Effects of Water-to-Cement Ratios on the Properties of Magnesium Potassium Phosphate Cement Prepared with Lithium-Extracted Magnesium Residue
Abstract
1. Introduction
2. Materials and Experimental Procedure
2.1. Raw Materials
2.2. Raw Material Handling
2.3. Mixing Process
2.4. Test Methods
3. Results
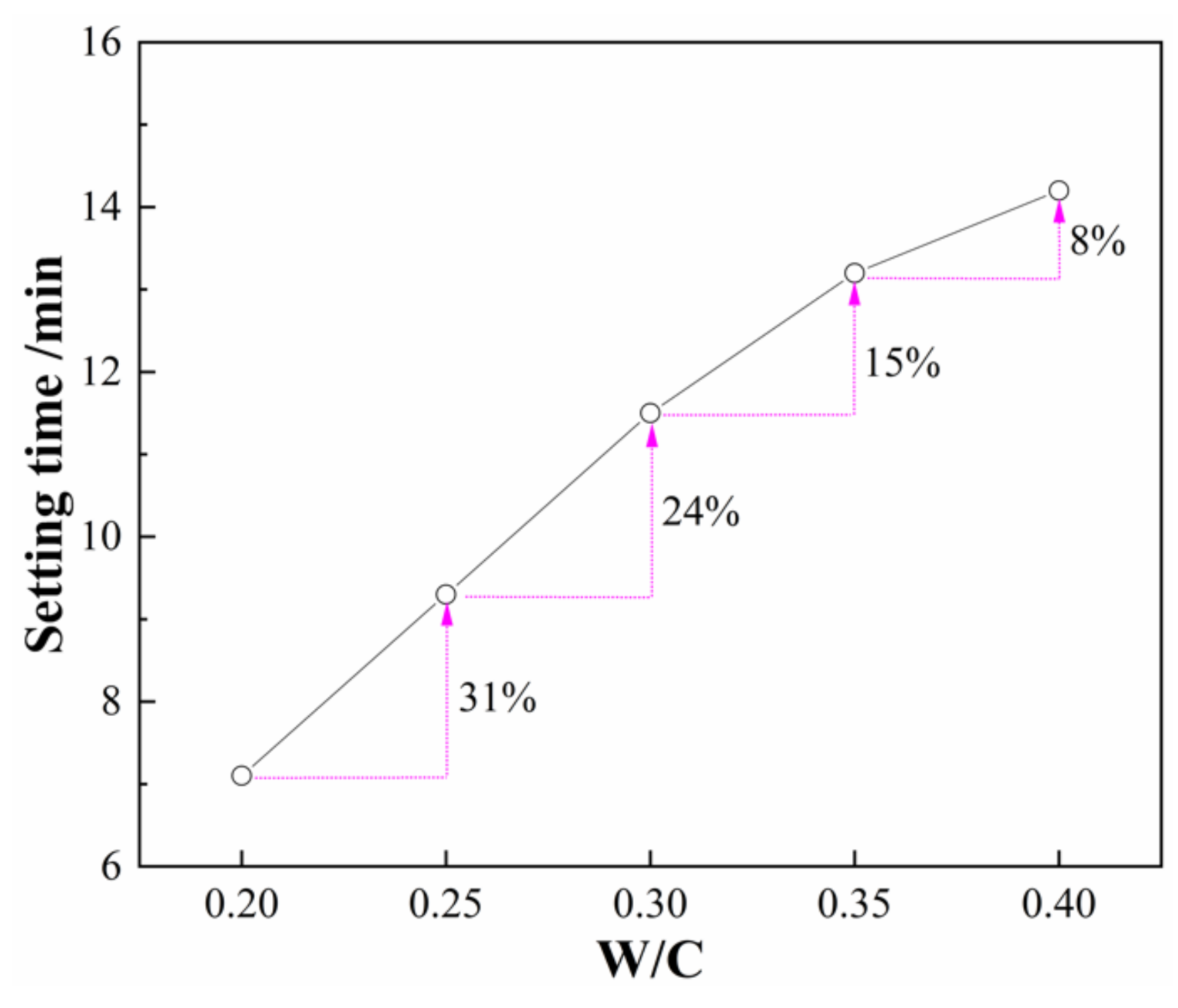
3.1. Effect of W/C Ratios on the Setting Time of SLMKPC Slurry
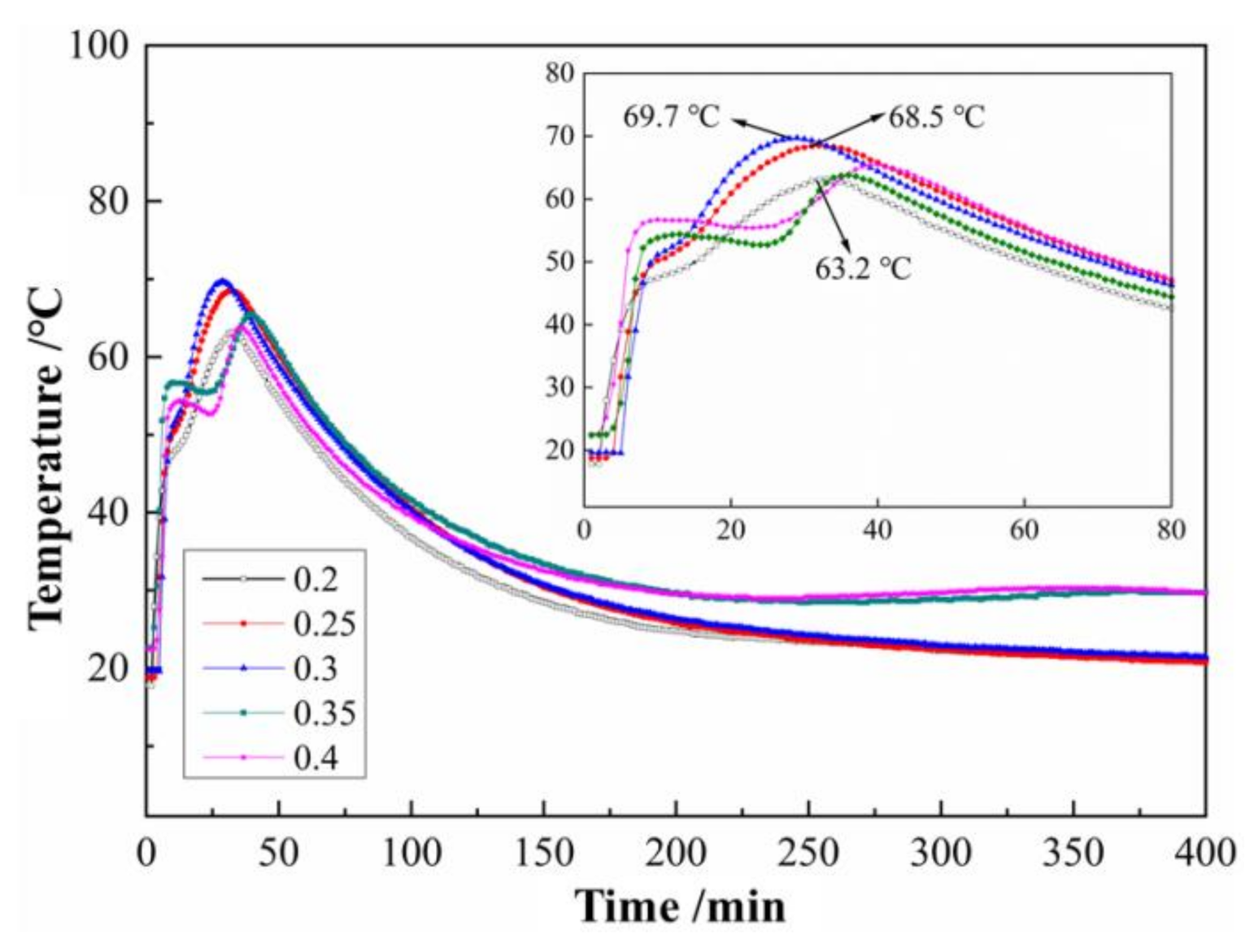
3.2. Effects of W/C Ratios on the Hydration Temperature Curve of SLMKPC Slurry
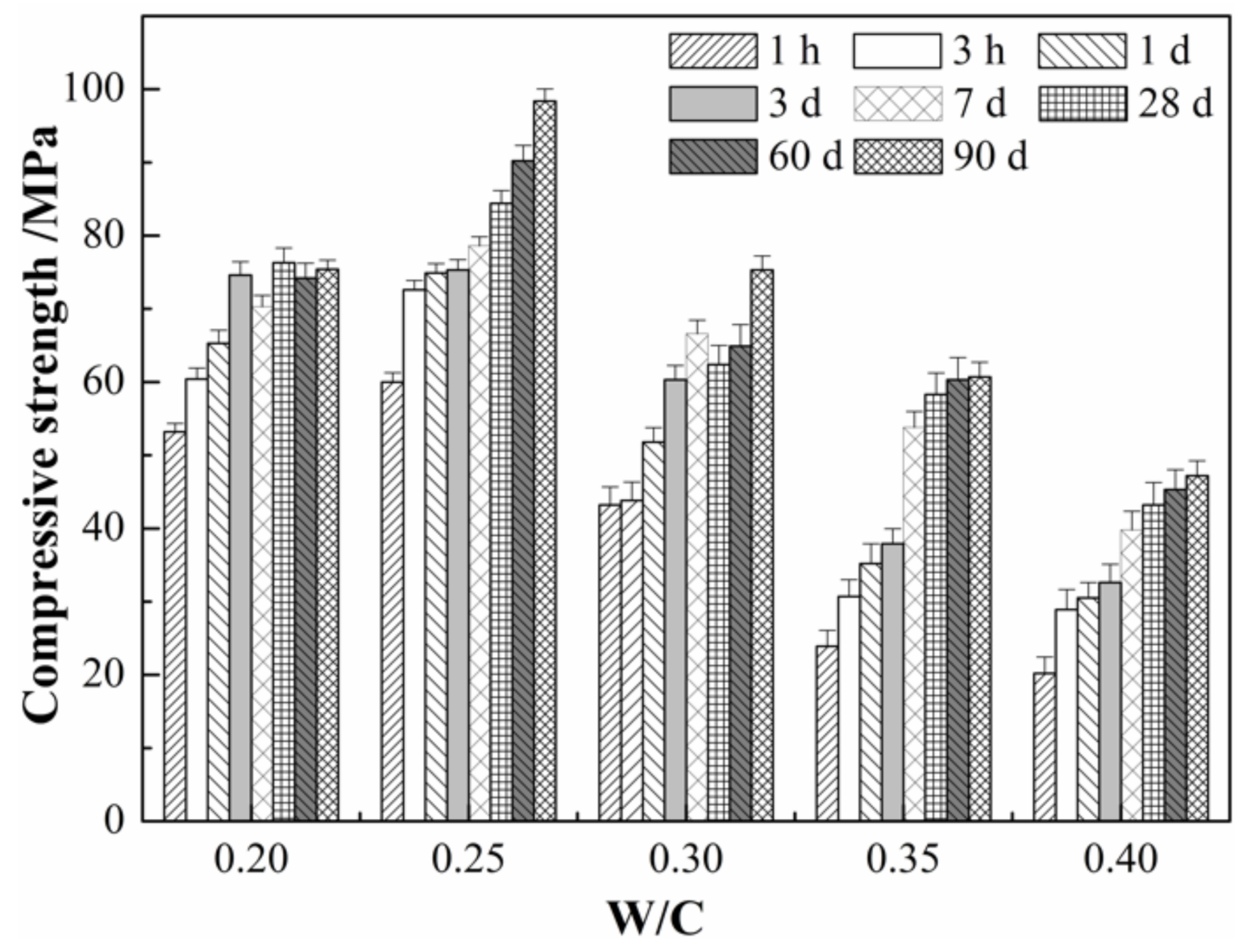
3.3. Effects of W/C Ratios on the Compressive Strength of SLMKPC
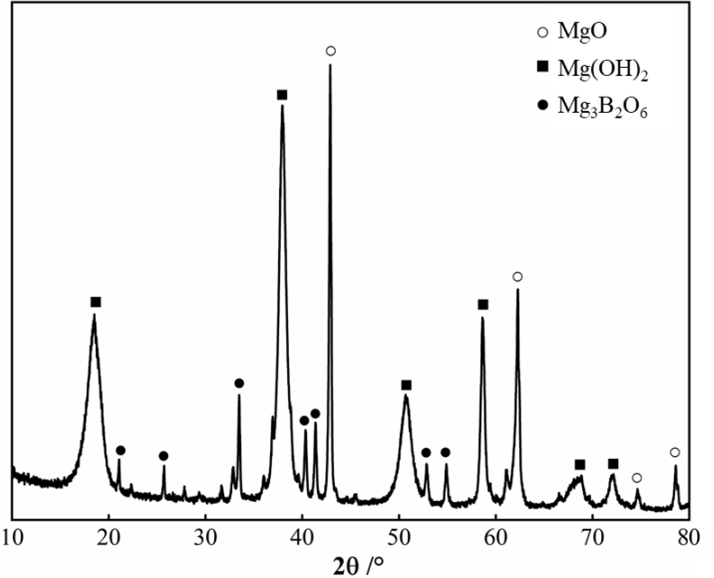
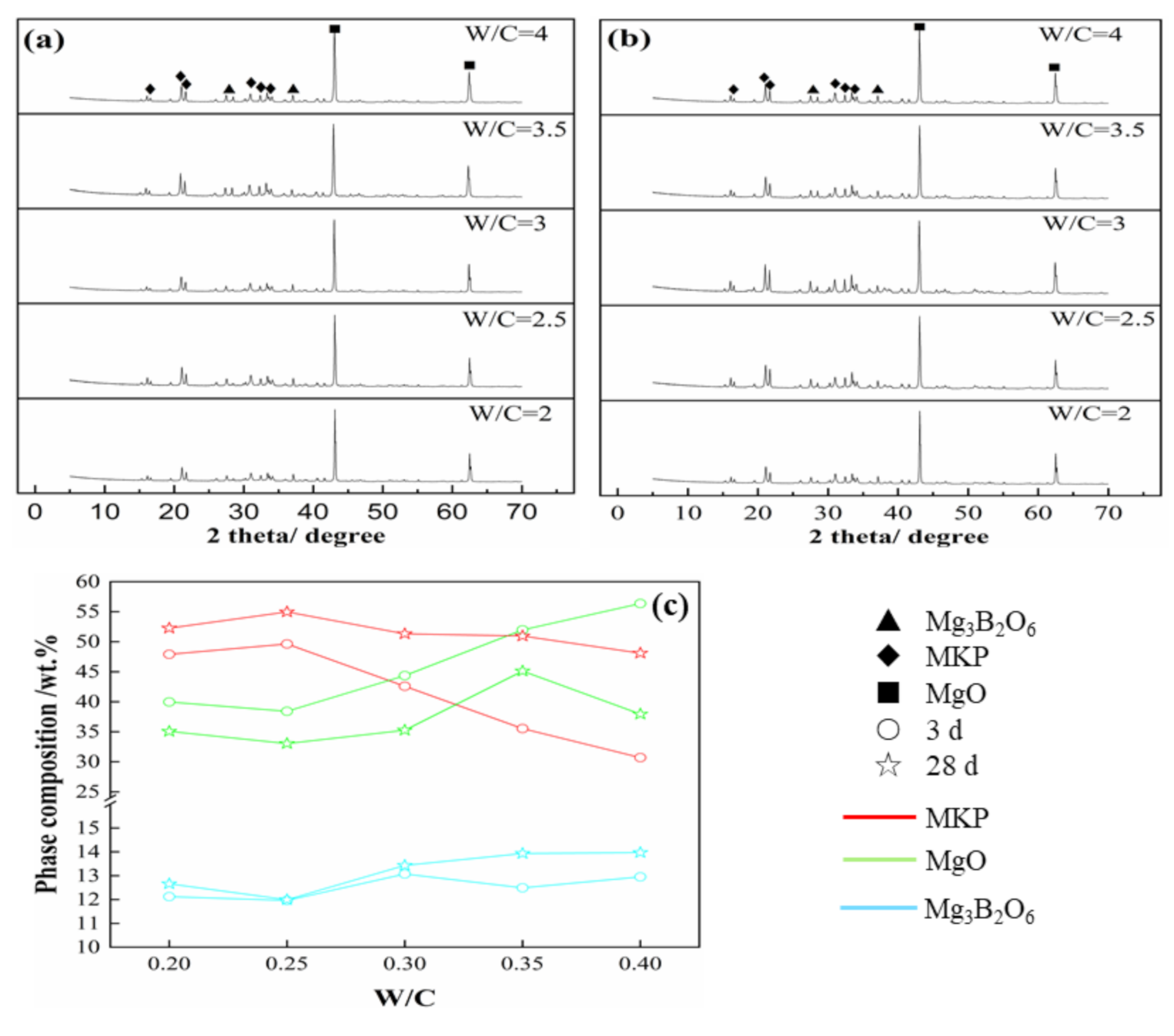
3.4. Effecst of W/C Ratios on the Phase Composition of SLMKPC
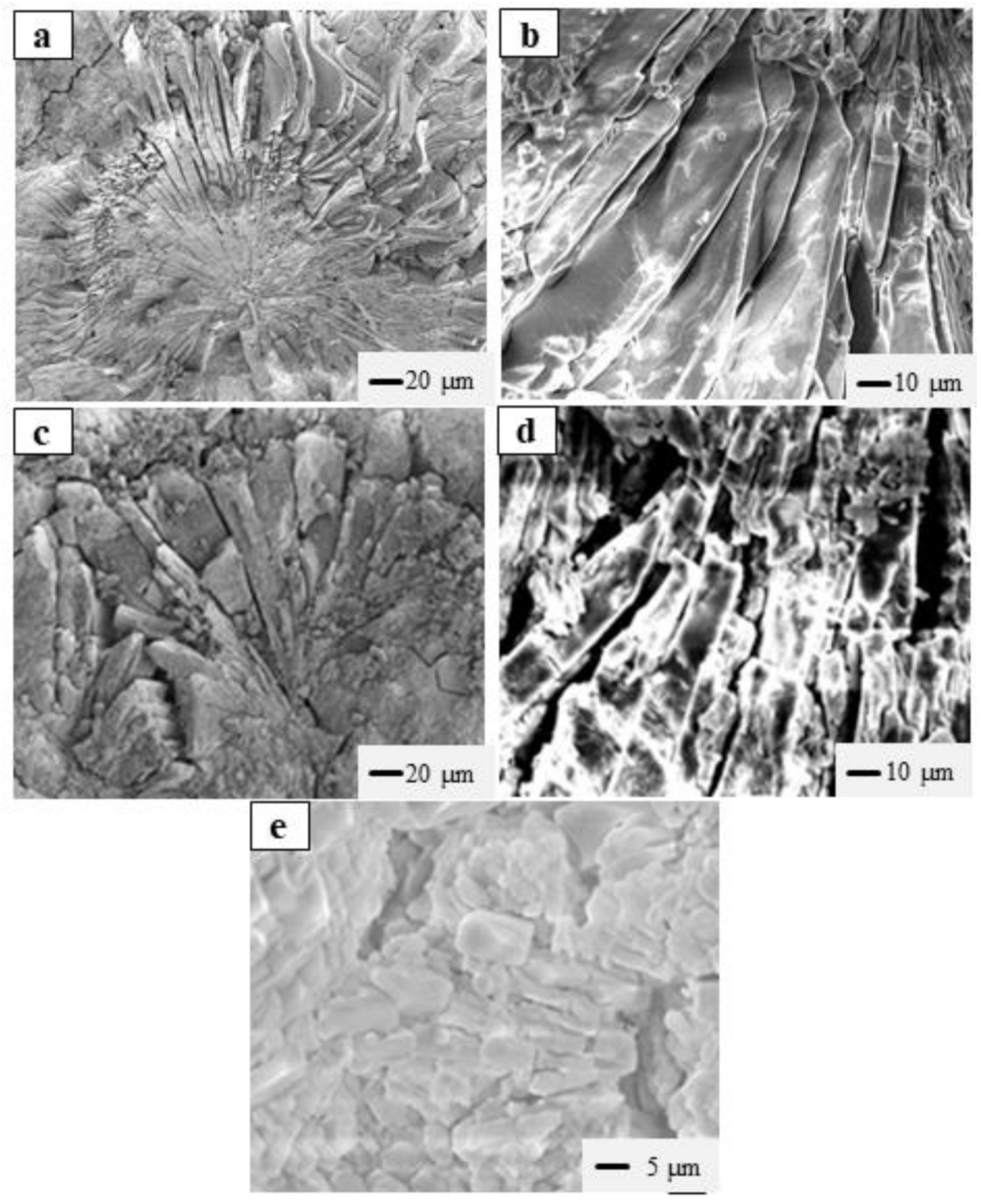
3.5. Effects of W/C Ratios on the Microstructure of SLMKPC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The beneficiation of lithium minerals from hard rock ores: A review. Miner. Eng. 2019, 131, 170–184. [Google Scholar] [CrossRef]
- Angermeier, S.; Ketterer, J.; Karcher, C. Liquid-Based Battery Temperature Control of Electric Buses. Energies 2020, 13, 4990. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Wang, Y.; Yun, R.; Xu, X. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine. Sep. Purif. Technol. 2021, 256, 117807. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Li, B.; Wu, J.; Lu, J. Life cycle assessment considering water-energy nexus for lithium nanofiltration extraction technique. J. Clean. Prod. 2020, 261, 121152. [Google Scholar] [CrossRef]
- Kong, R.; Xue, F.; Wang, J. Research on Mineral Resources and Environment of Salt Lakes in Qinghai Province based on System Dynamics Theory. Resour. Policy 2017, 52, 19–28. [Google Scholar] [CrossRef]
- Campos, M.D.; Davy, C.A.; Djelal, N.; Rivenet, M.; Garcia, J. Development of a stoichiometric magnesium potassium phosphate cement (MKPC) for the immobilization of powdered minerals. Cem. Concr. Res. 2021, 142, 106346. [Google Scholar] [CrossRef]
- Li, Y.; Shi, T.; Li, Y.; Bai, W.; Lin, H. Damage of magnesium potassium phosphate cement under dry and wet cycles and sulfate attack. Constr. Build. Mater. 2019, 210, 111–117. [Google Scholar] [CrossRef]
- Brückner, T.; Meininger, M.; Groll, J.; Kübler, A.C.; Gbureck, U. Magnesium Phosphate Cement as Mineral Bone Adhesive. Materials 2019, 12, 3819. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Z.; Zhang, W.; Peng, S.; Chen, P. Flexural Properties and Microstructure Mechanisms of Renewable Coir-Fiber-Reinforced Magnesium Phosphate Cement-Based Composite Considering Curing Ages. Polymers 2020, 12, 2556. [Google Scholar] [CrossRef]
- Du, Y.; Gao, P.; Yang, J.; Shi, F. Research on the Chloride Ion Penetration Resistance of Magnesium Phosphate Cement (MPC) Material as Coating for Reinforced Concrete Structures. Coatings 2020, 10, 1145. [Google Scholar] [CrossRef]
- Huete-Hernandez, S.; Maldonado-Alameda, A.; Giro-Paloma, J.; Chimenos, J.M.; Formosa, J. Fabrication of sustainable magnesium phosphate cement micromortar using design of experiments statistical modelling: Valorization of ceramic-stone-porcelain containing waste as filler. Ceram. Int. 2021, 47, 10905–10917. [Google Scholar] [CrossRef]
- Pyo, J.; Um, W.; Heo, J. Magnesium potassium phosphate cements to immobilize radioactive concrete wastes generated by decommissioning of nuclear power plants. Nucl. Eng. Technol. 2021. [Google Scholar] [CrossRef]
- Ding, Z.; Dong, B.; Xing, F.; Han, N.; Li, Z. Cementing mechanism of potassium phosphate based magnesium phosphate cement. Ceram. Int. 2012, 38, 6281–6288. [Google Scholar] [CrossRef]
- Tang, Y.; Yao, J.; Yin, W.; Kelebek, S. Molecular Dynamics Simulation of Cetyl Phosphate Adsorption in Flotation of Magnesite and Pertinent Chemical Aspects. Minerals 2020, 10, 761. [Google Scholar] [CrossRef]
- Qiao, F.; Chau, C.; Li, Z. Setting and compressive strength characteristics of magnesium phosphate cement paste. Adv. Cem. Res. 2009, 21, 175–180. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, L.; Peng, X.; Li, L.; Song, F.; Nie, F.; Ji, L.; Zhang, Y. Extraction of lithium from salt lake brine containing boron using multistage centrifuge extractors. Desalination 2018, 441, 44–51. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, Y.; Song, J.; Xing, L.; Kong, D.; Li, X.; He, T. Extraction of boron from salt lake brine using 2-ethylhexanol. Hydrometallurgy 2016, 160, 129–136. [Google Scholar] [CrossRef]
- Hall, D.A.; Stevens, R.; El-Jazairi, B. The effect of retarders on the microstructure and mechanical properties of magnesia–phosphate cement mortar. Cem. Concr. Res. 2001, 31, 455–465. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Paula, G.R.; Morelli, M.R. Effect of boric acid content on the properties of magnesium phosphate cement. Constr. Build. Mater. 2019, 214, 557–564. [Google Scholar] [CrossRef]
- Formosa, J.; Chimenos, J.M.; Lacasta, A.M.; Niubo, M. Interaction between low-grade magnesium oxide and boric acid in chemically bonded phosphate ceramics formulation. Ceram. Int. 2012, 38, 2483–2493. [Google Scholar] [CrossRef]
- Jeon, I.K.; Qudoos, A.; Kim, H.G. Influence of carbonation curing on hydration and microstructure of magnesium potassium phosphate cement concrete. J. Build. Eng. 2021, 38, 102203. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, H.; Li, Y.; Wu, C.; Dong, J.; Wen, J. Magnesium potassium phosphate cement prepared by the byproduct of magnesium oxide after producing Li2CO3 from salt lakes. Ceram. Int. 2014, 40, 13543–13551. [Google Scholar] [CrossRef]
- Chen, W.; Wu, C.; Yu, H.; Chen, Y.; Zheng, S. Effect of Calcined MgO-rich Byproduct from the Extraction of Li2CO3 on the Performance of Magnesium Phosphate Cement. J. Adv. Concr. Technol. 2017, 15, 749–759. [Google Scholar] [CrossRef][Green Version]
- Dong, J.; Xiao, X.; Li, Y.; Wen, J.; Zheng, W.; Cheng, C.; Yu, H. The influences of raw materials mass ratio on properties and microstructure of salt lake magnesium potassium phosphate cement. Mater. Rep. B 2020, 34, 10041–10045. [Google Scholar]
- Lahalle, H.; Coumes, C.C.D.; Mercier, C.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Influence of the w/c ratio on the hydration process of a magnesium phosphate cement and on its retardation by boric acid. Cem. Concr. Res. 2018, 109, 159–174. [Google Scholar] [CrossRef]
- Hou, D.; Yan, H.; Zhang, J.; Wang, P.; Li, Z. Experimental and computational investigation of magnesium phosphate cement mortar. Constr. Build. Mater. 2016, 112, 331–342. [Google Scholar] [CrossRef]
- Xu, B.; Winnefeld, F.; Kaufmann, J.; Lothenbach, B. Influence of magnesium-to-phosphate ratio and water-to-cement ratio on hydration and properties of magnesium potassium phosphate cements. Cem. Concr. Res. 2019, 123, 105781. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Chen, B. Experimental study of magnesia and M/P ratio influencing properties of magnesium phosphate cement. Constr. Build. Mater. 2014, 65, 177–183. [Google Scholar] [CrossRef]
- Graeser, S.; Postl, W.; Bojar, H.P.; Berlepsch, P.; Armbruster, T.; Raber, T.; Ettinger, K.; Walter, F. Struvite-(K), KMgPO4·6H2O, the potassium equivalent of struvite—A new mineral. Eur. J. Miner. 2008, 20, 629–663. [Google Scholar] [CrossRef]
- Sugama, T.; Kukacka, L.E. Characteristics of magnesium polyphosphate cements derived from ammonium polyphosphate solutions. Cem. Concr. Res. 1983, 13, 499–506. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, Z.; Liu, C.; Gao, Y.; Qi, Y. Properties of red mud blended with magnesium phosphate cement paste: Feasibility of grouting material preparation. Constr. Build. Mater. 2020, 260, 119704. [Google Scholar] [CrossRef]
- Xu, B.; Lothenbach, B.; Winnefeld, F. Influence of wollastonite on hydration and properties of magnesium potassium phosphate cements. Cem. Concr. Res. 2020, 131, 106012. [Google Scholar] [CrossRef]
- Gardner, L.J.; Walling, S.A.; Corkhill, C.L.; Bernal, S.A.; Lejeune, V.; Stennett, M.C.; Provis, J.L.; Hyatt, N.C. Temperature transformation of blended magnesium potassium phosphate cement binders. Cem. Concr. Res. 2021, 141, 106332. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Danilov, S.S.; Belova, K.Y.; Rodionova, A.A.; Vinokurov, S.E. Optimization of the Solidification Method of High-Level Waste for Increasing the Thermal Stability of the Magnesium Potassium Phosphate Compound. Energies 2020, 13, 3789. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-based cements: A journey of 150years, and cements for the future. Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]
- Zheng, D.; Ji, T.; Wang, C.; Sun, C.; Lin, X.; Hossain, K.M.A. Effect of the combination of fly ash and silica fume on water resistance of Magnesium-Potassium Phosphate Cement. Constr. Build. Mater. 2016, 106, 415–421. [Google Scholar] [CrossRef]
- Qian, Z.; Yu, B.; Zhou, S.; Qiao, Y.; Liu, X.; Wang, S.; Qin, Q.; Ma, J.; Wu, X. Influence of silica fume on radioactive fluoride concentrate liquid waste solidified by magnesium potassium phosphate cement. J. Radioanal. Nucl. Chem. 2020, 324, 1013–1019. [Google Scholar] [CrossRef]
- Shi, C.; Yang, J.; Yang, N.; Chang, Y. Effect of waterglass on water stability of potassium magnesium phosphate cement paste. Cem. Concr. Compos. 2014, 53, 83–87. [Google Scholar] [CrossRef]
- Bouaoun, I.; Hammi, H.; Aït-Mokhtar, A.; Hamami, A.E.; M’Nif, A. Effect of calcination temperature of magnesium silicate on the properties of magnesium phosphate cement. J. Aust. Ceram. Soc. 2017, 53, 351–359. [Google Scholar] [CrossRef]
- Zheng, W.; Xiao, X.; Chang, C.; Dong, J.; Wen, J.; Huang, Q.; Zhou, Y.; Li, Y. Characterizing properties of magnesium oxychloride cement concrete pavement. J. Cent. South Univ. 2019, 26, 3410–3419. [Google Scholar] [CrossRef]
- Zheng, W.; Xiao, X.; Wen, J.; Chang, C.; An, S.; Dong, J. Water-to-Cement Ratio of Magnesium Oxychloride Cement Foam Concrete with Caustic Dolomite Powder. Sustainability 2021, 13, 2429. [Google Scholar] [CrossRef]
- Ma, H.; Xu, B.; Li, Z. Magnesium potassium phosphate cement paste: Degree of reaction, porosity and pore structure. Cem. Concr. Res. 2014, 65, 96–104. [Google Scholar] [CrossRef]
- Liu, R.; Yang, Y.; Sun, S. Effect of M/P and Borax on the Hydration Properties of Magnesium Potassium Phosphate Cement Blended with Large Volume of Fly Ash. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 33, 1159–1167. [Google Scholar] [CrossRef]
- Lahalle, H.; Céline, C.D.C.; Mesbah, A.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Investigation of magnesium phosphate cement hydration in dilutedsuspension and its retardation by boric acid. Cem. Concr. Res. 2016, 87, 77–86. [Google Scholar] [CrossRef]
- Gardner, L.J.; Bernal, S.A.; Walling, S.A.; Corkhill, C.L.; Provis, J.L.; Hyatt, N.C. Characterization of magnesium potassium phosphate cements blended with fly ash and ground granulated blastfurnace slag. Cem. Concr. Res. 2015, 74, 78–87. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Li, L.; Han, J.; Zhang, B.; Hou, D.; Wang, J.; Lin, C. A preliminary study of the properties of potassium phosphate magnesium cement-based grouts admixed with metakaolin, sodium silicate and bentonite. Constr. Build. Mater. 2020, 262, 119893. [Google Scholar] [CrossRef]


| Component | Mg2+ | Ca2+ | B2O3 | SiO2 | Al3+ | Fe2+ | K+ | Li+ | Na+ | Cl- | SO42- | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 39.55 | 0.58 | 4.22 | 0.029 | 0.022 | 0.21 | 0.044 | 0.019 | 0.12 | 0.88 | 0.32 | 25.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Dong, J.; Wen, J.; Chang, C.; Xiao, X. Effects of Water-to-Cement Ratios on the Properties of Magnesium Potassium Phosphate Cement Prepared with Lithium-Extracted Magnesium Residue. Appl. Sci. 2021, 11, 4193. https://doi.org/10.3390/app11094193
Zheng W, Dong J, Wen J, Chang C, Xiao X. Effects of Water-to-Cement Ratios on the Properties of Magnesium Potassium Phosphate Cement Prepared with Lithium-Extracted Magnesium Residue. Applied Sciences. 2021; 11(9):4193. https://doi.org/10.3390/app11094193
Chicago/Turabian StyleZheng, Weixin, Jinmei Dong, Jing Wen, Chenggong Chang, and Xueying Xiao. 2021. "Effects of Water-to-Cement Ratios on the Properties of Magnesium Potassium Phosphate Cement Prepared with Lithium-Extracted Magnesium Residue" Applied Sciences 11, no. 9: 4193. https://doi.org/10.3390/app11094193
APA StyleZheng, W., Dong, J., Wen, J., Chang, C., & Xiao, X. (2021). Effects of Water-to-Cement Ratios on the Properties of Magnesium Potassium Phosphate Cement Prepared with Lithium-Extracted Magnesium Residue. Applied Sciences, 11(9), 4193. https://doi.org/10.3390/app11094193





