analysis of Sr-Bearing Ye’elimite. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1694–1702. [Google Scholar] [CrossRef]
- Cuesta, A.; de la Torre, Á.G.; Losilla, E.; Santacruz, I.; Aranda, M. Pseudocubic crystal structure and phase transition in doped ye’elimite. Cryst. Growth Des. 2014, 14, 5158–5163. [Google Scholar] [CrossRef] [Green Version]
- Cuesta, A.; Pinazo, G.; Sanfélix, S.; Peral, I.; Aranda, M.; De la Torre, A. Hydration mechanisms of two polymorphs of synthetic Ye’elimite. Cem. Concr. Res. 2014, 63, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Lazic, B.; Krüger, H.; Kahlenberg, V.; Konzett, J.; Kaindl, R. Incommensurate structure of Ca2Al2O5 at high temperatures—Structure investigation and Raman spectroscopy. ACS Catal. 2010, 64, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.C.H.; Zussman, J. The crystal structure of anhydrite (CaSO4). Acta Crystallogr. 1963, 16, 767–769. [Google Scholar] [CrossRef] [Green Version]
- Boysen, H.; Lerch, M.; Stys, A.; Senyshyn, A. Structure and oxygen mobility in mayenite (Ca12Al14O33): A high-temperature neutron powder diffraction study. Acta Crystallogr. Sect. B Struct. Sci. 2007, 63, 675–682. [Google Scholar] [CrossRef]
- Mondal, P.; Jeffery, J.W. The crystal structure of tricalcium aluminate, Ca3Al2O6. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1975, 31, 689–697. [Google Scholar] [CrossRef]
- Verbraeken, M.; Suard, E.; Irvine, J. Structural and electrical properties of calcium and strontium hydrides. J. Chem. Educ. 2009, 19, 2766–2770. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
- Bao, X.; Zhao, P.; Liang, C.; Li, Q.; Wang, S.; Cheng, X. Phase formation mechanism and kinetics in solid-state synthesis of Ba-doped Ye′elimite: The effect of Ba-doping concentration on C4−xBxA3$ systems. Ceramics-Silikáty 2020, 64, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Chang, J. Kinetic Analysis for Formation Process of Sr-Bearing Ye’elimite. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1861–1869. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Scholten, T.; Scrivener, K.; Ben Haha, M.; Wolter, A. Formation, composition and stability of Ye’elimite and iron-bearing solid solutions. Cem. Concr. Res. 2020, 131, 106009. [Google Scholar] [CrossRef]



| Phases | Formula (Abbreviation) | COD Code | Reference |
|---|---|---|---|
| Typical solid-solution ye’elimite | Ca3.8Na0.2Al5.6Fe0.2Si0.2SO16 (ss-C4A3$) | 45,119,60 | [21] |
| Monocalcium aluminate | CaAl2O4 (CA) | 43,080,75 | [23] |
| Anhydrite | CaSO4 (C$) | 50,000,40 | [24] |
| Mayenite | Ca12Al14O33 (C12A7) | 21,029,55 | [25] |
| Tricalcium aluminate | Ca3Al2O6 (C3A) | 90,159,66 | [26] |
| Calcium oxide | CaO (C) | 72,006,86 | [27] |
| Symbol | Models | f(α) | g(α) |
|---|---|---|---|
| D1 | One-dimensional diffusion | α2 | |
| D2 | Two-dimensional diffusion | −1/ln(1 − α) | α + (1 − α)ln(1 − α) |
| D3 | Jander | 3(1 − α)2/3/{2[1 − (1 − α)1/3]} | [1 − (1 − α)1/3]2 |
| (Three-dimensional diffusion) | |||
| D4 | Ginstling–Brounstein | 3/{2[(1 − α)−1/3 − 1]} | 1 − (2α/3) − (1 − α)2/3 |
| (Three-dimensional) | |||
| R3 | Model for interface reaction | 1 − (1 − α)1/3 | |
| A3 | Avrami–Erofeyev | [−ln(1 − α)]1/3 | |
| F1 | Crystal nucleation growth model | −ln(1 − α) |
| Types of Ye’elimite | Crystallographic System (Space Group) | Lattice Parameters (Å) | Sources |
|---|---|---|---|
| Stoichiometric | Orthorhombic | a = 13.041, b = 13.036, c = 9172 | This paper |
| (Pcc2) | a = 13.037, b = 13.035, c = 9168 | [15] | |
| Typical solid-solution | Cubic | a = 9191 | This paper |
| (I-43 m) | a = 9197 | [21] |
| Temperature | Kinetic Models | ||||||
|---|---|---|---|---|---|---|---|
| (°C) | D1 | D2 | D3 | D4 | A3 | R3 | F1 |
| 1150 | 0.9608 | 0.9791 | 0.9942 | 0.9848 | 0.7357 | 0.9031 | 0.9293 |
| 1200 | 0.9314 | 0.9590 | 0.9869 | 0.9693 | 0.7655 | 0.9106 | 0.9442 |
| 1250 | 0.9994 | 0.9886 | 0.9682 | 0.9800 | 0.8658 | 0.9888 | 0.9986 |
| 1300 | 0.9937 | 0.9998 | 0.9961 | 0.9996 | 0.9195 | 0.9807 | 0.9931 |
| Average | 0.9713 | 0.9816 | 0.9864 | 0.9834 | 0.8216 | 0.9458 | 0.9663 |
| Standard deviation | 0.0274 | 0.0150 | 0.0110 | 0.0109 | 0.0743 | 0.0392 | 0.0301 |
| Target Minerals | Dopants | Model | Ea (kJ/mol) | Sources |
|---|---|---|---|---|
| Ca3.8Na0.2Al5.6Fe0.2Si0.2SO16 | Na+, Fe3+, Si4+ | D3 | 285.6 | This paper |
| Ca4Al6SO16 | − | D3 | 300.8 | [30] |
| Ca4Al6SO16 | − | D3 | 304.0 | [29] |
| Ca4−xBaxAl6SO16 1 | Ba2+ | D3 | 304 + 71.9x | [29] |
| Ca3SrAl6SO16 | Sr2+ | D3 | 367.9 | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Huang, J.; Yu, C.; Cui, C. Synthesis and Formation Process of a Typical Doped Solid-Solution Ye’elimite (Ca3.8Na0.2Al5.6Fe0.2Si0.2SO16): Experiments and Kinetic Analysis. Appl. Sci. 2021, 11, 8015. https://doi.org/10.3390/app11178015
Zhao J, Huang J, Yu C, Cui C. Synthesis and Formation Process of a Typical Doped Solid-Solution Ye’elimite (Ca3.8Na0.2Al5.6Fe0.2Si0.2SO16): Experiments and Kinetic Analysis. Applied Sciences. 2021; 11(17):8015. https://doi.org/10.3390/app11178015
Chicago/Turabian StyleZhao, Jiuye, Jiazhi Huang, Chunyang Yu, and Chunyi Cui. 2021. "Synthesis and Formation Process of a Typical Doped Solid-Solution Ye’elimite (Ca3.8Na0.2Al5.6Fe0.2Si0.2SO16): Experiments and Kinetic Analysis" Applied Sciences 11, no. 17: 8015. https://doi.org/10.3390/app11178015
APA StyleZhao, J., Huang, J., Yu, C., & Cui, C. (2021). Synthesis and Formation Process of a Typical Doped Solid-Solution Ye’elimite (Ca3.8Na0.2Al5.6Fe0.2Si0.2SO16): Experiments and Kinetic Analysis. Applied Sciences, 11(17), 8015. https://doi.org/10.3390/app11178015





