Single-Stage Fractionation of Vine Shoots Using Microwave Heating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Pretreated Vine Shoots
2.2. Fractionation Treatments
2.3. Refining of the Aqueous Phase from Fractionation Treatment
2.3.1. Membrane Processing
- DDVR of AqO was performed using a Millipore membrane of 1 kDa molecular weight cut-off (MWCO). Water was added at a volumetric ratio of 2.33 mL/mL AqO, and operation was continued until the volume of retentate 1 (denoted R1) corresponded to 38% of the feed volume;
- Concentration of the solution coming from the enzymatically processing of R1 (denoted E) was performed using a 0.3 kDa MWCO membrane (GE Osmonics Inc., Minnetonka, MN, USA). Operation continued until the volume of retentate (denoted R2) corresponded to 66% of R1.
2.3.2. Enzymatic Processing
2.4. Enzymatic Hydrolysis of the Processed Solid from Fractionation Treatment
2.5. Analytical Procedures
- HPLC, to assess the type and amount of OS structural units and substituents;
- High performance size exclusion chromatography (HPSEC), to assess the DP distribution of OS;
- High performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD), to provide a quantitative assessment on the small-DP OS derived from hemicelluloses;
- Matrix-assisted laser desorption and ionization time of flight mass spectrometry (MALDI-TOF-MS) to elucidate the DP and substitution pattern of OS composed of backbones containing between 3 and 7 anhydroxylose units.
3. Results and Discussion
3.1. Composition of Water-Extracted Vine Shoots
3.2. Fractionation
3.3. Refining of OS
3.4. Characterization of Oligosaccharides from Vine Shoot Hemicelluloses
3.5. Enzymatic Hydrolysis of the Processed Solid from Fractionation Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- OIV, International Organisation of Vine and Wine. Statistical Report of World Vitiviniculture. 2019. Available online: https://www.oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf (accessed on 2 July 2021).
- Nabais, J.M.V.; Laginhas, C.; Carrott, P.J.M.; Carrott, M.M.L.R. Thermal conversion of a novel biomass agricultural residue (vine shoots) into activated carbon using activation with CO2. J. Anal. Appl. Pyrolysis 2010, 87, 8–13. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Vine-shoot waste aqueous extracts for re-use in agriculture obtained by different extraction techniques: Phenolic, volatile, and mineral compounds. J. Agric. Food Chem. 2014, 62, 10861–10872. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Maciel, T.C.; Gudiña, E.; Miguel, E.C.; Rodrigues, L.R.; Rodrigues, S. Multivariate analysis as a tool for selecting the vine pruning pretreatment towards the highest enzymatic hydrolysis yield. Biomass Bioenergy 2020, 140, 105653. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Rivas, S.; Moldes, A.B.; Domínguez, J.M. Simultaneous lactic acid and xylitol production from vine trimming wastes. J. Sci. Food Agric. 2007, 87, 1603–1612. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, B.; Alonso, J.L.; Labidi, J.; Gullón, P. Vine shoots as a new source for the manufacture of prebiotic oligosaccharides. Carbohydr. Polym. 2019, 207, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Buratti, C.; Barbanera, M.; Lascaro, E. Ethanol production from vineyard pruning residues with steam explosion pretreatment. Environ. Prog. Sustain. Energy 2015, 34, 802–809. [Google Scholar] [CrossRef]
- Jesús, M.S.; Romaní, A.; Genisheva, Z.; Teixeira, J.A.; Domingues, L. Integral valorization of vine pruning residue by sequential autohydrolysis stages. J. Clean. Prod. 2017, 168, 74–86. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Rodríguez, N.; García-Bernet, D.; Domínguez, J.M. Effects of enzymatic hydrolysis and ultrasounds pretreatments on corn cob and vine trimming shoots for biogas production. Bioresour. Technol. 2016, 221, 130–138. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, P.; Andrés, M.A.; Labidi, J. Coproduction of lignin and glucose from vine shoots by ecofriendly strategies: Toward the development of an integrated biorefinery. Bioresour. Technol. 2017, 23, 328–337. [Google Scholar] [CrossRef]
- Ginni, G.; Kavitha, S.; Yukesh Kannah, R.; Bhatia, S.K.; Adish Kumar, S.; Rajkumar, M.; Kumar, G.; Pugarzhendhi, A.; Chi, N.L.T.; Rajesh Banu, J. Valorization of agricultural residues: Different biorefinery Routes. J. Environ. Chem. Eng. 2021, 9, 105435. [Google Scholar] [CrossRef]
- Rivas, S.; Rigual, V.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Parajó, J.C.; Rodriguez, F. A biorefinery strategy for the manufacture and characterization of oligosaccharides and antioxidants from poplar hemicelluloses. Food. Bioprod. Process. 2020, 123, 398–408. [Google Scholar] [CrossRef]
- Rivas, S.; Moure, A.; Parajó, J.C. Pretreatment of hazelnut shells as a key strategy for the solubilization and valorization of hemicelluloses into bioactive compounds. Agronomy 2020, 10, 760. [Google Scholar] [CrossRef]
- Mahmood, H.; Moniruzzaman, M.; Iqbal, T.; Khan, M.J. Recent advances in the pretreatment of lignocellulosic biomass for fuels and value-added products. Curr. Opin. Green Sustain. Chem. 2019, 20, 18–24. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for the efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122774. [Google Scholar] [CrossRef] [PubMed]
- Dávila, I.; Robles, E.; Andrés, M.A.; Gullón, P. Delignification alternatives of spent solid from autohydrolysis of vine shoots. Chem. Eng. Trans. 2017, 57, 85–90. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Matsakas, L.; Rova, U.; Christakopoulos, P. Recent advances in organosolv fractionation: Towards biomass fractionation technology of the future. Bioresour. Technol. 2020, 306, 123189. [Google Scholar] [CrossRef] [PubMed]
- Teramura, H.; Sasaki, K.; Oshima, T.; Matsuda, F.; Okamoto, M.; Shirai, T.; Kawaguchi, H.; Ogino, C.; Hirano, K.; Sazuka, T.; et al. Organosolv pretreatment of sorghum bagasse using a low concentration of hydrophobic solvents such as 1-butanol or 1-pentanol. Biotechnol. Biofuels 2016, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teramura, H.; Sasaki, K.; Oshima, T.; Kawaguchi, H.; Ogino, C.; Sazuka, T.; Kondo, A. Effective usage of sorghum bagasse: Optimization of organosolv pretreatment using 25% 1-butanol and subsequent nanofiltration membrane separation. Bioresour. Technol. 2018, 252, 157–164. [Google Scholar] [CrossRef]
- Schmetz, Q.; Teramura, H.; Morita, K.; Oshima, T.; Richel, A.; Ogino, C.; Kondo, A. Versatility of a dilute acid/butanol pretreatment investigated on various lignocellulosic biomasses to produce lignin, monosaccharides and cellulose in distinct phases. ACS Sustain. Chem. Eng. 2019, 7, 11069–11079. [Google Scholar] [CrossRef] [Green Version]
- Romo, J.E.; Bollar, N.V.; Zimmermann, C.J.; Wettstein, S.G. Conversion of sugars and biomass to furans using heterogeneous catalysts in biphasic solvent systems. ChemCatChem 2018, 10, 4805–4816. [Google Scholar] [CrossRef] [Green Version]
- Gunasekaran, V.; Ramesh, S.; Sathiasivan, K.; Shankar, M.; Rajesh, M.; Tamilarasan, K. Simultaneous organosolv pretreatment and detoxification of agro-biomass for efficient lignin extraction and characterization. Chem. Pap. 2019, 74, 273–283. [Google Scholar] [CrossRef]
- Chin, D.W.K.; Lim, S.; Pang, Y.L. Fundamental review of organosolv pretreatment and its challenges in emerging consolidated bioprocessing. Biofuels Bioprod. Biorefining 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Romaní, A.; Larramendi, A.; Yáñez, R.; Cancela, A.; Sánchez, A.; Teixeira, J.A.; Domingues, L. Valorization of Eucalyptus nitens bark by organosolv pretreatment for the production of advanced biofuels. Ind. Crop. Prod. 2019, 132, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moure, A.; Gullón, P.; Domínguez, H.; Parajó, J.C. Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals. Process. Biochem. 2006, 41, 1913–1923. [Google Scholar] [CrossRef]
- Vegas, R.; Alonso, J.L.; Domínguez, H.; Parajó, J.C. Enzymatic processing of rice husk autohydrolysis products for obtaining low molecular weight oligosaccharides. Food Biotechnol. 2008, 22, 31–46. [Google Scholar] [CrossRef]
- Gómez, B.; Míguez, B.; Veiga, A.; Parajó, J.C.; Alonso, J.L. Production, purification and in vitro evaluation of the prebiotic potential of arabinoxylooligosaccharides from brewer’s spent grain. J. Agric. Food Chem. 2015, 63, 8429–8438. [Google Scholar] [CrossRef]
- Sun, F.F.; Hong, J.; Hu, J.; Saddler, J.N.; Fang, X.; Zhang, Z.; Shen, S. Accesory enzymes influence cellulose hydrolysis of the model substrate and the realistic lignocellulosic biomass. Enzym. Microb. Technol. 2015, 79, 42–48. [Google Scholar] [CrossRef]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; NREL/TP-510-42619, Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42619.pdf (accessed on 28 June 2021).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618, Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2012. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 28 June 2021).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; NREL/TP-510-42622, Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42622.pdf (accessed on 26 June 2021).
- Rivas, S.; Conde, E.; Moure, A.; Domínguez, H.; Parajó, J.C. Characterization, refining and antioxidant activity of saccharides derived from hemicelluloses of wood and rice husks. Food Chem. 2013, 141, 495–502. [Google Scholar] [CrossRef]
- Gullón, P.; González-Muñoz, M.J.; van Gool, M.P.; Schols, H.A.; Hirsch, J.; Ebringerová, A.; Parajó, J.C. Production, refining, structural characterization and fermentability of rice husk xylooligosaccharides. J. Agric. Food Chem. 2010, 58, 3632–3641. [Google Scholar] [CrossRef] [PubMed]
- Rivas, S.; Gullón, B.; Gullón, P.; Alonso, J.L.; Parajó, J.C. Manufacture and properties of bifidogenic saccharides derived from wood mannan. J. Agric. Food Chem. 2012, 60, 4296–4305. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Kim, T.H.; Kim, J.S. Flow-though pretreatment of corn stover by recycling organosolv to reduce waste solvent. Energies 2018, 11, 879. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, L.; Angulo, V.; Ramos, E.; De la Torre, M.J.; Ferrer, J.L. Comparison of various pulping processes for producing pulp from vine shoots. Ind. Crops Prod. 2006, 23, 122–130. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Q.; Si, C.-L.; Wang, Z.; Huo, D.; Hong, Y.; Li, Z. Recovery of oligosaccharides from prehydrolysis liquors of poplar by microfiltration/ultrafiltration membranes and anion Exchange resin. ACS Sustain. Chem. Eng. 2016, 4, 937–943. [Google Scholar] [CrossRef]
- Rivas, S.; Santos, V.; Parajo, J.C. Aqueous fractionation of hardwood: Selective glucuronoxylan solubilisation and purification of the reaction products. J. Chem. Technol. Biotechnol. 2016, 92, 367–374. [Google Scholar] [CrossRef]
- Kiran, E.U.; Akpinar, O.; Bakir, U. Improvement of enzymatic xylooligosaccharides production by the co-utilization of xylans from different origins. Food Bioprod. Process. 2013, 91, 565–574. [Google Scholar] [CrossRef]
- López, L.; Rivas, S.; Moure, A.; Vila, C.; Parajó, J.C. Development of Pretreatment Strategies for the Fractionation of Hazelnut Shells in the Scope of Biorefinery. Agronomy 2020, 10, 1568. [Google Scholar] [CrossRef]
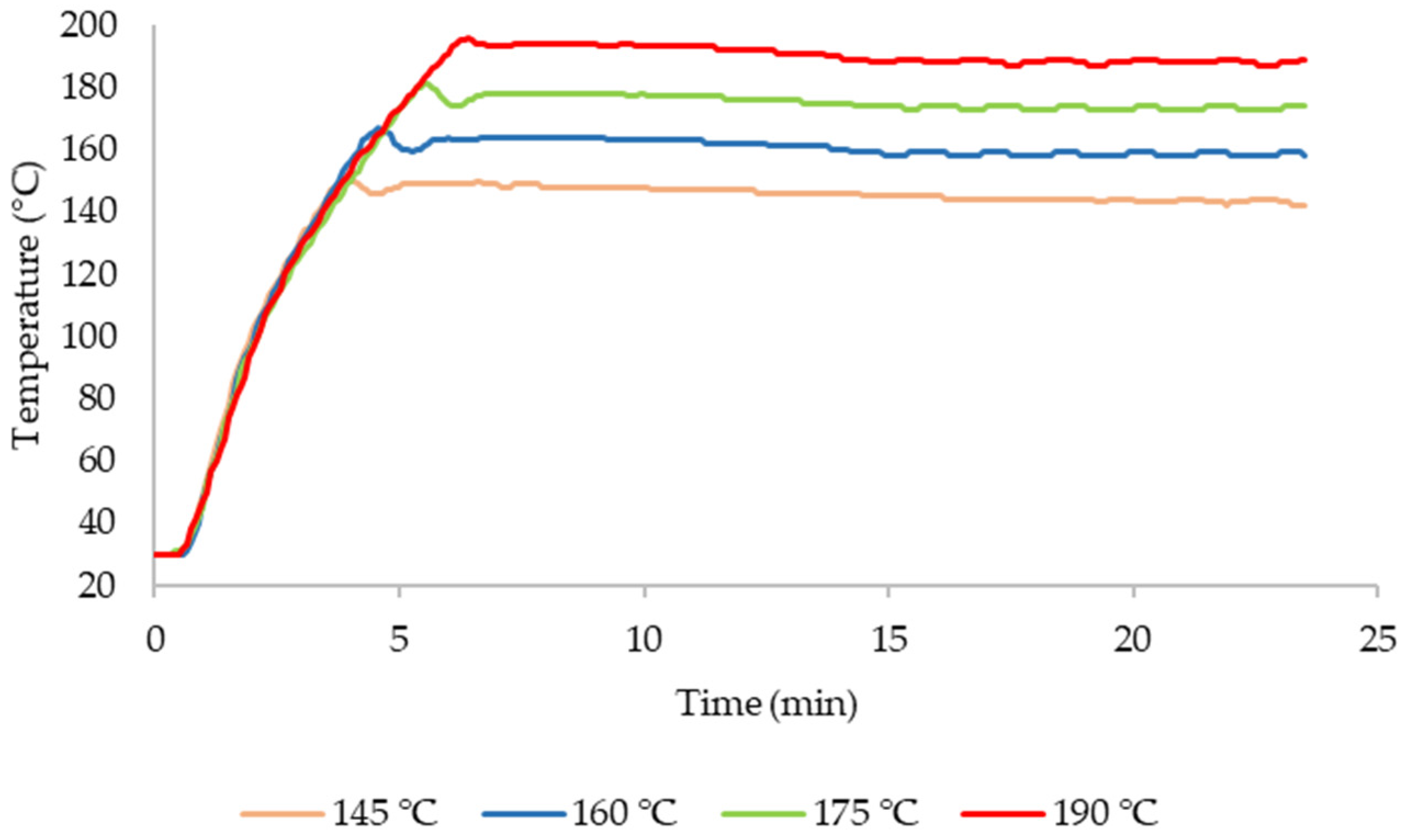
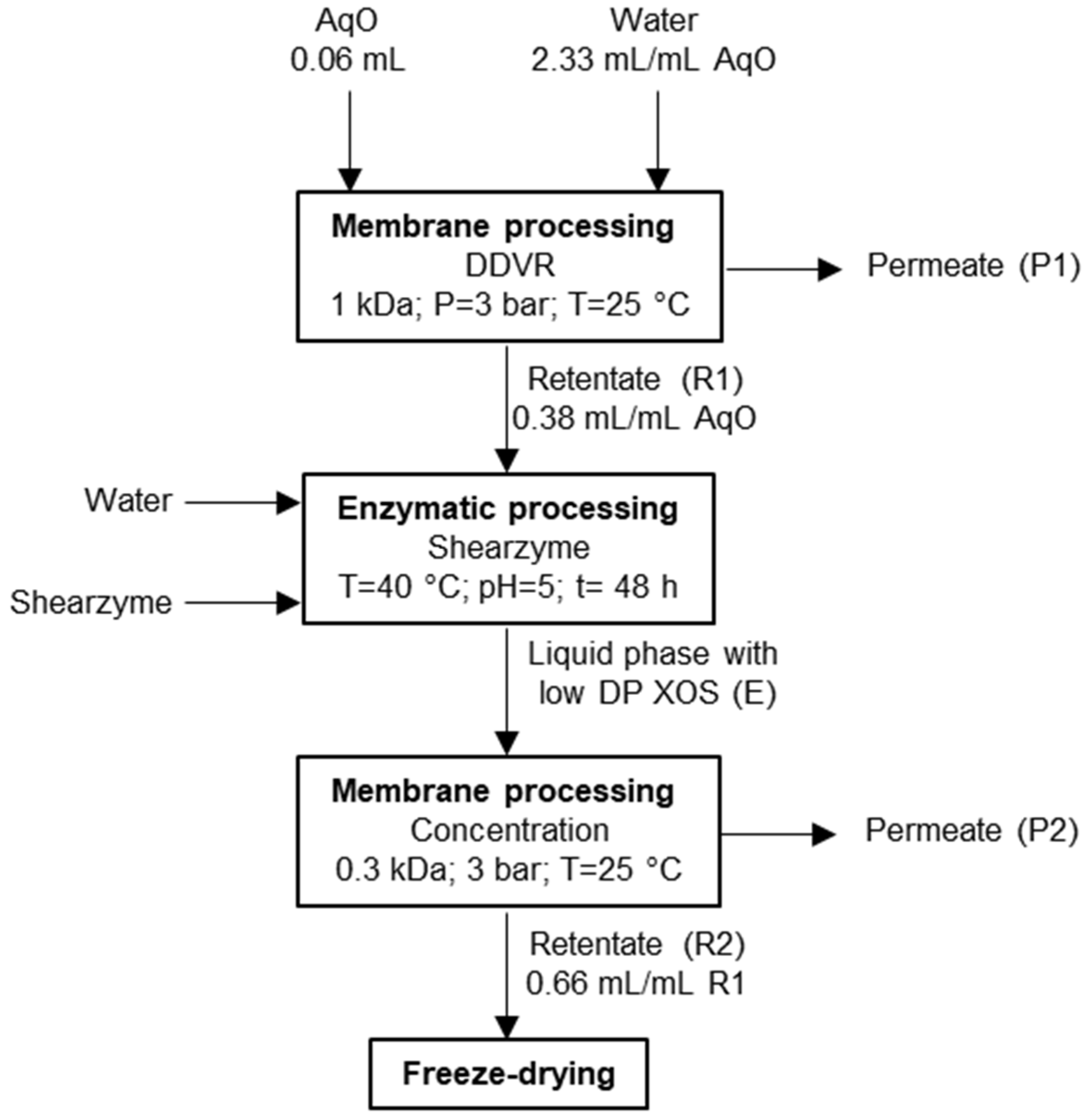
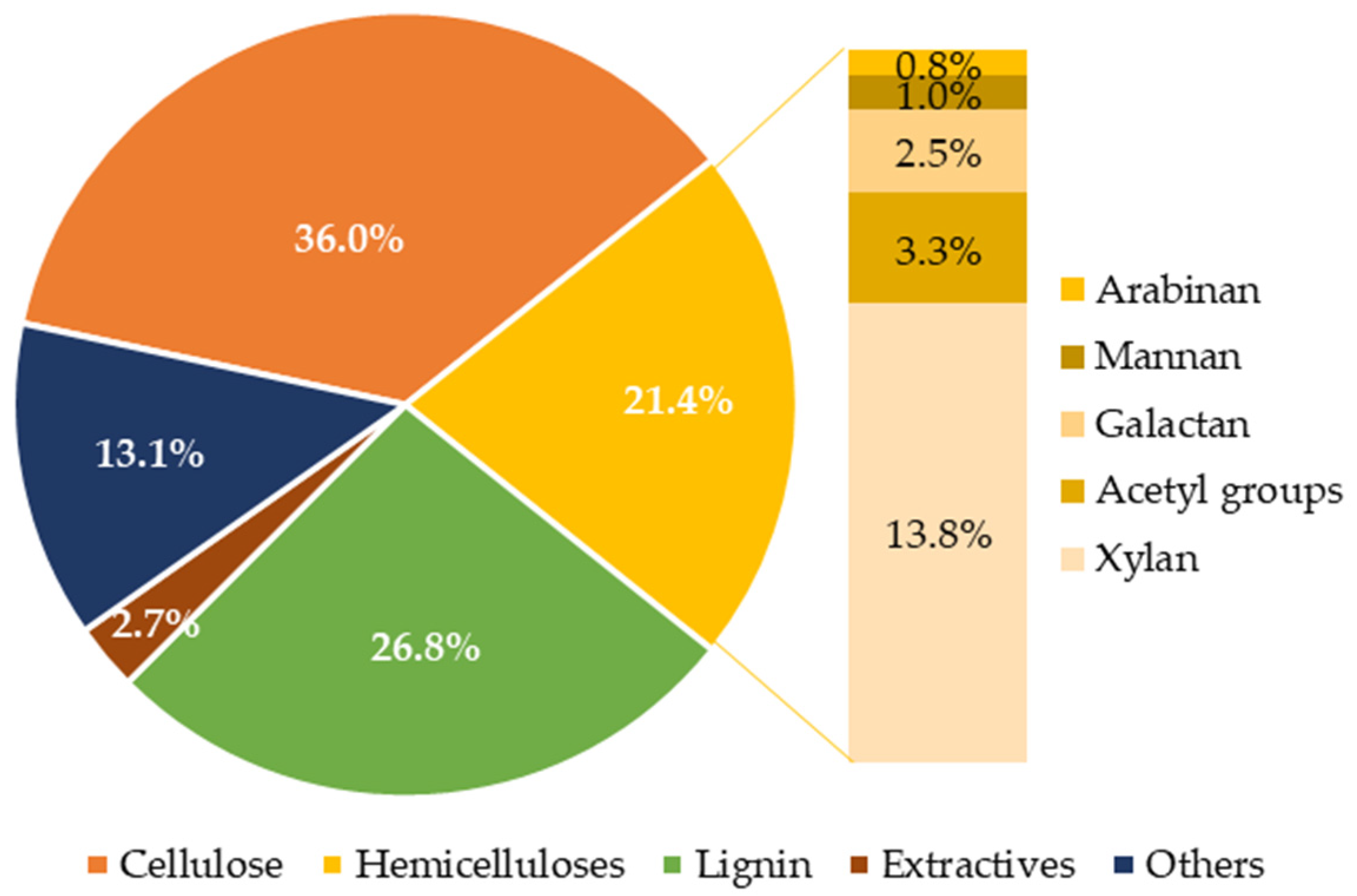
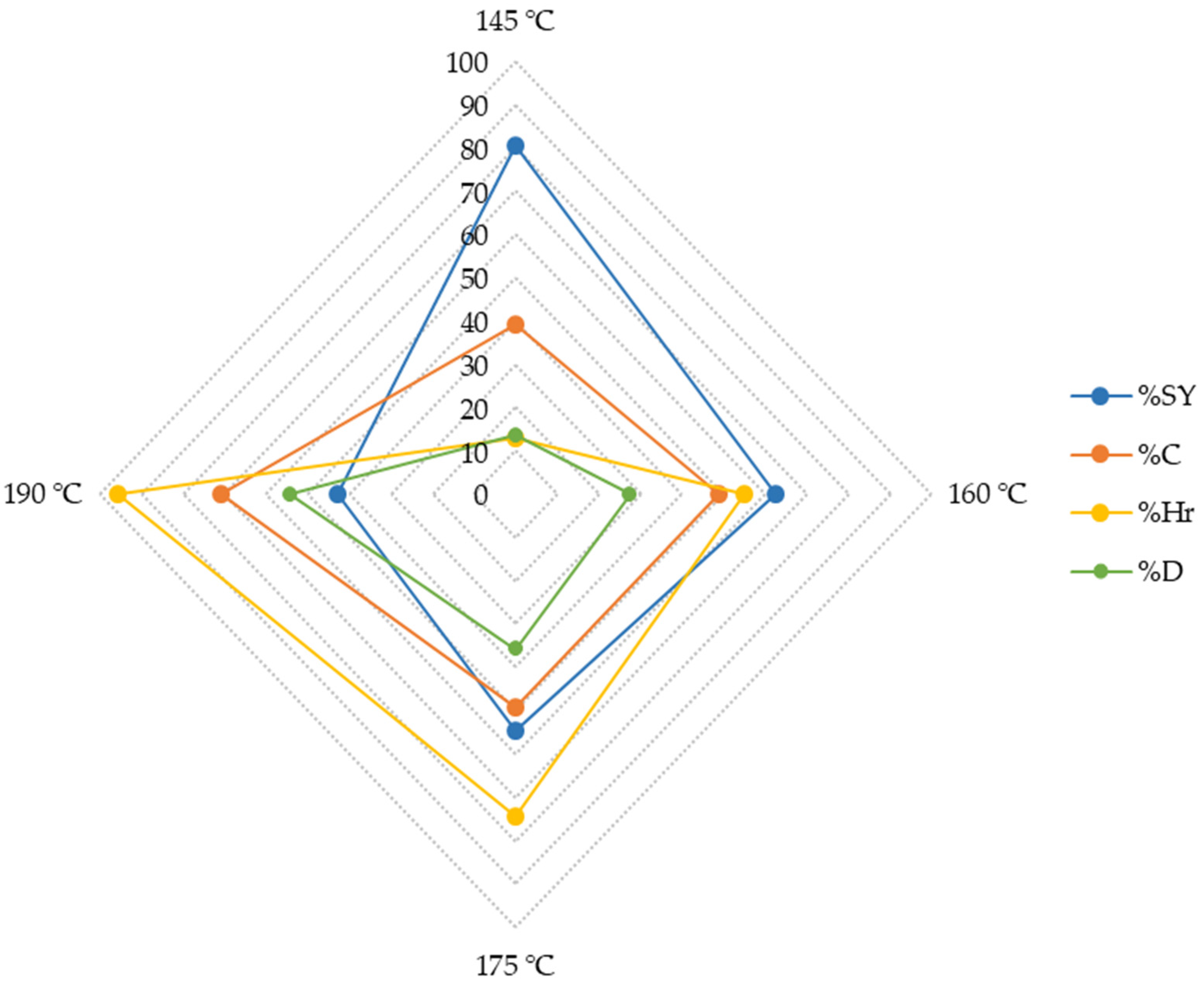
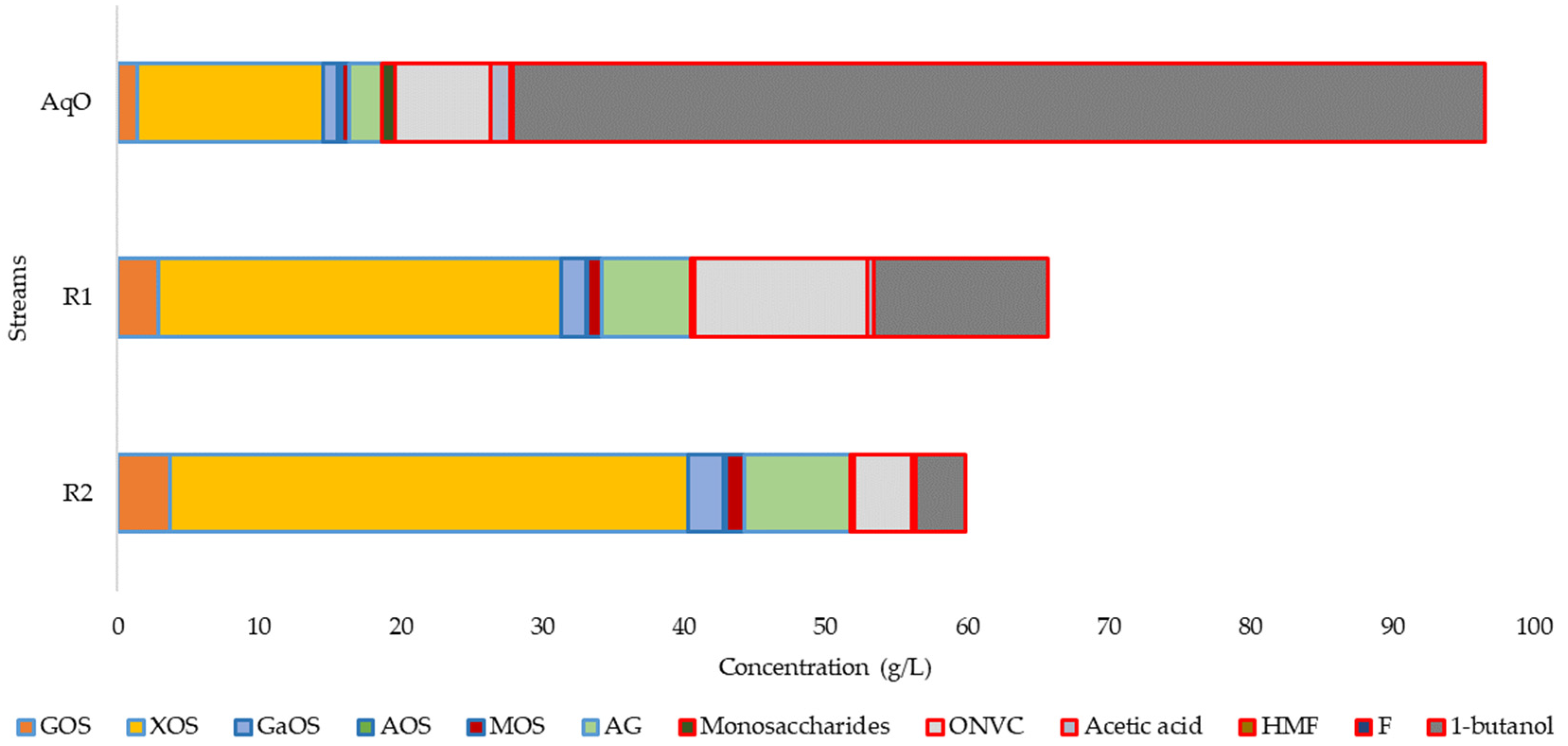
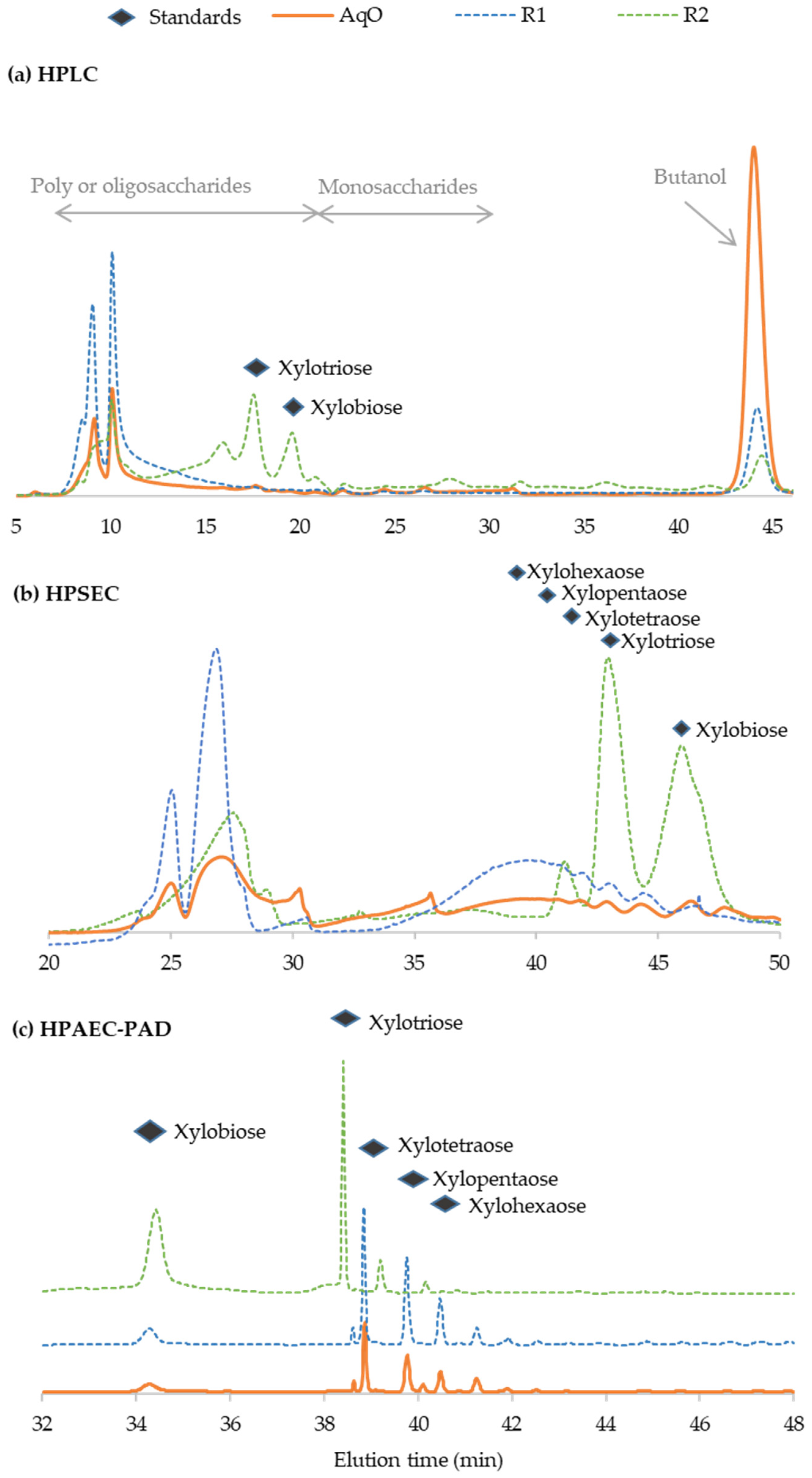
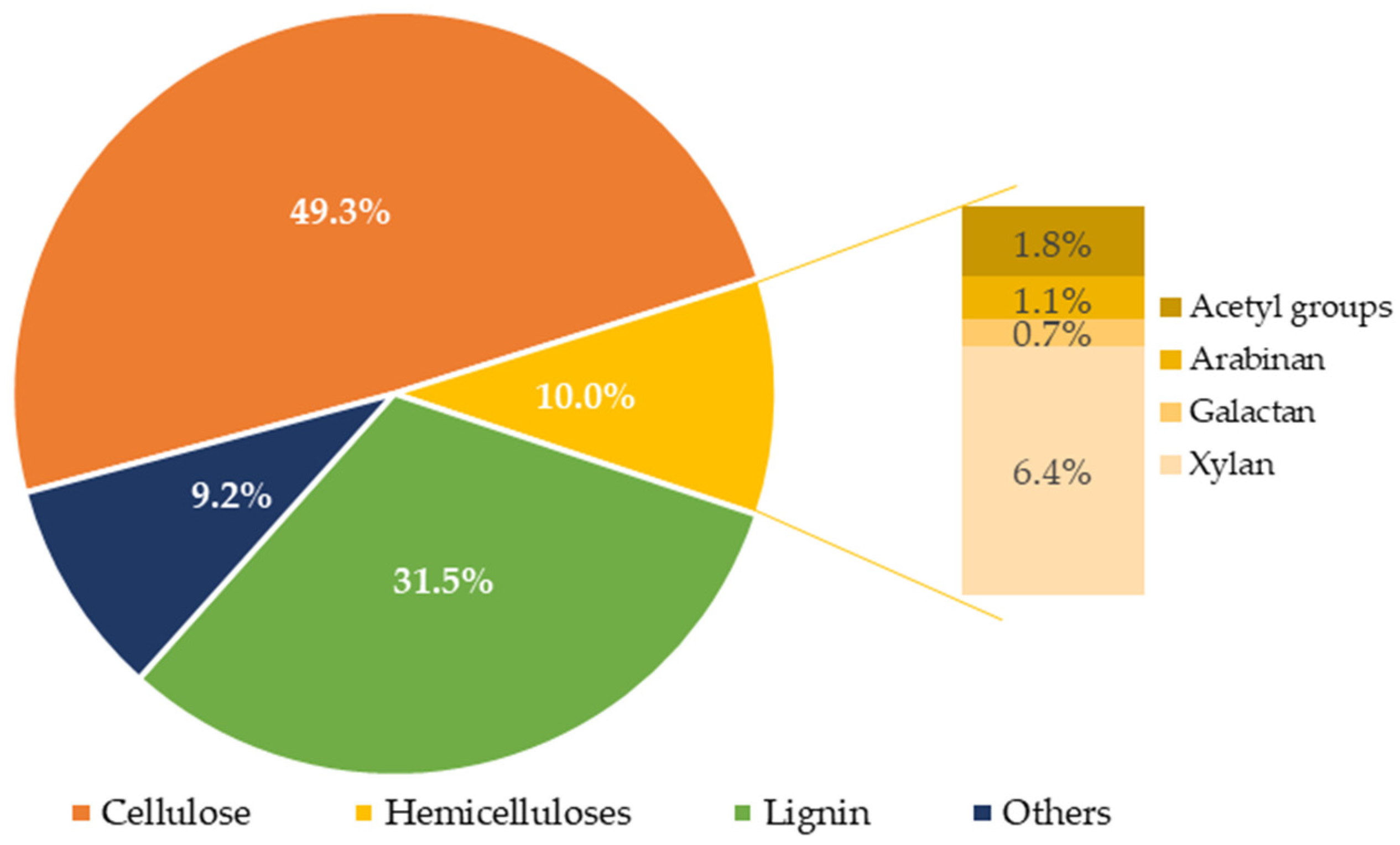
| Composition (g/L) | 145 °C | 160 °C | 175 °C | 190 °C |
|---|---|---|---|---|
| NVC | ||||
| Glucose | 0.07 ± 0.00 | 0.10 ± 0.01 | 0.23 ± 0.01 | 0.38 ± 0.02 |
| Xylose | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.21 ± 0.01 | 3.56 ± 0.21 |
| Galactose | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.30 ± 0.02 | 0.00 ± 0.00 |
| Arabinose | 0.18 ± 0.02 | 0.25 ± 0.02 | 0.25 ± 0.01 | 0.00 ± 0.00 |
| Mannose | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| GOS | 4.32 ± 0.22 | 4.42 ± 0.25 | 1.44 ± 0.10 | 1.79 ± 0.08 |
| XOS | 0.89 ± 0.06 | 7.96 ± 0.09 | 13.1 ± 0.11 | 2.14 ± 0.20 |
| GaOS | 0.97 ± 0.10 | 1.46 ± 0.04 | 1.03 ± 0.04 | 0.00 ± 0.00 |
| AOS | 0.81 ± 0.05 | 0.59 ± 0.03 | 0.25 ± 0.02 | 0.00 ± 0.00 |
| MOS | 0.17 ± 0.02 | 0.10 ± 0.00 | 0.50 ± 0.03 | 0.00 ± 0.00 |
| AG | 0.40 ± 0.02 | 2.20 ± 0.12 | 2.35 ± 0.03 | 1.66 ± 0.08 |
| ONVC | 3.66 ± 0.23 | 5.53 ±0.15 | 6.78 ± 0.22 | 9.57 ± 0.45 |
| Volatile compounds | ||||
| Acetic acid | 0.20 ± 0.02 | 0.71 ± 0.02 | 1.41 ± 0.04 | 2.21 ± 0.09 |
| HMF | 0.00 ± 0.00 | 0.03 ± 0.00 | 0.15 ± 0.02 | 0.28 ± 0.02 |
| F | 0.00 ± 0.00 | 0.04 ± 0.00 | 0.08 ± 0.01 | 0.75 ± 0.05 |
| Glucose Concentration (g/L) | Conversion of Cellulose into Glucose (%) | |||||
|---|---|---|---|---|---|---|
| Time (h) | ESR = 10 | ESR = 15 | ESR = 20 | ESR = 10 | ESR = 15 | ESR = 20 |
| 0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 24 | 16.7 ± 1.1 | 18.9 ± 0.2 | 22.8 ± 0.6 | 44.3 ± 2.0 | 49.9 ± 0.5 | 60.4 ± 1.2 |
| 48 | 22.8 ± 0.4 | 27.2 ± 0.1 | 29.3 ± 0.4 | 60.3 ± 0.8 | 71.9 ± 0.1 | 77.5 ± 0.7 |
| 72 | 28.4 ± 0.1 | 31.5 ± 0.8 | 32.9 ± 0.4 | 75.2 ± 0.2 | 83.5 ± 1.4 | 87.0 ± 0.7 |
| 96 | 31.4 ± 0.5 | 31.3 ± 0.4 | 34.9 ± 0.0 | 83.1 ± 0.9 | 82.8 ± 0.7 | 92.5 ± 0.1 |
| Xylose Concentration (g/L) | Conversion of Xylan into Xylose (%) | |||||
| Time (h) | ESR = 10 | ESR = 15 | ESR = 20 | ESR = 10 | ESR = 15 | ESR = 20 |
| 0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 24 | 2.4 ± 0.1 | 2.6 ± 0.2 | 2.6 ± 0.1 | 53.1 ± 0.6 | 57.5 ± 2.3 | 56.5 ± 0.8 |
| 48 | 3.0 ±0.1 | 3.6 ± 0.1 | 3.3 ± 0.3 | 66.1 ± 1.5 | 77.5 ± 1.5 | 71.6 ± 4.2 |
| 72 | 3.8 ± 0.1 | 4.2 ± 0.0 | 4.1 ± 0.3 | 82.0 ± 1.0 | 90.2 ± 0.4 | 89.2 ± 5.3 |
| 96 | 4.2 ± 0.0 | 4.2 ± 0.2 | 4.3 ± 0.2 | 91.0 ± 0.5 | 91.8 ± 2.4 | 93.6 ± 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas, S.; Parajó, J.C. Single-Stage Fractionation of Vine Shoots Using Microwave Heating. Appl. Sci. 2021, 11, 7954. https://doi.org/10.3390/app11177954
Rivas S, Parajó JC. Single-Stage Fractionation of Vine Shoots Using Microwave Heating. Applied Sciences. 2021; 11(17):7954. https://doi.org/10.3390/app11177954
Chicago/Turabian StyleRivas, Sandra, and Juan Carlos Parajó. 2021. "Single-Stage Fractionation of Vine Shoots Using Microwave Heating" Applied Sciences 11, no. 17: 7954. https://doi.org/10.3390/app11177954
APA StyleRivas, S., & Parajó, J. C. (2021). Single-Stage Fractionation of Vine Shoots Using Microwave Heating. Applied Sciences, 11(17), 7954. https://doi.org/10.3390/app11177954






