Abstract
Allium fistulosum is a perennial plant species grown worldwide belonging to the family Liliaceae. In Korean medicine, it is referred to as Chongbaek (CB), and it is prescribed for symptoms associated with the common cold due to its antipyretic properties. This study examined the effects of aqueous (CBW) and 30% ethanol (CBE) extracts on bone growth using a calcium- and vitamin D-deficient animal model. In an in vitro experiment, the alkaline phosphate activities of the extracts were examined using MC3T3-E1 and MG63 cells, and both the aqueous and ethanolic extracts had significant alkaline phosphate activities. In vivo, a serum analysis indicated that the CB extracts promoted bone growth based on the osteogenic markers ALP, calcium, osteocalcin, and collagen type 1 and increased the bone mineral content (BMC), bone mineral density (BMD), and growth plate length. Overall, our results indicate that both CBW and CBE of A. fistulosum can be utilized to facilitate bone growth and increase BMD in children and adolescents by lengthening the growth plate without adverse side effects, such as metabolic disorders or the release of obesity-inducing hormones.
1. Introduction
Allium fistulosum is a perennial plant species grown worldwide belonging to the family Liliaceae. This perennial herbaceous plant grows to a height of 30–50 cm, has an overall spicy odor, and releases a spicy mucus when cut. The bulb of the plant is known for its medicinal properties, and is known as Chongbaek (CB) in Korea [1]. The Donguibogam, a book of Korean medicine, describes the various uses of CB, including treating chills and fever associated with the common cold, alleviating swelling of the face and eyes from exposure to heavy wind, treating sore throats, stabilizing fetal movements, improving vision, and facilitating circulation throughout the five viscera [2]. Moreover, the detoxifying effect of this plant, specifically its role in removing “evil qi” from the liver, has been recorded. Previous studies have reported the effects of A. fistulosum on obesity [3], cardiovascular disease [4], antioxidant activities [5], nonalcoholic fatty liver [6], anti-influenza viruses [7], and bone growth [8].
In general, growth occurs continuously as a result of growth hormones (GHs) released internally and externally, and the GH increases the cell count in cartilage from birth to adolescence. Growth of the growth plate plays the most crucial role in this process, and an interaction between the zone of proliferation and zone of hypertrophy, which facilitates maturation and enlargement, stimulates longitudinal bone growth [9]. Furthermore, the insulin-like growth factor (IGF), which is present in bone tissue, is activated by various regulatory mechanisms. Transforming growth factor-beta1 (TGF-β1) suppresses osteoblast differentiation by inhibiting IGF-1 expression and downregulating the PI3K/Akt pathway, whereas IGF synthesis induces osteoblast differentiation, including cell replication, collagen synthesis, and matrix attachment [10,11].
Vitamin D mostly acts as a regulator of calcium homeostasis, maintaining a balance between bone formation and resorption, and associates with nutrients and hormones by regulating gene expression transcriptionally. Thus, Vitamin D generally maintains bone health, cell proliferation, differentiation, the immune system, and serum calcium homeostasis by regulating calcium metabolism [12]. Calcium plays an important role in a wide range of biologic functions, either in the form of its free ion or bound complexes. The most important function as bound calcium is in skeletal mineralization. Particularly, in bone, calcium provides skeletal strength and stores to maintain the calcium pools [13]. Calcium in the body serves as a messenger involved in the process of insulin secretion, and vitamin D, which plays an essential role in calcium metabolism, increases IGF-1 and TGF-β [14]. Furthermore, vitamin D is a key factor involved in various immune cells that regulate the onset and prevention of diseases [15,16,17].
In this study, we investigated the efficacy of CB using an animal model with a calcium and vitamin D deficiency, which is closely related to bone weakening. Ko et al. (2019) reported the efficacy of 70% ethanolic extract of CB on bone growth [8]. However, in regard to optimization for ethanolic extraction, no research has been conducted on the efficacy of any other CB extracts on bone growth. Therefore, this study aimed to compare the effects of aqueous and 30% ethanolic extracts of CB on bone growth in children using a calcium- and vitamin D-deficient animal model.
2. Materials and Methods
2.1. Samples and Extractions
The CB extracts used in this study were obtained from raw materials cultivated in Gongju Chungcheongnam-to, South Korea. The samples, which were washed under clean water, were prepared for experiments using only the bulbs. Three kilograms of the chopped samples were extracted in distilled water (3 L) and ethanol (3 L) by using a reflux extractor at 100 and 80–85 °C for 2 h, respectively. The filtered extracts of water and ethanol were evaporated until 40–41 brix, dried, and turned into powder by freeze-drying. The extracts were stored at 4 °C.
2.2. Quantitative Analysis of Marker Compounds Using High-Performance Liquid Chromatography
In our previous research, the five cinnamic acid amides and two decursidate isomers were isolated and fully validated for the standardization of CB using high-performance liquid chromatography (HPLC) [18]. We demonstrated that, among them, the only the trans-isomers were originally nature compounds. Therefore, the four trans-compounds N-trans-coumaroyl tyramine (1), N-trans-feruloyltyramine (2), N-trans-feruloyl-3′-methoxytyramine (3), and N-trans-decursidate (4) were selected for verification of the CB extracts. Standards and samples for HPLC analysis were prepared and proceeded according to the methods reported in the previous literature [18].
2.3. Cell Culture and Treatment
MC3T3-E1 mouse preosteoblast cells (CRL-2595TM, ATCC, Manassas, VA, USA) were used to study the activity of alkaline phosphatase (ALP). The MC3T3-E cells were cultured in 100 cells/100 mm tissue culture dish (Falcon, Corning®, NY, USA) at 37 °C under a 5% CO2 atmosphere in an alpha-modified Eagle medium (α-MEM, Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco, ThermoFisher Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin (Gibco, ThermoFisher Scientific, USA). Differentiation was induced by the addition of 100 nM dexamethasone, 50 μg/mL ascorbic acid, and 10 mM β-glycerophosphate. MG63 human osteosarcoma osteoblast cells (CRL-1427TM, ATCC, Manassas, VA, USA) were used to study the effect of ALP as another cell line. MG63 cells were cultured in tissue culture polystyrene flasks (Falcon, Corning®, NY, USA) at 37 °C in a 5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, ThermoFisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco, Waltham, MA, USA) and 1% penicillin/streptomycin (Gibco, ThermoFisher Scientific, Waltham, MA, USA). Cells were harvested after treatment with 0.25% trypsin-ethylenediaminetetraacetic acid (Gibco, ThermoFisher Scientific, Waltham, MA, USA).
2.4. MTT
Each cell strain was added to the plate at a density of 1 × 104 cells/well and incubated for 24 h. After treatment with distilled water (control) and varying concentrations of CB, the cells were incubated again for 24 h. After removing the media, 50 µL of a serum-free medium and 50 µL of MTT reagent were added to each well using the MTT Assay Kit (ab211091, USA), and the cells were incubated for 3 h. Then, 150 µL of MTT solvent was added, and 15 min later, the absorbance was determined at 590 nm using an ELISA reader (Synergy HTX, BIOTEK, Winooski, VT, USA).
2.5. Alkaline Phosphate Assay
MC3T3-E1 cells were seeded in a 12-well plate at 1 × 103 cells/well and incubated for 24 h. CB extracts (CBW and CBE separately) were added to a differentiation medium containing 10 nM dexamethasone, 10 mM β-calcium glycerophosphate, and 50 μg/mL of ascorbate at varying concentrations (1, 10, and 50 μg/mL) and incubated in an incubator for three days at 5% CO2 and 37 °C. The supernatant was used for the analysis. MG63 cells were added to a 96-well plate at 2 × 104 cells/well and incubated for 24 h. CB extracts (CBW and CBE separately) were added to a differentiation medium containing 10 nM dexamethasone, 10 mM β-calcium glycerophosphate, and 50 μg/mL of ascorbate at varying concentrations (1, 10, and 50 μg/mL) and incubated in an incubator for three days at 5% CO2 and 37 °C. Cell lysates were then used for the analysis. An alkaline phosphatase assay was performed for the two cell lines using an alkaline phosphatase assay kit (Abcam, ab83369, Cambridge, UK), and the absorbance was determined at 405 nm using a multiplate reader. The relationship between the supernatant, lysate protein concentration, and absorbance was formulated based on the absorbance of the standard, and the formula was used to calculate the protein concentration of the supernatant of each group.
2.6. Animal Experiments
Four-week-old C57BL/6 mice (Central Lab Animal, Seoul, Korea) were used for the experiment, and the mice were acclimated to the specific pathogen-free (SPF) experimental animal study center at the Korea Institute of Oriental Medicine (KIOM) for a week with ad libitum feeding. Five-week-old mice acclimated for one week were fed a vitamin D- and calcium-deficient diet for five weeks to reduce bone growth. For the positive control group (Eutropin, 0.2 mg/kg) and CB (CBW; CBE, 150 or 450 mg/kg) groups, the corresponding samples were included in the AIN-76A feeds at the experimental concentrations for ad libitum feeding, and the improvement in reduced bone growth was assessed four weeks later. This experiment was approved by the Institutional Animal Care and Use Committee of the KIOM (KIOM-19-066).
2.7. Serum Analysis
Serum Calcium, ALP, Osteocalcin, Pro-Collagen I-Alpha Analysis
Prior to the autopsy, the mice were anesthetized with avertin, and whole-blood samples were collected from the hearts. The collected whole-blood samples were transferred to a serum separating tube (SST) and centrifuged at 3000 rpm for 10 min, and the supernatant serum was collected for experimental analysis. Leftover serum was stored in an ultra-low temperature freezer, and the serum calcium (Abcam, ab102505, Cambridge, UK), ALP (Abcam, ab83369, Cambridge, UK), osteocalcin (TaKaRa, MK127, Kyoto, Japan), and collagen (pro-collagen I alpha 1, Abcam, ab210579, Cambridge, UK) contents were analyzed by ELISA.
2.8. BMD, BMC, and Growth Plate Analysis
After completing the animal study, the removed femur and tibia samples were used to measure the BMD, BMC, and growth plate thickness. The femoral BMD and BMC contents were measured via dual-energy X-ray absorptiometry (DEXA; Norland pDEXA Sabre; Fort Atkinson, WI, USA) after fixing the sample in 10% neutral formalin for three days and removing muscle and vascular tissues, and the data were analyzed using the Sabre Research (v3.6) software. Growth plate thickness in the tibia was observed by preparing 5 μm paraffin blocks using the protocol described above, followed by pre-treatment, decalcification, embedding, microtome cutting, and staining the blocks with hematoxylin and eosin stain. Thickness was measured using the Cellsens standard Olympus software (v1.16).
2.9. Statistical Analysis
Values expressed as the mean ± SD were subjected to one-way analysis of variance (ANOVA) at a 95% confidence interval. In addition, Tukey’s test was performed using GraphPad Prism version 9.0 for Windows (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Content Comparison of Four Standards in Two Different Extracts by Using HPLC Analysis
As mentioned in the Materials and Methods Section, four trans-isomers were selected for quality comparison standards. These were quantified with aqueous and 30% ethanolic samples. While the 30% ethanolic extracts showed a higher content of the compounds than the aqueous extract, all four compounds were detected in both solvent extracts. The contents differed between the two types of solvents and were calculated as follows: aqueous extractions: 0.1614 (1), 0.6735 (2), 0.4112 (3), and 0.1967 (4); 30% ethanol extractions: 0.2245 (1), 1.1054 (2), 0.6226 (3), and 0.5716 (4). The linear range (mg/mL) of the quantitative curve of the four compounds was 0.0005–0.25, and R2 was >0.9996 (Figure 1 and Table 1).
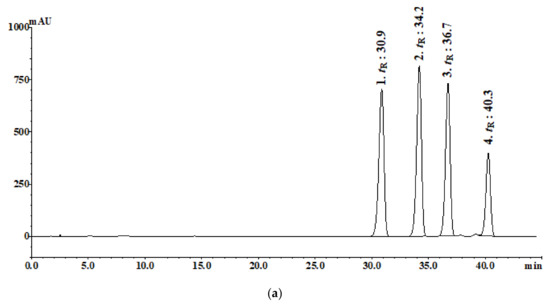
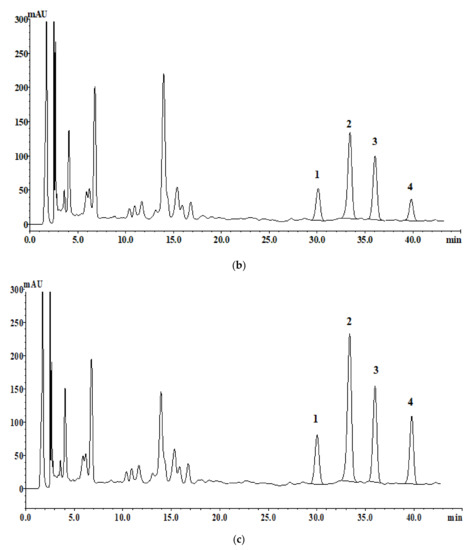
Figure 1.
HPLC chromatograms analysis of the four standards: (a) (0.35 mg/mL), (b) aqueous extraction (100 mg/mL), and (c) 30% ethanolic extraction (100 mg/mL).

Table 1.
Contents of the four compounds in the aqueous and 30% ethanolic extracts of A. fistulosum.
3.2. MTT Assay
The cytotoxicity of CB against the MC3T3-E1 and MG63 cells was determined by the MTT assay. This assay revealed that the viability of the MC3T3-E1 cells increased significantly after 24 h of treatment with the 30% CB ethanolic extract compared to the control group, which was determined by analyzing the serial dilutions (4–125 µg/mL). However, the water extract did not show cytotoxicity (Figure 2a). In addition, in the other MG63 cells, cell viability was not affected by the water and 30% ethanolic extracts (Figure 2b). Therefore, 100 µg/mL was determined to be the optimal concentration for experimental use.
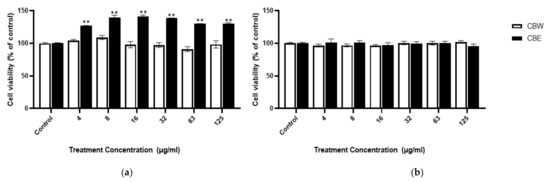
Figure 2.
Cell viability assay: relative cell viability of (a) MC3T3-E1 and (b) MG63 after incubation with CBW and CBE for 24 h. T-test was performed for the control and treatment groups. Data show the mean ± SD (n = 3). ** p < 0.0001 compared with the control group.
3.3. ALP Activity
ALP is an important enzyme in osteoid formation and mineralization and also serves as an osteogenic marker. In this study, the ALP activity was significantly elevated in the normal control, CBW, and CBE groups compared to the control group. Moreover, significant changes were observed in the MC3T3-E1 cells of both the aqueous and ethanolic extracts at 10 and 50 μg/mL (Figure 3a), whereas in the MC63 cells, significant changes were observed in the aqueous and 30% ethanolic extracts at concentrations of 1, 10, and 50 μg/mL (Figure 3b).
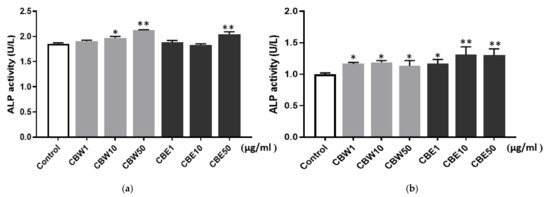
Figure 3.
Alkaline phosphatase (ALP) activity of CBW and CBE in (a) MC3T3-E1 and (b) MG63 cells. The bar represents the mean ± SD (n = 3), and a statistically significant difference was determined between the two groups (* p < 0.05, ** p < 0.01).
3.4. Effect of CB Extractions on Serum Bone Markers
ALP, osteocalcin, calcium, and collagen are bone turnover markers that are included in matricellular proteins and enzymes produced and released from osteoblasts or osteoclasts. In this study, bone metabolism was assessed by measuring these bone resorption markers as well as bone formation. In our examination of changes in the bone markers among the different solvent extracts of CB, high concentrations (450 mg/kg) of the aqueous and 30% ethanolic extracts were observed to produce the most significant changes. In particular, the blood calcium concentration significantly increased in the 30% ethanolic extracts at both low and high concentrations (Figure 4). Overall, these results indicated that the administration of CB extract in a bone growth-suppressed model improved bone growth.
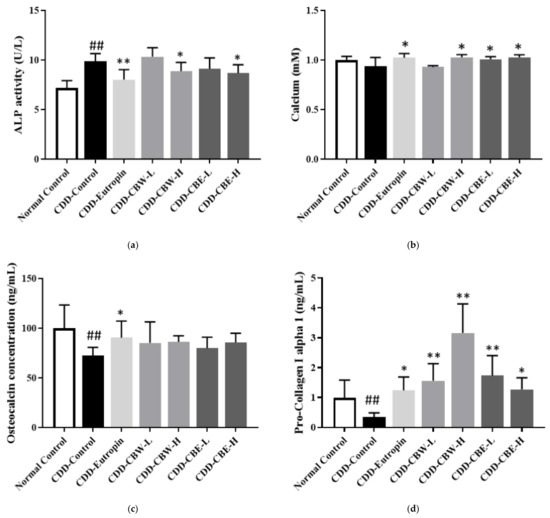
Figure 4.
The serum alkaline phosphatase (ALP) activity, calcium, osteocalcin (OC), and pro-collagen I alpha 1 level: (a) 1/10 serum ALP, (b) 1/20 serum calcium, (c) 1/20 serum OC concentration, and (d) 1/100 serum pro-collagen I alpha 1. All data are presented as the mean ± SD (n = 8 per group). ## p < 0.001 compared with the normal control group, * p < 0.05, and ** p < 0.01 compared with the CDD-control group.
3.5. Effect of CB Extractions on BMC, BMD, and Growth Plate Analysis
BMC and BMD in the femur and tibia were measured using dual-energy X-ray absorptiometry, which revealed that the effects on BMC and BMD were similar to those observed in the positive control group that received a growth hormone injection. Moreover, regarding the changes in BMD, BMC, and bone growth improvement facilitated by CB extracts, the mice given these extracts showed increased BMD and BMC compared to the negative control group that was not administered these extracts. Moreover, these effects were similar to those observed in the positive control group that received somatotropin injections (Figure 5). As shown in Figure 6, thicker growth plates were observed in the mice that were administered the 30% ethanolic extracts of CB.
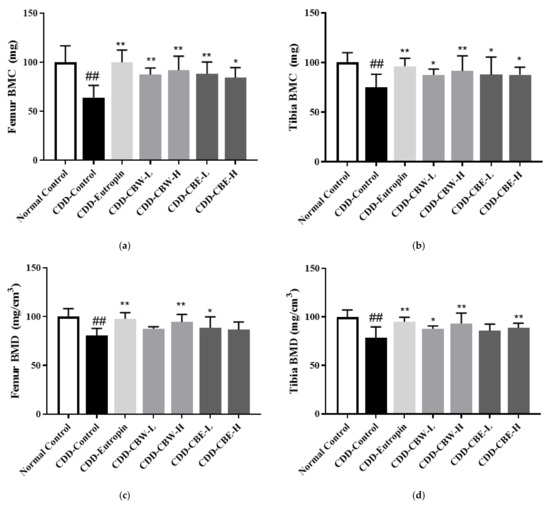
Figure 5.
(a,b) Femur and tibia bone mineral content (BMC) and (c,d) bone mineral areal density (BMD). All data are presented as the mean ± SD (n = 8 in each group). ## p < 0.001 compared with the normal control group, * p < 0.05, and ** p < 0.01 compared with the CDD-control group.
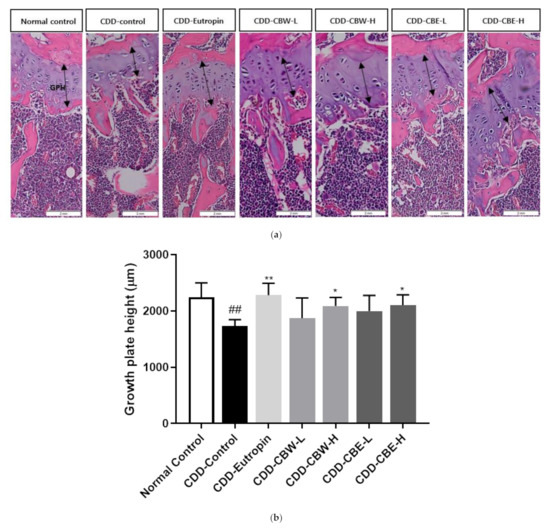
Figure 6.
(a) Morphology of paraffin sections from CBW and CBE in tibial growth plates; magnification, 10X and (b) total growth plate height. The arrow indicates the growth plate height (GPH). All data are presented as the mean ± SD, and differences were compared by one-way ANOVA. ## p < 0.001 compared with the normal control group, * p < 0.05, and ** p < 0.01 compared with the CDD-control group.
4. Discussion
Welsh onion is a known health food because of its rich lipid-soluble vitamins, calcium, potassium, magnesium, and iron contents. Moreover, A. fistulosum (CB), which refers to the white part of this perennial herbaceous plant, is used in Korean medicine to treat symptoms of the common cold due to its antipyretic, antibacterial, and peptogenic effects. A previous study reported that a 70% ethanolic extract of CB improved blood calcium concentration, BMC, BMD, and growth plate length [8]. In an effort to expand the application of CB, we will focus on developing recognized health-functional foods using CB to promote bone growth and maintain or increase BMD in children and adolescents without causing metabolic disorders or obesity in future research. Thus, we further explored whether the bone growth-promoting effects of CB vary depending on the extraction solvent.
In relation to the activation results of the different solvent extracts examined in this study, observations of the content of previously isolated and fully validated compounds in the samples showed that all four compounds were detected in both the aqueous and 30% ethanolic extracts, with a higher content in the ethanolic extracts than the aqueous extracts. This suggests that the activation of each compound requires further study. Compared to the activity results of this study, further data might provide important information regarding the correlation between the compounds and the activity.
Four natural compounds without altered chemical structures (N-trans-coumaroyltyramine, N-trans-feruloyltyramine, N-cis-feruloyl-3’-methoxytyramine, and N-trans-decursidate) from seven characterizing compounds of CB (N-trans-coumaroyltyramine, N-cis-feruloyltyramine, N-trans-feruloyltyramine, N-cis-feruloyl-3’-methoxytyramine, N-trans-feruloyl-3’-methoxytyramine, N-trans-decursidate, and N-cis-decursidate) reported by Hwang et al. [18] were also used. All four compounds were detected in both the aqueous and the 30% ethanolic extracts, with a higher content in the ethanolic extracts. This suggests that the amount of extracted compounds varies depending on the fractionation method used, and further research is required to compare the active ingredients.
In vitro, we analyzed the activities of ALP, an osteogenic biomarker, using MC3T3-E1 and MG63 cells. Our results indicated that the ALP activity was elevated in both the aqueous and the 30% ethanolic extracts of CB. Moreover, we observed that the ALP enzyme increased the activity of early-differentiated cells and mature osteoblasts. It has been reported that herbal extracts such as Astragalus membranaceus, Palmul-tang, and Areca catechu increase ALP activity during early bone formation [19,20,21].
Vitamin D and hormone metabolites act on intestinal mucosal cells and form calcium-binding proteins. These proteins facilitate the absorption of calcium and magnesium and affect phosphorus absorption. Vitamin D metabolites maintain calcium and phosphorus homeostasis along with the parathyroid hormone (PTH) and calcitonin [22]. Moreover, a vitamin D deficiency hinders calcium and phosphorus absorption and metabolism, resulting in inadequate calcification of the bone. Thus, we administered four-week old male mice a calcium- and vitamin D-deficient diet for five weeks to induce nutritional deficiency, followed by high concentrations of aqueous and 30% ethanolic extracts (150 and 450 mg/kg, respectively) of CB for four weeks to determine the effects on bone growth. Eutropin (0.2 mg/kg), which was used as the positive control in this experiment, is a growth hormone agent proven for its biological efficacy and safety, and it is used to treat growth hormone deficiencies. Seven experimental groups of eight mice were administered AIN-76A feed, and for six of these groups, varying concentrations of each extract were added to the feed. Bone growth and resorption are regulated by osteoblasts and osteoclasts, and bone turnover occurs through repeated bone formation and resorption. Moreover, osteocyte differentiation is closely linked to bone matrix protein expression, and its markers include ALP, pro-collagen I alpha 1, osteocalcin, and calcium. In our study, all the administered aqueous and 30% ethanolic extracts of CB stimulated bone growth, with significantly higher stimulations observed in the mice administered high extract concentrations. However, only a trend of increased bone growth was observed for osteocalcin. Furthermore, the normal group and calcium- and vitamin D-deficient diet groups significantly differed in all markers, with the exception of calcium, indicating that the calcium- and vitamin D-deficient diet induced bone growth suppression. Calcium is generally present in abundance in the bones, and a small amount is present in the blood, body fluids, and muscles. Calcium is regulated by PTH, and PTH increases blood calcium concentration by increasing the amount of calcium released into the blood pool. While calcium is released from the bones to the blood by osteoclasts, the same amount of calcium is simultaneously transported from the blood into the bone matrix by osteoblasts. Although the calcium- and vitamin D-deficient diet groups did not show a significant difference in calcium concentration compared to the normal group, these groups tended to have a lower calcium concentration. These results suggest that blood calcium concentration is influenced by repeated bone remodeling processes involving bone formation and resorption.
BMD is the basis of BMC and is a marker of bone health. Compared to the normal group, the calcium- and vitamin D-deficient diet group had significantly reduced BMD and BMC in the femur and tibia, and administering high concentrations of aqueous and ethanolic extracts significantly increased these values.
The growth plate is the site of longitudinal bone growth, which occurs through the process of chondrocyte proliferation and enlargement through cell division in soft cartilage tissues and osteogenic differentiation. Both the aqueous and ethanolic extracts of CB significantly lengthened the growth plate in the tibia compared to the control group.
According to Ko et al. (2019), CB significantly increased the length of the femur and tibia in a growing rat model. While this study used a different solvent, our results were consistent with those of [8].
GHs are known to induce cellular proliferation by directly acting on osteoblasts. In general, the GH increases IGF-1 production in the liver, but considering that the amount of IGF-1 is separately regulated by the GH, it is believed that the GH is not an absolute determinant of local IGF-1 production in the bones and that various factors, such as estrogen, PTH, and cortisol, are involved in the regulation of IGF-1 [11,23]. Previous studies have reported that the GH induces IGF-1 expression in osteoblasts; however, whether the GH stimulates the IGF-1 expression in human osteoblasts remains unknown. IGF-1 activity in the bone is determined by various insulin-like growth factor binding proteins (IGFBPs) and IGF-1 concentrations, and IGFBP-3, IGFBP-4, and IGFBP-5 are generally involved in this process. In this study, we did not examine the osteogenic mechanisms of CB because Ko et al. (2019) confirmed that A. fistulosum stimulates cell growth through TGF-β and Wnt signaling in osteoblasts [8]. A previous study reported that CB dose-dependently increased the smad4 expression in TGF-beta and downregulated DKK-1 expression, which is a negative regulator of TGF-beta, ultimately enhancing TGF-beta signaling and increasing cell division in bones [8]. Furthermore, when LRP5, which is involved in Wnt signaling, is activated, β-catenin is not phosphorylated and shifts from the cytoplasm to the nucleus to increase the expression of Wnt target genes, thereby facilitating the Wnt signaling pathway [24,25]. Moreover, a high concentration of CB has been reported to increase LRP5 and the beta-catenin mRNA expression. To develop the ingredients of CB, we will aim to establish an extraction process for CB, examine the effect of CB on bone growth stimulation, and investigate the mechanisms of IGF1 and TGF-beta related to bones in future research.
Growth, a key characteristic of childhood, refers to a series of processes in which the weight of body organs, height, and body weight quantitatively increase with advancing age. Growth impairment is classified into primary growth disorders caused by skeletal defects; secondary growth disorders caused by environmental factors; idiopathic short stature with normal GH secretion; and no malnutrition, chronic diseases, or endocrine disorders. The GH injection, which is used to treat low stature caused by GH deficiencies in pre-pubertal children, has been widely used for diseases characterized by a short stature [26,27]. However, the high cost and need for advanced testing due to potential blood glucose elevation are some of its drawbacks. For these reasons, the results of this study are highly significant, as they provide the data required to obtain evidence for clinical trials on CB extracts, which can be used to stimulate growth in children and adolescents with fewer adverse reactions.
In conclusion, CBW and CBE of A. fistulosum might contribute to grow longitudinal bone when the effects of BMD and serum level occur in vivo. Therefore, the findings of this study will contribute to the development of a functional food product that stimulates bone growth by enlarging the growth plate and increasing BMD in children and adolescents without inducing metabolic disorders or obesity.
Author Contributions
Conceptualization, B.S.K., J.A.R. and H.J.K.; methodology, H.J.K. and J.A.R.; validation, J.T.H.; writing—original draft preparation, J.A.R.; writing—review and editing, H.J.K., J.T.H. and B.S.K.; supervision, B.S.K.; project administration, B.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the INNOPOLIS Foundation of Korea, grant number 2020-DD-RD-0413 and a National Research Council of Science & Technology (NST) grant from the Korean government (MSIT), grant number CAP-16-07-KIOM.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Animal Care and Use Committee of the Korea Institute of Oriental Medicine (protocol code KIOM-19-066 and date of approval).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are not publicly available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Central Pharmaceutical Affairs Council. The Korean Herbal Pharmacopoeia; Central Pharmaceutical Affairs Council: Seoul, Korea, 2021; p. 523. [Google Scholar]
- Yoon, S.; Kim, H. Donguibogam; Donguibogam Publishing Company: Seoul, Korea, 2006; pp. 297–2189. [Google Scholar]
- Sung, Y.Y.; Yoon, T.; Kim, S.J.; Yang, W.K.; Kim, H.K. Anti-obesity activity of Allium fistulosum L. extract by down-regulation of the expression of lipogenic genes in high-fat diet-induced obese mice. Mol. Med. Rep. 2011, 4, 431–435. [Google Scholar] [CrossRef]
- Chen, J.H.; Tsai, S.J.; Chen, H.I. Welsh onion (Allium fistulosum L.) extracts alter vascular responses in rat aortae. J. Cardiovasc. Pharmacol. 1999, 33, 515–520. [Google Scholar] [CrossRef]
- Harada, K.; Wada, R.; Yaguchi, S.; Maeda, T.; Date, R.; Tokunaga, T.; Kazumura, K.; Shimada, K.; Matsumoto, M.; Wako, T.; et al. Supplementation with Japanese bunching onion (Allium fistulosum L.) expressing a single alien chromosome from shallot increases the antioxidant activity of Kamaboko fish jelly paste in vitro. Biomed. Rep. 2013, 1, 355–358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hwang, J.T.; Shin, E.J.; Chung, M.Y.; Park, J.H.; Chung, S.; Choi, H.K. Ethanol extract of Allium fistulosum inhibits development of non-alcoholic fatty liver disease. Nutr. Res. Pract. 2018, 12, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Miyake, S.; Umetsu, R.; Hayashi, K.; Chijimatsu, T.; Hayashi, T. Anti-influenza A virus effects of fructan from Welsh onion (Allium fistulosum L.). Food Chem. 2012, 134, 2164–2168. [Google Scholar] [CrossRef]
- Ko, B.S.; Ryuk, J.A.; Hwang, J.T.; Zhang, T.; Wu, X.G.; Kim, H.J.; Yi, Q.J.; Park, S. Allium fistulosum (Welsh onion) and Portulaca oleracea increase longitudinal bone growth in weanling rats possibly by promoting TGF-beta and IGF-1 signaling. J. Funct. Foods 2019, 58, 151–160. [Google Scholar] [CrossRef]
- Wu, S.; Yang, W.; De Luca, F. Insulin-Like Growth Factor-Independent Effects of Growth Hormone on Growth Plate Chondrogenesis and Longitudinal Bone Growth. Endocrinology 2015, 156, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, H.; Okada, S.; Saito, A.; Hoshi, K.; Yamashita, H.; Takato, T.; Azuma, T. Inhibition of insulin-like growth factor-1 (IGF-1) expression by prolonged transforming growth factor-beta1 (TGF-beta1) administration suppresses osteoblast differentiation. J. Biol. Chem. 2012, 287, 22654–22661. [Google Scholar] [CrossRef] [PubMed]
- Minuto, F.; Palermo, C.; Arvigo, M.; Barreca, A.M. The IGF system and bone. J. Endocrinol. Investig. 2005, 28, 8–10. [Google Scholar]
- Cantorna, M.T.; McDaniel, K.; Bora, S.; Chen, J.; James, J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp. Biol. Med. (Maywood) 2014, 239, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010, 5, S23–S30. [Google Scholar] [CrossRef] [PubMed]
- An, J.L.; Zhang, W.; Zhang, J.; Lian, L.C.; Shen, Y.; Ding, W.Y. Vitamin D improves the content of TGF-beta and IGF-1 in intervertebral disc of diabetic rats. Exp. Biol. Med. (Maywood) 2017, 242, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Lv, C.; Wang, F.; Gan, K.; Zhang, M.; Tan, W. Modulatory effect of 1,25-dihydroxyvitamin D 3 on IL1 beta -induced RANKL, OPG, TNF alpha, and IL-6 expression in human rheumatoid synoviocyte MH7A. Clin. Dev. Immunol. 2013, 2013, 160123. [Google Scholar] [CrossRef]
- He, R.; Shen, J.; Liu, F.; Zeng, H.; Li, L.; Yu, H.; Lu, H.; Lu, F.; Wu, Q.; Jia, W. Vitamin D deficiency increases the risk of retinopathy in Chinese patients with type 2 diabetes. Diabet. Med. 2014, 31, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Shimo, N.; Yasuda, T.; Kaneto, H.; Katakami, N.; Kuroda, A.; Sakamoto, F.; Takahara, M.; Irie, Y.; Horikawa, K.; Miyashita, K.; et al. Vitamin D deficiency is significantly associated with retinopathy in young Japanese type 1 diabetic patients. Diabetes Res. Clin. Pract. 2014, 106, e41–e43. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Ryuk, J.A.; Kim, H.J.; Jung, D.H.; Ko, B.S. Validation study on the geometric isomers from bulbs of Allium fistulosum and their conversion. Appl. Biol. Chem. 2020, 63, 1–12. [Google Scholar] [CrossRef]
- Pu, X.; Chai, Y.; Guan, L.; Li, W.; Gao, J.; Jiang, Z.; Li, Q.; Wu, Y.; Chen, Y. Astragalus improve aging bone marrow mesenchymal stem cells (BMSCs) vitality and osteogenesis through VD-FGF23-Klotho axis. Int. J. Clin. Exp. Pathol. 2020, 13, 721. [Google Scholar] [PubMed]
- Choi, L.Y.; Kim, M.H.; Nam, Y.K.; Kim, J.H.; Cho, H.-Y.; Yang, W.M. Palmul-Tang, a Korean Medicine, Promotes Bone Formation via BMP-2 Pathway in Osteoporosis. Front. Pharmacol. 2021, 12, 453. [Google Scholar] [CrossRef] [PubMed]
- Meng, K.; Mei, F.; Zhu, L.; Xiang, Q.; Quan, Z.; Pan, F.; Xia, G.; Shen, X.; Yun, Y.; Zhang, C. Arecanut (Areca catechu L.) seed polyphenol improves osteoporosis via gut-serotonin mediated Wnt/β-catenin pathway in ovariectomized rats. J. Funct. Foods 2021, 84, 104598. [Google Scholar] [CrossRef]
- Yi, X.; Sun, J.; Li, L.; Wei, Q.; Qian, Y.; Chen, X.; Ma, L. 1,25-Dihydroxyvitamin D3 Deficiency is Involved in the Pathogenesis of Diabetic Retinopathy in the Uygur Population of China. IUBMB Life 2016, 68, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, B.; Zhao, J.; Pan, W.; Xu, J.; Chen, S. IGF-I induces adipose derived mesenchymal cell chondrogenic differentiation in vitro and enhances chondrogenesis in vivo. In Vitro Cell Dev. Biol. Anim. 2016, 52, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Zhao, J.; Jiang, D. Allicin inhibits oxidative stress-induced mitochondrial dysfunction and apoptosis by promoting PI3K/AKT and CREB/ERK signaling in osteoblast cells. Exp. Ther. Med. 2016, 11, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Zhang, T.; Li, K.; Gao, K. Bone marrow mesenchymal stem cells improve spinal function of spinal cord injury in rats via TGF-β/Smads signaling pathway. Exp. Ther. Med. 2020, 19, 3657–3663. [Google Scholar] [CrossRef] [PubMed]
- Lal, R.A.; Hoffman, A.R. Perspectives on long-acting growth hormone therapy in children and adults. Arch. Endocrinol. Metab. 2020, 63, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Ergun-Longmire, B.; Wajnrajch, M.P. Growth and Growth Disorders. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279142/ (accessed on 20 July 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).