Abstract
Application of wound-healing/dressing biomaterials is amongst the most promising approaches for wound repair through protection from pathogen invasion/contamination, maintaining moisture, absorbing exudates, modulating inflammation, and facilitating the healing process. A wide range of materials are used to fabricate wound-healing/dressing biomaterials. Active wound-healing/dressings are next-generation alternatives for passive biomaterials, which provide a physical barrier and induce different biological activities, such as antibacterial, antioxidant, and proliferative effects. Cellulose-based biomaterials are particularly promising due to their tunable physical, chemical, mechanical, and biological properties, accessibility, low cost, and biocompatibility. A thorough description and analysis of wound-healing/dressing structures fabricated from cellulose-based biomaterials is discussed in this review. We emphasize and highlight the fabrication methods, applied bioactive molecules, and discuss the obtained results from in vitro and in vivo models of cellulose-based wound-healing biomaterials. This review paper revealed that cellulose-based biomaterials have promising potential as the wound-dressing/healing materials and can be integrated with various bioactive agents. Overall, cellulose-based biomaterials are shown to be effective and sophisticated structures for delivery applications, safe and multi-customizable dressings, or grafts for wound-healing applications.
1. Introduction
Skin is the largest organ of the human body, with distinct functions, such as protecting other organs from pathogeneses invasion, dehydration, mechanical impacts, radiation, and chemicals. Skin also serves to regulate body temperature, is an integral part of the sensory system, and acts as a reservoir for Vitamin D synthesis [1,2]. Therefore, any skin damage must be treated appropriately, not only for the visual appearance but also to recover its functions, as mentioned above. Accordingly, specific consideration has been dedicated to developing proper wound-care/healing biomaterials/structures [3]. It is estimated that the global wound-care market reaches 22 billion US dollars by 2022 from the current share of 18.35 billion dollars, with a Global Compound Annual Growth Rate (CAGR) of 3.7% [4]. Moreover, out of various commercial wound-care/biomaterials, wound-dressings are the most prevalent. Different forms of wound-dressing biomaterials have been developed, such as transparent adhesive bandages, medicated bandages, hydrogel dressings, foams dressings, hydrocolloid dressings, and film dressings [5,6,7].
From another perspective, wound-dressings can be categorized as passive, active, and interactive wound-dressings [8]. Passive dressings simply serve as a protective barrier and do not intervene in the healing process. Functional dressings provide a moist environment and facilitate the healing process. On the other hand, interactive wound-dressings provide a moist environment and interact with the wound bed components to further enhance the healing process [9,10]. For instance, interactive dressings can adjust moisture retention, reduce microorganism colonization, improve collagen matrix formation, modulate inflammatory responses, reduce exudate levels, and protect epithelialization.
Moreover, various types of bioactive substances have been implemented in the interactive dressings, such as anti-microbial, anti-inflammatory, and wound-healing agents. In addition to the structure, the composition of wound-dressings is another determinant factor. Wound-dressings have been made from different natural, synthetic, and semi-synthetic polymers. Among them, natural polymers have attracted more attention due to toxicity issues and, in most cases, more abundance [11,12].
It is crucial for an ideal wound-dressing to be biocompatible and to match in vivo physicochemical properties. While most natural polymers are biocompatible, they often have weak mechanical properties and low processability [13,14]. Cellulose-based biomaterials not only have negligible biocompatibility, but also tunable physical, chemical, and mechanical properties. Various types of wound-dressing have been developed from cellulose-based biomaterials and exhibited promising results [15,16].
This review’s primary focus is to provide a comprehensive assessment of cellulose-based biomaterials’ studies as wound-dressing materials. We first elucidate the wound-dressings’ role in the wound-healing process and then discuss the challenges regarding the wound-healing process and ideal wound-dressing requirements. Finally, cellulose-based wound-dressings are reviewed, their challenges are discussed, and practical perspectives are presented.
2. Wound Healing Process: The Role of the Wound-Dressing
Wound healing is a complicated, multi-step, and multifactorial process, which involves different cells, signals, and biochemical substances. The wound-healing phases include hemostasis, inflammatory, proliferative, and maturation, in which different molecules, cells, and signals/stimuli are involved (Figure 1). Hemostasis begins at the onset of injury, aiming to stop the bleeding. It is reported that uncontrolled bleeding from a wound, hemorrhage, is the leading cause of complications and death. While passive wound-dressings cannot initiate or accelerate blood clotting, unprecedented attempts have been conducted to design interactive hemostatic wound-dressings capable of controlling bleeding. Moreover, there are different specialized hemostatic wound-dressings on the market, such as HemCon Bandage PRO and Stasilon®, Entegrion, Inc. of Durham, NC, USA, which exhibit promising performance [17].
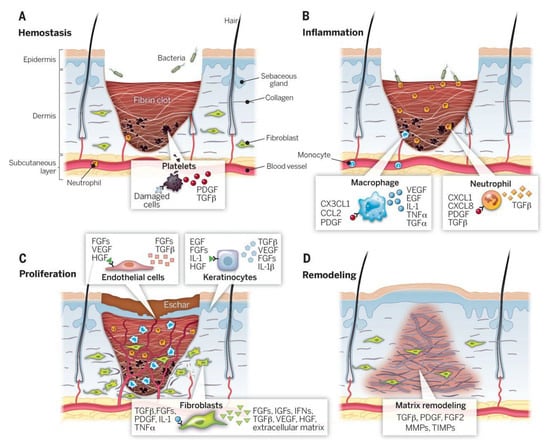
Figure 1.
The wound-healing stages and various involved elements. (A) the hemostasis, (B) the inflammation, (C) the proliferation, and (D) the remodeling stages. PDGF: platelet-derived growth factor, TNF: tumor necrosis factor, TGF: transforming growth factor, KGF: keratinocyte growth factor, FGFs: fibroblast growth factors, IGF: insulin-like growth factor, IL-1: interleukin-1, IFN: interferon, TIMP: tissue inhibitor of metalloproteinase, VEGF: vascular endothelial growth factor, MMP: matrix metalloproteinase, HGF: hepatocyte growth factor. Reproduced with permission from Reference [18].
The second step of wound-healing is the defensive/inflammatory phase, which prepares the wound bed for new tissue growth by destroying bacteria and removing debris. Generally, anti-microbial substances have been tried to incorporate into the interactive wound-dressing to facilitate the defensive/inflammatory wound-healing phase. Since providing a moist environment facilitates the body’s enzymatic debridement, several studies have tried to develop a wound-dressing structure for this purpose [19,20,21]. Although inflammation is a vital step in the wound-healing process, chronic inflammation is an undesirable condition that makes chronic wounds and subsequently induces some complications, such as mature scar formation. Wound dressings containing anti-inflammatory agents have been developed to modulate the inflammatory responses and prevent chronic wound progression [22].
The transition from the defensive/inflammatory phase to the proliferative phase is critical during the wound-healing process. In this phase, the provisional fibrin matrix, formed in the previous stage, replaces a new matrix of fibronectin, proteoglycans, and collagen fibers. Moreover, angiogenesis, granulation tissue formation, and epithelialization are vital processes in this step [1]. Fibroblasts and keratinocytes have critical roles in this phase, and several substances have been investigated to modulate their migration and activities. Several studies applied wound-dressings as the drug-delivery vehicle. These studies included efforts to deliver various growth factors such as epidermal growth factor (EGF), fibroblast growth factor (FGF), insulin-like growth factor (IGF-1), and platelet-derived growth factor (PDGF) to modulate the functions of these cells. Moreover, different phytochemicals and naturally derived substances have been combined with wound-dressings to improve their efficacy [22,23,24].
The remodeling stage, also called the maturation stage, is the final stage of wound-healing, in which collagen type III remodels to type I, and the wound fully closes. During this process, the disorganized collagens align along tension lines, absorb water, and crosslink together. These events make the skin area of the wound stronger and reduce scar thickness. Although wound-dressings are more applicable in the first phases of the wound-healing process, it is possible to modulate the latest wound-healing stage using proper wound-dressings and reduce scar formation [3,5].
3. Wound-Healing Challenges in the Clinic
Despite significant advancements in medicine, pharmacy, and materials sciences, wound healing and management have encountered critical challenges. Some relate to the type of wound and some to the treatment options. Chronic wounds’ recovery is the most challenging clinical issue, which induces several burdens on hospitals and health systems [25]. Chronic wounds have different etiologies, such as diabetes, aging, infections, arterial insufficiency, and other systemic diseases. These conditions further complicate any healing strategy due to active disruption in the healing process [26]. Moreover, high protease activity, long-term infection, extra inflammation, and hypoxia are often seen in chronic wounds [27]. Indeed, in spite of wide attempts to develop therapeutic policies for efficient treatment of chronic wounds, imperfect clinical achievement has been accomplished until now. Some reasons comprise (1) complexity of wound environment, (2) increasing percentage of aging populations, and (3) several products on the market having limited clinical efficiencies [28]. Additionally, patients with chronic wounds often have one or more additional diseases that must be managed for optimal healing. For example, patients with diabetic foot wounds often have kidney and cardiac disease related to their diabetes. Taking into account this complexity at both the clinical and cellular level, it is not surprising that studies on wound healing have often failed to generate significant results [29].
Biomaterial candidates have critical roles in chronic wound-healing along with controlling the underlying etiologies. Wound healing materials can facilitate different debridement processes, a crucial step in acute and chronic wound-healing. They can promote the autolytic and enzymatic/chemical debridement methods. Anti-bacterial wound-healing materials have decisive roles in chronic wound-healing management. They can be either fabricated from anti-bacterial polymers, such as chitosan, or loaded with anti-bacterial agents, such as antibiotics. Anti-inflammatory and antioxidant drugs and substances are applied to modulate the inflammatory responses in chronic wounds. Wound-healing materials containing anti-inflammatory substances have shown promising results in chronic wound-healing. Moreover, cell-laden structures are an emerging concept with fascinating potential in wound-healing applications [30,31,32]. In this regard, one of the most important challenges is adjusting a delivery method for the biological products. For example, RNAi is a powerful gene-silencing tool with massive potential for wound-healing application. Thus, by improving delivery methods, RNAi therapeutics can be developed into a biological product with the potential to apply a transformational effect on modern regenerative medicine [33]. Additionally, nanomedicine approaches such as microfluidic systems as drug delivery systems, as well as controlled release applications of the biological product, can be considered for clinical wound-healing therapy in the future [33].
4. The Ideal Wound-Healing/Dressing Materials
The wound-healing/dressing materials have a significant and determinant role in the wound-healing process. Accordingly, they must possess some critical requirements. Biocompatibility and non-immunogenicity are the most essential requirements. They should control the moisture of the wound bed, allow gas exchange, absorb the exudates, prevent microbial invasion, contain bioactive substances, possess adequate mechanical properties, be quickly changed and removed, and have a low production cost [34]. A wide range of natural, synthetic, and semi-synthetic polymers have been evaluated as the wound-healing/dressing materials. Among them, natural polymers have received significant attention due to low toxicity issues and proper biocompatibility. Although, they suffer from lower mechanical strength and processability compared with synthetic ones. Cellulose is a natural polysaccharide with fascinating prosperities, with a wide range of applications in various fields of science and industries [35,36]. Various cellulose derivatives have been applied as the wound-healing/dressing materials.
5. Cellulose-Based Biomaterials in Wound-Healing Augmentation
5.1. Cellulose Acetate (CA)
Cellulose acetate (CA) has been recognized as a suitable semi-synthetic biomaterial for anti-bacterial wound-healing. It possesses proper biodegradation, biocompatibility, hydrolytic stability, mechanical strength, insolubility in water, availability, and low cost. Moreover, it is an excellent scaffolding biomaterial for loading drugs with anti-microbial, antioxidant, anti-inflammatory, and antiviral activity to repair wounds. Depending on the application, CA-based biomaterials can be exploited in various hydrogels, nanofibers, thin-film, and nanoparticles, each with advantages and disadvantages. Two forms of CA hydrogel and CA nanofibers are mostly preferred for dressings [37,38].
5.2. CA-Based Nanofibers
Various nanofibers have been fabricated as a membranous mat to cover skin wounds using various natural, semi-synthetic, and synthetic polymers. CA is another polymer used to fabricate nanofibers for wound-dressing, alone or in combination with other polymers. Sultana and colleagues fabricated membranous mats for wound-dressing with electrospinning a CA solution in a mixture of acetic acid/acetone solvents (3:1). Nanofibers with a concentration of 10% to 14% (w/v) CA were produced, and in mats with the highest concentration of CA, no beads were found. To load tetracycline hydrochloride in nanofibers, 2 w/v% of the drug was dissolved in the acetic acid/acetone mixture (3:1). The loaded antibiotic inhibited the growth of both Gram-negative and Gram-positive bacteria on the mat. These nanofibers showed a remarkable ability to absorb high amounts of water in examinations. Cytotoxicity was also investigated using human skin fibroblasts against a tissue culture without the membrane. The results showed more than 90% of cell viability for the membrane after 48 h compared to the control. Generally, these tetracycline-loaded mats proved high water uptake and hydrophilic properties and excellent antibacterial activity as a wound-dressing in in vitro studies [39,40].
In various studies, CA nanofibers have shown proper mechanical strengths and benefits in wound-dressing alone. Various other studies have examined the results of their association with additional materials and polymers on wound-healing. One of these attempts by Panichpakdee et al. examined the effect of different concentrations (0.005, 0.01, 0.05, and 0.1 wt.%) of emodin on CA nanofibers. This emodin-loaded mat morphology did not differ much from that of neat mats, indicating that emodin was well-compatible with CA polymer. Although the diameters of emodin-loaded nanofibers increased with emodin content, this increase was not significantly different from the diameters of neat nanofibers. At the concentration of 0.1 wt.% of emodin, the highest amount of release was observed. Besides the non-toxicity feature, these emodin-loaded mats showed enhanced collagen synthesis at concentrations of 0.05 and 0.1 wt.% compared to neat CA mats [41].
In another study by Ullah et al., different amounts of Manuka honey were incorporated in CA nanofibers. The results showed that increasing the amount of Manuka honey increases the antibacterial and antioxidant activities of these nanofibers and their diameters. It is also worth noting that the neat CA mat’s strength was higher than the Manuka honey-loaded mat, which was mostly attributed to the lack of mechanical interaction between Manuka honey and nanofibers. Water uptake and water vapor transmission processes, which play an essential role in wound-healing, were disrupted in Manuka honey-containing mats due to diameter increase and surface reduction of nanofibers, causing wound dehydration and healing disruption. However, in vitro studies have shown that cell growth and proliferation rates in these mats were higher than those of the neat mats. Despite their enhanced cytocompatibility and antibacterial activities, Manuka honey-loaded CA mats’ weakness lies in their unfavorably increased diameter and lower hydrophilicity compared to neat mats. Therefore, so far, more research was required to find a combination of materials for fabricating mats with anti-microbial and antioxidant properties and a high ability to absorb, retain, and transfer water [42].
Khoshnevisan and colleagues used propolis-coated nanofibrous mats, which are also plant-based honeybees and have high antibacterial and antioxidant properties. The CA/poly(caprolactone) nanofibrous mats were impregnated with propolis. They had an inhibiting effect on the growth of both Gram-negative and Gram-positive bacteria, in addition to a high capacity to absorb and retain water for hydrating the wound. These results made the fabricated mats a suitable dressing for diabetic wounds, burns, and skin defects [43].
Curcumin’s distinctive features, such as antibacterial, antifungal, antioxidant, anti-inflammatory, and antitumor properties, have convinced many researchers to strive to fabricate curcumin-containing mats for the wound dressing. The material alone has low solubility and low heat resistance. Studies have established that loading the drug on nanofibrous composites in the presence of poly(vinyl pyrrolidone) (PVP) shows the highest release rate due to the increased solubility induced by this polymer. This fact was emphasized in a study by Tsekova et al. In their study, curcumin was loaded on CA nanofibrous mats, as well as CA/PVP mats. Mats with PVP released a higher amount of curcumin faster, and they had higher hydrophilicity compared to curcumin-loaded CA nanocomposites. Anti-microbial tests also showed that matrices with a higher release amount of curcumin have higher anti-microbial activity [44].
Thymoquinone is another anti-microbial agent that can inhibit the growth of many microbes at the wound site. Systematic administration of this drug can have lower efficacy and higher side effects than topical drug use. On the other hand, it has some limitations for local use as well. However, loading in scaffolds such as CA nanofibrous mats, which have a high bioavailability, stability, bioactivity, and hydrophilicity, can enhance the treatment outcomes [45]. Although nanofibrous mats made of various polymers are known as a great mechanical and physical barrier for covering wounds, this is not the only requirement for a wound-healing process. Combining an antibacterial agent in a wound-dressing expedites the healing process and prevents possible wound infections or even systemic infections. Poly (lactic acid) (PLA) draws particular attention in biomedical applications, especially for wound-dressing, due to its biocompatibility, biodegradability, as well as mechanical durability and elasticity. However, a severe limitation of PLA is its hydrophobicity and low water absorption capacity. Gomaa et al. attempted to fabricate a thymoquinone-loaded PLA/CA nanocomposite. They tested the composites on mice in terms of cell proliferation, re-epithelization, and wound site closure speed (Figure 2). Their results were promising. In addition to an optimal anti-microbial activity shown by this nanocomposite mat, H&E staining studies confirmed more tissue granulation and faster re-epithelization for this dressing than the control group [46].

Figure 2.
Overall representation of fabricating and testing of thymoquinone-loaded PLA/CA nanocomposite. (A) SEM micrograph of nanofibers, (B) SEM micrograph of cells on nanofibers, (C) overall design on the construct, and (D) histopathological results. Reprinted with permission from Reference [46].
Another nanocomposite was made from polyurethane, CA, and zein materials, presenting higher cell viability and proliferation and blood clotting ability, and hydrophilicity. In addition to that, loading streptomycin onto this nanocomposite mat as an anti-microbial agent provided all the wound requirements to heal. The results of in vitro and in vivo studies of the fabricated mat indicated that all the vital biological and physiological properties for a wound-dressing are enhanced in this nanocomposite [47].
One of the most critical wounds is a diabetic wound. These wounds go through a more extended recovery period and are more likely to become infected since they have inefficient blood flow and nerve conduction. Moreover, in the case of infection of these wounds, infection progression is very high, and the possibility of recovery is lower. Besides, the low blood supply to these wounds makes systemic antibacterial agents less effective and necessitates higher doses of the medication, which leads to more side effects. With this in mind, as far as these diabetic wounds are concerned, proper dressing has critical importance to prevent infection and accelerate its healing process and the provision of the anti-microbial drug for local release at the wound site. Therefore, CA-zein nanocomposites were fabricated containing a high dose of sesamol to be tested on diabetic rats. The in vivo study revealed an accelerated wound-healing improvement in diabetic mice [48]. Another system was a CA/gelatin nanofibrous mat containing berberine. It showed that loading berberine does not significantly affect CA/gelatin nanofiber physiology. However, it has a significant beneficial effect on biological activity and is a suitable dressing for diabetic wounds [49].
5.3. CA-Based Hydrogels
Hydrogel nanocomposites are high potent biomaterials for biomedical applications. Their mechanical strength, controlled drug release ability, stimuli-responsive behavior, biological interactions, and high elasticity encourage researchers to incorporate various nanoparticles in hydrogel networks. There are two methods for incorporating nanoparticles into a matrix hydrogel: one is to mix monomers’ solutions and then produce the polymers, and the other one is to mix the produced polymers after the polymerization process. CA is a polymer that forms a biocompatible hydrogel. Bajpai et al. [50] fabricated semi-interpenetrating polymer network (IPN) films based on CA and crosslinked poly(acrylic acid) using a photopolymerization approach. They applied poly(acrylic acid), N,N′ methylene bisacrylamide (MB), and benzophenone as the monomer, crosslinker, and photo-initiator, respectively. The photopolymerization was conducted using a UV illumination system (UVITEK Crosslinker, Germany) at the wavelength of 365 nm, with the intensity of 1850 µW/cm3 for one hour. The process resulted in transparent semi-IPN films with adjustable mechanical strength, water vapor, and oxygen permeation performance. They reported that the tensile strength and percent elongation of the film improved with increasing CA content. The water vapor transmission rate reduced with CA and the photo-initiator concentration and increased with monomer content. The in vitro studies showed antibacterial and antifungal activities against E. coli and Aspergillus flavus. The authors proposed that the physical properties of the semi-IPN film can be tailored for various wounds and loaded with different bioactive agents as the promising wound-healing biomaterial/dressing.
Abd El-Mohdy et al. produced hydrogels by incorporating Ag NPs into poly(vinyl alcohol), CA, and gelatin. In these hydrogels, the amounts of loaded Ag NPs were different, and the diameter of produced nanocomposites was increased with the concentration of AgNO3 loaded on the hydrogels. In fact, at concentrations of 5, 10, and 20 mM, yielded diameters were 38.6, 56.8, and 60.1 nm, respectively. The results of this study showed that these hydrogel membranes exhibited sufficient strength. These samples, formed by combining the polymers mentioned above and incorporation Ag NPs, showed higher thermal stability than single-polymer hydrogels and other sample groups. Additionally, Ag NPs in this hydrogel prevented the crystallization of PVA and the formation of NPs in the hydrogel.
Moreover, Ag NPs induced long-term anti-microbial activity of these hydrogels against a wide range of fungi and bacteria. This anti-microbial activity was clearly higher in higher concentrations of AgNO3. Overall, this study showed that these hydrogels are useful biomaterials for wound-healing and dressing applications. However, extensive studies have not treated these hydrogels’ construction in much detail due to nanofibers’ prominent features and the possibility of easily manipulating their structure, especially at small diameters, as mentioned earlier [51,52]. Some other studies conducted on CA-based wound-healing biomaterials have been summarized in Table 1.

Table 1.
CMC-based biomaterials fabricated and applied for wound-healing applications. The fonts are not the same in all cases of the table.
6. Carboxymethyl Cellulose (CMC)
Carboxymethyl cellulose (CMC) is a semi-synthetic water-soluble polysaccharide and a derivative of cellulose. It is composed of multiple carboxyl groups, with applications ranging from tissue engineering to protein delivery [67,68]. CMC is a highly versatile biomaterial due to numerous useful properties, including tunable biodegradability, the ability to construct hydrogels in the presence of metallic ions, potential antioxidant activity, and biocompatibility [69,70,71]. Due to these desirable properties, different forms and combinations of CMC are used in various biomedical engineering research [72,73].
Upon a systematic survey of CMC’s different biomedical applications, wound-healing and wound-dressing applications have been one of the most investigated areas of research for these applications [74,75,76,77,78]. This fact can be attributed to the fact that it has been shown that cellulose derivatives such as CMC contribute to mimic healthy physiological conditions that damage skin tissue [79,80]. Besides, antioxidant capacity, close adhesion to the wound bed, minimal interference with new tissue formation, and high fluid absorbency are other promising aspects of CMC for wound-healing applications, which have been thoroughly investigated [81,82]. Various strategies have been employed to fabricate and characterize CMC-based wound-dressings to take advantage of these attributes [72,82,83].
Like other cellulose derivatives, a majority of CMC-based wound-dressing fabrication strategies can be categorized into the use of CMC nanofibrous scaffolds and CMC-based hydrogels. Both of these main strategies will be addressed [84,85]. A summary of key studies on CMC-based wound-dressings is provided in Table 2. Studies on CMC nanofibers and CMC-based hydrogels have been heavily focused on modifying and improving CMC-based structures, either through chemical and physical modification of CMC-based wound-dressings or by combining other promising wound-dressing biomaterials [78,81]. These trends exhibit the continuous evolution of the CMC-based wound-dressing subfield, resulting in significant progress in the polymeric wound-dressing field if directed towards application-based studies [86].

Table 2.
CA-based biomaterials fabricated and applied for wound-healing applications.
6.1. CMC-Based Nanofibers
2D CMC nanofibrous wound dressings have been extensively studied due to their microstructure similarity to intact skin tissue and extracellular matrix (ECM) and their high potential as drug carriers, liquid absorbents, and moisturizers. Various strategies can fabricate CMC nanofiber dressings, including ultrasonication, casting, freeze-drying, and electrospinning [91,104,105]. Electrospinning is the most widely used CMC nanofiber dressing fabrication technique due to its low costs, green fabrication, simplicity, reliability, and versatility [87,92,106,107].
The majority of electro-spun CMC nanofiber wound dressings either involve CMC nanofibers’ fabrication with/without a type of modification or co-electrospinning of a mixture of CMC and complement materials [91,92,105]. For instance, the fabrication of CMC electro-spun nanofibers was the focus of one interesting study. Silver nanoparticles were added to the structure to induce antibacterial activity for wound-dressing applications [91]. Anti-bacterial studies showed that the antibacterial efficiency against several bacteria, including E. coli and Staphylococcus aureus, was up to 100%, indicating a significant potential for wound-dressing anti-microbial capability [91].
The other approach for producing CMC-containing electro-spun nanofibers through mixing with another material with complementary properties is more prevalent in the literature, especially pronounced in recent years [87,92,105,108]. Fabrication and characterization of CMC/poly (ethylene oxide) (PEO) nanofibers were investigated in a study in which the mixture of the two materials was co-electro-spun to obtain a structure with tetracycline hydrochloride as a model drug, which can be used for wound-dressing applications [105]. The drug-delivery profile identified an optimized combination of CMC 3% (w/v)/PEO 1% (w/v), and it showed excellent bactericidal activity against various bacteria, indicating a highly promising material for applications such as wound-dressing.
6.2. CMC-Based Hydrogels
Hydrogels based on CMC have been more extensively studied than nanofibrous mats because CMC is a generally good candidate for hydrogel formation due to its coordination ability and biodegradability and hydrogel formation in the presence of metallic ions [101,103,109]. Das et al. were pioneers of using CMC hydrogels as wound-dressings, and they prepared silver nanoparticle-containing CMC hydrogels by in situ silver nitrate reduction [103]. Their hydrogel dressing was found to be highly absorbent, biocompatible, and proliferation-inducing for skin cells. The 50 ppm composition of silver nanoparticles in the hydrogel was highly effective against Gram-negative and Gram-positive bacteria strains, indicating an ideal dressing for treatment of infected wounds [103]. More recent studies have noticeably shifted towards hydrogels with CMC and at least another polymer component such as collagen and poly(ethylene glycol) [81,102]. Through this strategy, the wound-dressing can take advantage of all the materials’ promising properties while potentially alleviating the less desirable properties [110,111,112].
Fan et al. reported one of the most important studies with this approach [81]. They used a mixture of collagen and CMC to take advantage of collagen’s antioxidant potential and its improvement capability for biocompatibility and hydrophilicity. They investigated the effects of different concentrations, molecular weights, and substitution degrees on modified CMC’s radical scavenging properties. They found that CMC’s scavenging effects improved upon increasing concentrations and degrees of substitution, showing a promising material that can reduce reactive oxygen species (ROS), a vital capability for wound-dressing applications [81].
7. Bacterial Cellulose
Bacterial cellulose (BC), also known as microbial cellulose (MC), is an inexpensive polysaccharide biopolymer of linear β-1,4-glucan chains, which is produced at a high yield by Gram-negative bacteria strains. This type of cellulose has a very similar structure as plant-extracted cellulose, with minor chemical and physical properties’ differences, such as a higher degree of polymerization in BC than plant-extracted cellulose [113,114,115]. BC is made of assembled microfibril bundles of hundreds of polyglucan chains and has an average fiber diameter of 20–100 nm, a much lower fiber diameter than plant-extracted cellulose, resulting in a significantly higher available surface area [114,116]. BC is used in a wide range of fields and applications, especially in biomedical applications, such as cornea and bone scaffolds, vessel grafts, diagnostic tools, and wound healing [114,117,118].
BC offers unique properties that have turned it into one of the most sought-after biomaterials for various biomedical applications [118,119]. The characteristics that make BC a highly versatile biomaterial include high effective surface area, and a hydrophilic nature that gives BC a high liquid loading capacity, together with non-toxicity, biocompatibility, and abundant sources. Its natural structure also closely mimics many biological tissue properties, it has an ability for cell adhesion regulation, and its antigen immobilization capability is high for biosensor applications [82,115,116,120]. Additionally, BC production leads to very high purity and does not involve harsh chemicals, which further eliminates the chance for any residual cytotoxic impurity [120]. Most of the capabilities mentioned above are very promising features for wound-healing applications [115,120].
BC offer enormous potentials for wound-dressing applications and has been one of the top choices for wound-dressing biomaterials in recent years, as it can provide mechanical stability and flexibility, promotes cell proliferation, retain liquids associated with wounds, allows gas exchange due to high porosity and permeability, and controls wound exudates, while not inducing any undesirable inflammatory response from the host tissue [116,119]. Another essential aspect of BC is its potential for loading and controlled release of drugs, exploited in various forms of BC-based dressings and scaffolds [121,122]. Despite these advantages and the presence of a variety of commercial BC-based wound dressings such as Membracell®, BC shows no activity against bacterial infections, and it has a very rapid drug release profile due to higher than optimal biodegradability. Although it is a reasonably biocompatible material, it has been shown that in various wound-healing applications, its cell and tissue biocompatibility still have room for improvement [114,120,123]. Main strategies to circumvent these issues include surface modifications, immobilization of cell adhesion molecules such as RGD molecules, and combination with other biomaterials to prepare nanocomposite materials [114,115]. Additionally, anti-microbial components such as anti-microbial drugs have been addressed [124,125]. Like other cellulose derivatives, BC nanofiber synthesis and hydrogel formation are the main fabrication strategies for wound-dressing applications, which will be addressed [116,125,126].
7.1. BC-Based Nanofibers
In general, BC nanofiber dressings utilize two main strategies: (1) using BC nanofibrous structure modified by loading with a model drug or antibacterial nanoparticles, and (2) nanofibrous dressing using a combination of BC and another natural or synthetic polymers with or without further chemical or physical modifications [115,127,128,129,130]. Both these strategies have proven to be highly successful for wound-healing applications and are among the most successful cellulose-based wound-healing applications [127,129]. Among various techniques for the fabrication of BC nanofiber dressings, electrospinning has proven to be the most cost-effective and reproducible technique [115,128].
Impregnation of BC nanofiber dressings with silver nanoparticles has been undoubtedly the most successful preparation strategy over the years due to the perfect complementary combination of BC as an already successful biomaterial for wound-dressing and the proven anti-infection capability of silver nanoparticles [124,127,128]. The most influential and pioneering study was conducted by Maneerung et al. towards preparing nanocomposite nanofibrous BC wound-dressings with embedded silver nanoparticles [124]. They were one of the first groups that showed that continual release of silver nanoparticles under physiological conditions was not high enough to cause the normal human cells damage but was sufficient for prolonged anti-microbial activity. This was followed by a range of studies that addressed the same or similar combinations of silver particles and BC using different fabrication techniques and employing other potential modifications [125,131,132,133].
7.2. BC-Based Hydrogels
BC-based hydrogels provide an alternative strategy for wound-healing applications since they offer a straightforward path to embed stem cells or healthy skin cells, to load various antibacterial drugs, and they are moldable, hydrophilic materials with high-water content, desirable for wound-dressings with no adverse inflammatory response [134,135,136]. Loh et al. characterized cell-containing dressings for wound-healing applications [137]. They loaded human epidermal keratinocytes and dermal fibroblasts in BC-based hydrogels and evaluated their performance in full-thickness wounds. Their results showed maintained cell viability, cell transfer, in vivo wound closure, and overall accelerated wound-healing due to the hydrogel’s presence, both with and without embedded cells [137]. In another study [138], semi-interpenetrating network (semi-IPN) hydrogels based on BC and chitosan were fabricated through blending the slurry of BC with CS solution and crosslinking the polymers with glutaraldehyde (Figure 3). They showed that the prepared hydrogel exhibited proper thermal stability, significant mechanical properties, and strong anti-bacterial activities. They proposed that the fabricated semi-IPN hydrogels could be useful wound-healing biomaterials.
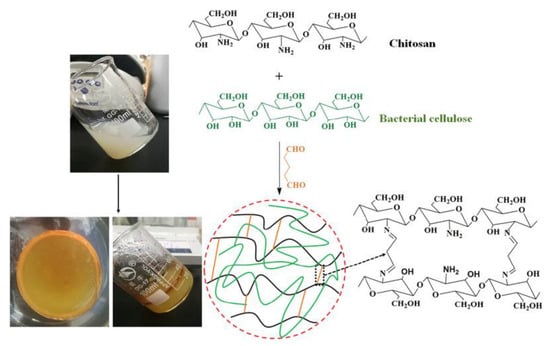
Figure 3.
Fabrication process of semi-IPN hydrogel synthesis. Reprinted with permission from Reference [138].
Almost all of the relevant studies refrain from using pure BC hydrogels, which can be attributed to the limited and poor rehydration of pure BC hydrogels following drying either during the intended wound-healing or during storage [120,134]. Most of these studies combine BC with other polymers, such as chitosan, dextran, and hyaluronan [134,138,139]. Yu et al. demonstrated the advantage of adding a physiologically relevant polymer such as hyaluronan. They prepared BC–hyaluronan nanocomposites with a 3D hydrogel via a modified solution impregnation method [139]. This combination exhibited improved water uptake capability, more pronounced growth, and viability of fibroblast cells compared to pure BC hydrogel, paving the way for subsequent studies with similar better wound-dressing outcomes [134,139,140]. Some studies conducted on BC-based wound-healing biomaterials have been provided in Table 3.

Table 3.
BC-based biomaterials fabricated and applied for wound-healing applications.
8. Conclusions and Future Prospective
Wound healing is a complicated, multi-step, and dynamic process with several involved cells, biochemical molecules, and signaling pathways. Moreover, various endogenous and exogenous interfering factors affect the process, such as acute inflammatory responses, pathogenic contaminations, wound bed dryness, exudates, and oxygen permeability. The passive wound-healing/dressing biomaterials solely act as the physical structures, which prevent bacterial invasion and modulate the wound bed’s moisture at the optimum situation. Many types of wounds, including an infected wound, diabetic ulcers, and burn wounds, required physical care and biological interventions, which are not accessible with the passive wound-healing/dressing biomaterials. Accordingly, bioactive interacting wound-healing/dressing biomaterials with different biological activities, such as antibacterial, antioxidant, and proliferative properties, are highly encouraged to improve and accelerate the wound-healing process.
Cellulose-based biomaterials are attractive for wound-healing/care applications due to their diversity, tunable physical, chemical, mechanical, and biological performances, accessibility, low cost, and biocompatibility. Moreover, cellulose can be obtained from various bacteria, tunicates, and plants. Cellulose-based biocompounds are fascinating for wound-healing/dressing materials because of their processability to multiple structures, such as nano/microfibers, hydrogels, films, and colloids. Their hydrophilic nature has been applied to modulate the performance of different natural and synthetic wound-healing/dressing materials. Various bioactive molecules such as antibiotics, vitamins, growth factors, peptides, and natural substances have been delivered to the wound site using cellulose-based structures. The biodegradation of cellulose-based biomaterials in the human body is challenging, which is not a big deal for wound-healing/dressing applications. They are easily removed from the wound bed after inducing the intended effect.
The application of toxic crosslinking agents (e.g., GA) is a concerning issue for fabricating biocompatible cellulose-based hydrogels. Accordingly, the development of safe, efficient, and biocompatible crosslinking approaches is necessary for the production of hydrogel-based biomaterials with appropriate physicochemical and biological features. The combination of the cell therapy concept with cellulose-based wound-healing biomaterials as the cell-laden structures is promising to accelerate the healing process in chronic wounds. The authors believe that the combination of wound-healing and stem cell therapy may result in more efficient clinical outcomes. In this review, we discussed the studies conducted on cellulose-based biomaterials as the wound-healing material and highlighted their outcomes and drawbacks beneficial for the future direction of wound-care/healing approaches.
Author Contributions
Conceptualization, M.H. and C.D.; Writing—original draft preparation, M.F.A., S.G., and S.Z.K.; writing—review and editing, M.F.A., S.G., S.Z.K., N.H.B., D.A., S.P., H.D., Z.A., S.M.A., M.H., and C.D. All authors have read and agreed to the published version of the manuscript.
Funding
There is no funding info for this manuscript.
Acknowledgments
Authors earnestly acknowledge the support from the Nano Drug Delivery Research Center (NDDRC), Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran (Grant Number: 980959).
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin acute wound healing: A comprehensive review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Joodaki, H.; Panzer, M.B. Skin mechanical properties and modeling: A review. Proc. Inst. Mech. Eng. Part H 2018, 232, 323–343. [Google Scholar] [CrossRef]
- Gonzalez, A.C.d.O.; Costa, T.F.; Andrade, Z.d.A.; Medrado, A.R.A.P. Wound healing-A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Polerà, N.; Badolato, M.; Perri, F.; Carullo, G.; Aiello, F. Quercetin and its natural sources in wound healing management. Curr. Med. Chem. 2019, 26, 5825–5848. [Google Scholar] [CrossRef]
- Nour, S.; Baheiraei, N.; Imani, R.; Khodaei, M.; Alizadeh, A.; Rabiee, N.; Moazzeni, S.M. A review of accelerated wound healing approaches: Biomaterial-assisted tissue remodeling. J. Mater. Sci. Mater. Med. 2019, 30, 120. [Google Scholar] [CrossRef] [PubMed]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Sahrapeyma, H.; Ghorbani, S. A promising wound dressing based on alginate hydrogels containing vitamin D3 cross-linked by calcium carbonate/d-glucono-δ-lactone. Biomed. Eng. Lett. 2020, 10, 309. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Maleki, H.; Larrañeta, E.; Fajardo, A.R.; Nik, A.B.; Shavandi, A.; Sheikhi, A.; Ghorbanpour, M.; Farokhi, M.; Govindh, P. Status and future scope of plant-based green hydrogels in biomedical engineering. Appl. Mater. Today 2019, 16, 213–246. [Google Scholar] [CrossRef] [Green Version]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for wound dressings: An up-to-date overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef]
- Aljghami, M.E.; Saboor, S.; Amini-Nik, S. Emerging innovative wound dressings. Ann. Biomed. Eng. 2019, 47, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Mala, R.; Rani, M.J.; Prasath, N.H.; Celsia, A.R. Nano composite material of chitosan for wound healing: A review. In Proceedings of the AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2019; p. 020013. [Google Scholar]
- Ranjith, R.; Balraj, S.; Ganesh, J.; Milton, M.J. Therapeutic agents loaded chitosan-based nanofibrous mats as potential wound dressings: A review. Mater. Today Chem. 2019, 12, 386–395. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stanciuc, A.-M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef]
- Obagi, Z.; Damiani, G.; Grada, A.; Falanga, V. Principles of Wound Dressings: A Review. Surg. Technol. Int. 2019, 35, 35. [Google Scholar]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural Polymer-Based Antimicrobial Hydrogels without Synthetic Antibiotics as Wound Dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef]
- Abdul Khalil, H.; Adnan, A.; Yahya, E.B.; Olaiya, N.; Safrida, S.; Hossain, M.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.; Oyekanmi, A. A Review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef]
- Suwantong, O.; Ruktanonchai, U.; Supaphol, P. Electrospun cellulose acetate fiber mats containing asiaticoside or Centella asiatica crude extract and the release characteristics of asiaticoside. Polymer 2008, 49, 4239–4247. [Google Scholar] [CrossRef]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, L.; Wang, F.; Guan, G.; Hu, X.; Xie, Q.; Wang, W.; King, M.W. Influence of structures on the mechanical and absorption properties of a textile pile debridement material and its biological evaluation. RSC Adv. 2015, 5, 87580–87588. [Google Scholar] [CrossRef]
- FU, Y.-J.; Wang, L.; Wang, F.-J.; Wang, W.-Z.; Meng, S.-Y. Preparation and mechanical properties of a novel textile pad for wound debridement. J. Donghua Univ. 2014, 31, 621–624. [Google Scholar]
- Shi, L.; Carson, D. Collagenase Santyl ointment: A selective agent for wound debridement. J. Wound Ostomy Cont. Nurs. 2009, 36, S12–S16. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Johnson, M.L.; Bilski, J.J.; Redmer, D.A.; Reynolds, L.P.; Abdullah, A.; Abdullah, K.M. Wound healing: The role of growth factors. Drugs Today 2003, 39, 787–800. [Google Scholar] [CrossRef]
- Pierce, G.F.; Mustoe, T.A.; Altrock, B.W.; Deuel, T.F.; Thomason, A. Role of platelet-derived growth factor in wound healing. J. Cell. Biochem. 1991, 45, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, M. Barriers to the implementation of best practice in wound care. WOUNDS UK 2005, 1, 74. [Google Scholar]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Baker, A.B. Biomaterials and nanotherapeutics for enhancing skin wound healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Gould, L.J. Opportunities and challenges of the management of chronic wounds: A multidisciplinary viewpoint. Chronic Wound Care Manag. Res. 2020, 7, 27–36. [Google Scholar] [CrossRef]
- Darwin, E.; Tomic-Canic, M. Healing chronic wounds: Current challenges and potential solutions. Curr. Dermatol. Rep. 2018, 7, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv. Healthc. Mater. 2020, 9, 1901502. [Google Scholar] [CrossRef]
- Stewart, P.S.; Bjarnsholt, T. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin. Microbiol. Infect. 2020. Available online: https://www.sciencedirect.com/science/article/pii/S1198743X20301312 (accessed on 12 August 2021). [CrossRef] [PubMed]
- Aitzetmüller, M.M.; Machens, H.-G.; Duscher, D. Challenges and Opportunities in Drug Delivery and Wound Healing. In Regenerative Medicine and Plastic Surgery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 27–38. [Google Scholar]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-based flexible functional materials for emerging intelligent electronics. Adv. Mater. 2020, 33, 2000619. [Google Scholar] [CrossRef] [PubMed]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef]
- Atila, D.; Keskin, D.; Tezcaner, A. Cellulose acetate based 3-dimensional electrospun scaffolds for skin tissue engineering applications. Carbohydr. Polym. 2015, 133, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Hamano, F.; Seki, H.; Ke, M.; Gopiraman, M.; Lim, C.T.; Kim, I.S. Cellulose acetate nanofiber mat with honeycomb-like surface structure. Mater. Lett. 2016, 169, 33–36. [Google Scholar] [CrossRef]
- Konwarh, R.; Karak, N.; Misra, M. Electrospun cellulose acetate nanofibers: The present status and gamut of biotechnological applications. Biotechnol. Adv. 2013, 31, 421–437. [Google Scholar] [CrossRef]
- Sultana, N.; Zainal, A. Cellulose acetate electrospun nanofibrous membrane: Fabrication, characterization, drug loading and antibacterial properties. Bull. Mater. Sci. 2016, 39, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Panichpakdee, J.; Pavasant, P.; Supaphol, P. Electrospun cellulose acetate fiber mats containing emodin with potential for use as wound dressing. Chiang Mai J. Sci. 2016, 43, 1249–1259. [Google Scholar]
- Ullah, A.; Ullah, S.; Khan, M.Q.; Hashmi, M.; Nam, P.D.; Kato, Y.; Tamada, Y.; Kim, I.S. Manuka honey incorporated cellulose acetate nanofibrous mats: Fabrication and in vitro evaluation as a potential wound dressing. Int. J. Biol. Macromol. 2020, 155, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Doostan, M.; Khorramizadeh, M.R. Antibacterial and antioxidant assessment of cellulose acetate/polycaprolactone nanofibrous mats impregnated with propolis. Int. J. Biol. Macromol. 2019, 140, 1260–1268. [Google Scholar] [CrossRef]
- Tsekova, P.B.; Spasova, M.G.; Manolova, N.E.; Markova, N.D.; Rashkov, I.B. Electrospun curcumin-loaded cellulose acetate/polyvinylpyrrolidone fibrous materials with complex architecture and antibacterial activity. Mater. Sci. Eng. C 2017, 73, 206–214. [Google Scholar] [CrossRef]
- Darakhshan, S.; Pour, A.B.; Colagar, A.H.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, S.F.; Madkour, T.M.; Moghannem, S.; El-Sherbiny, I.M. New polylactic acid/cellulose acetate-based antimicrobial interactive single dose nanofibrous wound dressing mats. Int. J. Biol. Macromol. 2017, 105, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Unnithan, A.R.; Gnanasekaran, G.; Sathishkumar, Y.; Lee, Y.S.; Kim, C.S. Electrospun antibacterial polyurethane–cellulose acetate–zein composite mats for wound dressing. Carbohydr. Polym. 2014, 102, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, X.; Wang, L.; Yan, X.; Ma, D.; Liu, Z.; Liu, X. Sesamol incorporated cellulose acetate-zein composite nanofiber membrane: An efficient strategy to accelerate diabetic wound healing. Int. J. Biol. Macromol. 2020, 149, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Zamiri, S.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Khastar, H.; Alam, M.; Ai, A.; Derakhshankhah, H.; Allahyari, Z. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In vitro and in vivo studies. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Bajpai, S.; Banger, P. Photopolymerized Cellulose Acetate/Poly (Acrylic Acid) Semi-IPN Films for Wound Dressing Applications. J. Macromol. Sci. A 2014, 51, 756–766. [Google Scholar] [CrossRef]
- Abd El-Mohdy, H. Radiation synthesis of nanosilver/poly vinyl alcohol/cellulose acetate/gelatin hydrogels for wound dressing. J. Polym. Res. 2013, 20, 177. [Google Scholar] [CrossRef]
- Melero, A.M.G.; Senna, A.; Domingues, J.; Hausen, M.; Duek, E.A.R.; Botaro, V. Hydrogel based on cellulose acetate used as scaffold for cell growth. Int. J. Chem. Eng. 2018, 12, 352–362. [Google Scholar]
- Elsayed, M.T.; Hassan, A.A.; Abdelaal, S.A.; Taher, M.M.; Khalaf Ahmed, M.; Shoueir, K.R. Morphological, antibacterial, and cell attachment of cellulose acetate nanofibers containing modified hydroxyapatite for wound healing utilizations. J. Mater. Res. Technol. 2020, 9, 13927–13936. [Google Scholar] [CrossRef]
- Barnthip, N.; Muakngam, A. Preparation of cellulose acetate nanofibers containing centella asiatica extract by electrospinning process as the prototype of wound-healing materials. J. Bionanosci. 2014, 8, 313–318. [Google Scholar] [CrossRef]
- Kurečič, M.; Maver, T.; Virant, N.; Ojstršek, A.; Gradišnik, L.; Hribernik, S.; Kolar, M.; Maver, U.; Kleinschek, K.S. A multifunctional electrospun and dual nano-carrier biobased system for simultaneous detection of pH in the wound bed and controlled release of benzocaine. Cellulose 2018, 25, 7277–7297. [Google Scholar] [CrossRef]
- Ahmed, M.; Menazea, A.; Abdelghany, A. Blend biopolymeric nanofibrous scaffolds of cellulose acetate/ε-polycaprolactone containing metallic nanoparticles prepared by laser ablation for wound disinfection applications. Int. J. Biol. Macromol. 2020, 155, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Farahani, H.; Barati, A.; Arjomandzadegan, M.; Vatankhah, E. Nanofibrous cellulose acetate/gelatin wound dressing endowed with antibacterial and healing efficacy using nanoemulsion of Zataria multiflora. Int. J. Biol. Macromol. 2020, 162, 762–773. [Google Scholar] [CrossRef]
- Gaydhane, M.K.; Kanuganti, J.S.; Sharma, C.S. Honey and curcumin loaded multilayered polyvinylalcohol/cellulose acetate electrospun nanofibrous mat for wound healing. J. Mater. Res. 2020, 35, 600–609. [Google Scholar] [CrossRef]
- López-Calderón, H.D.; Avilés-Arnaut, H.; Galán-Wong, L.J.; Almaguer-Cantú, V.; Laguna-Camacho, J.; Calderón-Ramón, C.; Escalante-Martínez, J.; Arévalo-Niño, K. Electrospun Polyvinylpyrrolidone-Gelatin and Cellulose Acetate Bi-Layer Scaffold Loaded with Gentamicin as Possible Wound Dressing. Polymers 2020, 12, 2311. [Google Scholar] [CrossRef]
- Liu, X.; Lin, T.; Gao, Y.; Xu, Z.; Huang, C.; Yao, G.; Jiang, L.; Tang, Y.; Wang, X. Antimicrobial electrospun nanofibers of cellulose acetate and polyester urethane composite for wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1556–1565. [Google Scholar] [CrossRef]
- Bie, X.; Khan, M.Q.; Ullah, A.; Ullah, S.; Kharaghani, D.; Phan, D.-N.; Tamada, Y.; Kim, I.S. Fabrication and characterization of wound dressings containing gentamicin/silver for wounds in diabetes mellitus patients. Mater. Res. Express 2020, 7, 045004. [Google Scholar] [CrossRef]
- Dos Santos, A.E.A.; dos Santos, F.V.; Freitas, K.M.; Pimenta, L.P.S.; de Oliveira Andrade, L.; Marinho, T.A.; de Avelar, G.F.; da Silva, A.B.; Ferreira, R.V. Cellulose acetate nanofibers loaded with crude annatto extract: Preparation, characterization, and in vivo evaluation for potential wound healing applications. Mater. Sci. Eng. C 2020, 118, 111322. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, R.E.; Madkour, T.M.; Azzam, R.A. Tailored-design of electrospun nanofiber cellulose acetate/poly (lactic acid) dressing mats loaded with a newly synthesized sulfonamide analog exhibiting superior wound healing. Int. J. Biol. Macromol. 2020, 164, 1984–1999. [Google Scholar] [CrossRef] [PubMed]
- Wutticharoenmongkol, P.; Hannirojram, P.; Nuthong, P. Gallic acid-loaded electrospun cellulose acetate nanofibers as potential wound dressing materials. Polym. Adv. Technol. 2019, 30, 1135–1147. [Google Scholar] [CrossRef]
- Khan, M.Q.; Kharaghani, D.; Shahzad, A.; Saito, Y.; Yamamoto, T.; Ogasawara, H.; Kim, I.S. Fabrication of antibacterial electrospun cellulose acetate/silver-sulfadiazine nanofibers composites for wound dressings applications. Polym. Test. 2019, 74, 39–44. [Google Scholar] [CrossRef]
- Samadian, H.; Salehi, M.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Sahrapeyma, H.; Goodarzi, A.; Ghorbani, S. In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 964–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wang, A. Nanocomposite of carboxymethyl cellulose and attapulgite as a novel pH-sensitive superabsorbent: Synthesis, characterization and properties. Carbohydr. Polym. 2010, 82, 83–91. [Google Scholar] [CrossRef]
- Ninan, N.; Muthiah, M.; Park, I.K.; Elain, A.; Thomas, S.; Grohens, Y. Pectin/carboxymethyl cellulose/microfibrillated cellulose composite scaffolds for tissue engineering. Carbohydr. Polym. 2013, 98, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Panda, N.N.; Mund, R.; Pramanik, K. Carboxymethyl cellulose enables silk fibroin nanofibrous scaffold with enhanced biomimetic potential for bone tissue engineering application. Carbohydr. Polym. 2016, 151, 335–347. [Google Scholar] [CrossRef]
- Joy, J.; Pereira, J.; Aid-Launais, R.; Pavon-Djavid, G.; Ray, A.R.; Letourneur, D.; Meddahi-Pellé, A.; Gupta, B. Gelatin—Oxidized carboxymethyl cellulose blend based tubular electrospun scaffold for vascular tissue engineering. Int. J. Biol. Macromol. 2018, 107, 1922–1935. [Google Scholar] [CrossRef]
- Tripathy, J.; Raichur, A.M. Designing carboxymethyl cellulose based layer-by-layer capsules as a carrier for protein delivery. Colloids Surf. B Biointerfaces 2013, 101, 487–492. [Google Scholar] [CrossRef]
- Amalraj, A.; Gopi, S.; Thomas, S.; Haponiuk, J.T. Cellulose Nanomaterials in Biomedical, Food, and Nutraceutical Applications: A Review. Macromol. Symp. 2018, 380, 1–9. [Google Scholar] [CrossRef]
- Pettignano, A.; Charlot, A.; Fleury, E. Carboxyl-functionalized derivatives of carboxymethyl cellulose: Towards advanced biomedical applications. Polym. Rev. 2019, 59, 510–560. [Google Scholar] [CrossRef]
- Ali, N.H.; Amin, M.C.I.M.; Ng, S.-F. Sodium carboxymethyl cellulose hydrogels containing reduced graphene oxide (rGO) as a functional antibiofilm wound dressing. J. Biomater. Sci. Polym. 2019, 30, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Beele, H.; Meuleneire, F.; Nahuys, M.; Percival, S.L. A prospective randomised open label study to evaluate the potential of a new silver alginate/carboxymethylcellulose antimicrobial wound dressing to promote wound healing. Int. Wound J. 2010, 7, 262–270. [Google Scholar] [CrossRef]
- Rakhshaei, R.; Namazi, H. A potential bioactive wound dressing based on carboxymethyl cellulose/ZnO impregnated MCM-41 nanocomposite hydrogel. Mater. Sci. Eng. C 2017, 73, 456–464. [Google Scholar] [CrossRef]
- Walker, M.; Hobot, J.A.; Newman, G.R.; Bowler, P.G. Scanning electron microscopic examination of bacterial immobilisation in a carboxymethyl cellulose (AQUACEL®) and alginate dressings. Biomaterials 2003, 24, 883–890. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef] [PubMed]
- Namazi, H.; Rakhshaei, R.; Hamishehkar, H.; Kafil, H.S. Antibiotic loaded carboxymethylcellulose/MCM-41 nanocomposite hydrogel films as potential wound dressing. Int. J. Biol. Macromol. 2016, 85, 327–334. [Google Scholar] [CrossRef]
- Cai, X.; Hu, S.; Yu, B.; Cai, Y.; Yang, J.; Li, F.; Zheng, Y.; Shi, X. Transglutaminase-catalyzed preparation of crosslinked carboxymethyl chitosan/carboxymethyl cellulose/collagen composite membrane for postsurgical peritoneal adhesion prevention. Carbohydr. Polym. 2018, 201, 201–210. [Google Scholar] [CrossRef]
- Fan, L.; Peng, M.; Zhou, X.; Wu, H.; Hu, J.; Xie, W.; Liu, S. Modification of carboxymethyl cellulose grafted with collagen peptide and its antioxidant activity. Carbohydr. Polym. 2014, 112, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Hipler, U.C. Polymer-based biomaterials as dressings for chronic stagnating wounds. Macromol. Symp. 2010, 294, 1–13. [Google Scholar] [CrossRef]
- Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; De Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment–A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onofrei, M.; Filimon, A. Cellulose-based hydrogels: Designing concepts, properties, and perspectives for biomedical and environmental applications. Polym. Sci. 2016, 108–120. [Google Scholar]
- Gopiraman, M.; Jatoi, A.W.; Hiromichi, S.; Yamaguchi, K.; Jeon, H.Y.; Chung, I.M.; Ick Soo, K. Silver coated anionic cellulose nanofiber composites for an efficient antimicrobial activity. Carbohydr. Polym. 2016, 149, 51–59. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, S.-J.; Oh, D.H.; Ku, S.K.; Li, D.X.; Yong, C.S.; Choi, H.-G. Wound healing evaluation of sodium fucidate-loaded polyvinylalcohol/sodium carboxymethylcellulose-based wound dressing. Arch. Pharm. Res. 2010, 33, 1083–1089. [Google Scholar] [CrossRef]
- Maver, T.; Kurečič, M.; Pivec, T.; Maver, U.; Gradišnik, L.; Gašparič, P.; Kaker, B.; Bratuša, A.; Hribernik, S.; Kleinschek, K.S. Needleless electrospun carboxymethyl cellulose/polyethylene oxide mats with medicinal plant extracts for advanced wound care applications. Cellulose 2020, 27, 4487–4580. [Google Scholar] [CrossRef]
- Alipour, R.; Khorshidi, A.; Shojaei, A.F.; Mashayekhi, F.; Moghaddam, M.J.M. Silver Sulfadiazine-loaded PVA/CMC Nanofibers for the Treatment of Wounds Caused by Excision. Fibers Polym. 2019, 20, 2461–2469. [Google Scholar] [CrossRef]
- Almasian, A.; Najafi, F.; Eftekhari, M.; Ardekani, M.R.S.; Sharifzadeh, M.; Khanavi, M. Polyurethane/carboxymethylcellulose nanofibers containing Malva sylvestris extract for healing diabetic wounds: Preparation, characterization, in vitro and in vivo studies. Mater. Sci. Eng. C 2020, 14, 111039. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.A.; Ali, E.A.; Soliman, A.A.; Shen, J.; Abou-Zeid, N.Y.; Hudson, S.M. Multi-layer dressing made of laminated electrospun nanowebs and cellulose-based adhesive for comprehensive wound care. Int. J. Biol. Macromol. 2020, 162, 629–644. [Google Scholar] [CrossRef]
- Shi, D.; Wang, F.; Lan, T.; Zhang, Y.; Shao, Z. Convenient fabrication of carboxymethyl cellulose electrospun nanofibers functionalized with silver nanoparticles. Cellulose 2016, 23, 1899–1909. [Google Scholar] [CrossRef]
- Maver, T.; Kurečič, M.; Smrke, D.M.; Kleinschek, K.S.; Maver, U. Electrospun nanofibrous CMC/PEO as a part of an effective pain-relieving wound dressing. J. Sol-Gel Sci. Technol. 2016, 79, 475–486. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.G.; Kang, S.W.; Lee, K.J. Nanofiber-Based Hydrocolloid from Colloid Electrospinning Toward Next Generation Wound Dressing. Macromol. Mater. Eng. 2016, 301, 818–826. [Google Scholar] [CrossRef]
- Esmaeili, A.; Haseli, M. Optimization, synthesis, and characterization of coaxial electrospun sodium carboxymethyl cellulose-graft-methyl acrylate/poly (ethylene oxide) nanofibers for potential drug-delivery applications. Carbohydr. Polym. 2017, 173, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Joorabloo, A.; Khorasani, M.T.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Fabrication of heparinized nano ZnO/poly (vinylalcohol)/carboxymethyl cellulose bionanocomposite hydrogels using artificial neural network for wound dressing application. J. Ind. Eng. Chem. 2019, 70, 253–263. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Miao, D.; Sui, S.; Deng, F.; Dong, C.; Zhang, L.; Zhu, P. Preparation and characterization of carboxymethylcellulose hydrogel fibers. J. Eng. Fibers Fabr. 2018, 13, 155892501801300302. [Google Scholar] [CrossRef] [Green Version]
- De Lima, G.G.; de Lima, D.W.; de Oliveira, M.J.; Lugão, A.B.; Alcântara, M.T.; Devine, D.M.; de Sá, M.J. Synthesis and in vivo behavior of PVP/CMC/agar hydrogel membranes impregnated with silver nanoparticles for wound healing applications. ACS Appl. Bio Mater. 2018, 1, 1842–1852. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Moreira, A.P.D.; Thiré, R.M.d.S.M.; Quilty, B.; Passos, T.M.; Simon, P.; Mancini, M.C.; McGuinness, G.B. Absorbent polyvinyl alcohol–sodium carboxymethyl cellulose hydrogels for propolis delivery in wound healing applications. Polym. Eng. Sci. 2017, 57, 1224–1233. [Google Scholar] [CrossRef]
- Gao, Z.; Yu, Z.; Huang, C.; Duan, L.; Gao, G.H. Carboxymethyl cellulose reinforced poly (vinyl alcohol) with trimethylol melamine as a chemical crosslinker. J. Appl. Polym. Sci. 2017, 134, 134. [Google Scholar] [CrossRef]
- Park, J.-S.; An, S.-J.; Jeong, S.-I.; Gwon, H.-J.; Lim, Y.-M.; Nho, Y.-C. Chestnut honey impregnated carboxymethyl cellulose hydrogel for diabetic ulcer healing. Polymers 2017, 9, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinklárková, L.; Masteiková, R.; Vetchý, D.; Doležel, P.; Bernatonienė, J. Formulation of novel layered sodium carboxymethylcellulose film wound dressings with ibuprofen for alleviating wound pain. Biomed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Ye, Y.; Li, D.; Li, X.; Mu, C. Biological properties of dialdehyde carboxymethyl cellulose crosslinked gelatin–PEG composite hydrogel fibers for wound dressings. Carbohydr. Polym. 2016, 137, 508–514. [Google Scholar] [CrossRef]
- Das, A.; Kumar, A.; Patil, N.B.; Viswanathan, C.; Ghosh, D. Preparation and characterization of silver nanoparticle loaded amorphous hydrogel of carboxymethylcellulose for infected wounds. Carbohydr. Polym. 2015, 130, 254–261. [Google Scholar] [CrossRef]
- Kawasaki, T.; Nakaji-Hirabayashi, T.; Masuyama, K.; Fujita, S.; Kitano, H. Complex film of chitosan and carboxymethyl cellulose nanofibers. Colloids Surf. B Biointerfaces 2016, 139, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili, A.; Haseli, M. Electrospinning of thermoplastic carboxymethyl cellulose/poly(ethylene oxide) nanofibers for use in drug-release systems. Mater. Sci. Eng. C 2017, 77, 1117–1127. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P. Electrospun Nanocomposites Containing Cellulose and Its Derivatives Modified with Specialized Biomolecules for an Enhanced Wound Healing. Nanomaterials 2020, 10, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Duan, X.-P.; Li, Y.-M.; Yang, D.-P.; Long, Y.-Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C 2017, 76, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Farbodi, M.; Khoshkbar Sadeghi, A.R. Preparation of Carboxymethyl Cellulose/Calcium Alginate/Polyvinyl Alcohol/Silver Nanocomposite by Electrospinning Method and Its Performance as Wound Dressing. Modares J. Biotechnol. 2018, 9, 593–601. [Google Scholar]
- Hebeish, A.; Sharaf, S. Novel nanocomposite hydrogel for wound dressing and other medical applications. RSC Adv. 2015, 5, 103036–103046. [Google Scholar] [CrossRef]
- Agarwal, R.; Alam, M.S.; Gupta, B. Polyvinyl alcohol-polyethylene oxide-carboxymethyl cellulose membranes for drug delivery. J. Appl. Polym. Sci. 2013, 129, 3728–3736. [Google Scholar] [CrossRef]
- Agarwal, R.; Sarwar Alam, M.; Gupta, B. Preparation of curcumin loaded poly(Vinyl Alcohol)-poly(Ethylene Oxide)-carboxymethyl cellulose membranes for wound care application. J. Biomater. Tissue Eng. 2013, 3, 273–283. [Google Scholar] [CrossRef]
- Salama, A.; El-Sakhawy, M.; Kamel, S. Carboxymethyl cellulose based hybrid material for sustained release of protein drugs. Int. J. Biol. Macromol. 2016, 93, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2014, 21, 1–30. [Google Scholar] [CrossRef]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications: A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, J.; Yang, G. Present status and applications of bacterial cellulose-based materials for skin tissue repair. Carbohydr. Polym. 2013, 92, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M. Microbial cellulose–The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. A 2006, 76, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Dahman, Y. Nanostructured biomaterials and biocomposites from bacterial cellulose nanofibers. J. Nanosci. Nanotechnol. 2009, 9, 5105–5122. [Google Scholar] [CrossRef]
- Kucińska-Lipka, J.; Gubanska, I.; Janik, H. Bacterial cellulose in the field of wound healing and regenerative medicine of skin: Recent trends and future prospectives. Polym. Bull. 2015, 72, 2399–2419. [Google Scholar] [CrossRef]
- Abeer, M.M.; Mohd Amin, M.C.I.; Martin, C. A review of bacterial cellulose-based drug delivery systems: Their biochemistry, current approaches and future prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Eslahi, N.; Mahmoodi, A.; Mahmoudi, N.; Zandi, N.; Simchi, A. Processing and properties of nanofibrous bacterial cellulose-containing polymer composites: A review of recent advances for biomedical applications. Polym. Rev. 2020, 60, 144–170. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trček, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Song, W.; Luan, J.; Wen, X.; Wu, Z.; Chen, X.; Wang, Q.; Guo, S. In situ synthesis of silver-nanoparticles/bacterial cellulose composites for slow-released antimicrobial wound dressing. Carbohydr. Polym. 2014, 102, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Xu, L.; Zhang, L.; Ye, H.; Zhao, J.; Liu, Z.; Rong, J. Superior hybrid hydrogels of polyacrylamide enhanced by bacterial cellulose nanofiber clusters. Mater. Sci. Eng. C 2016, 67, 221–230. [Google Scholar] [CrossRef]
- Sureshkumar, M.; Siswanto, D.Y.; Lee, C.K. Magnetic antimicrobial nanocomposite based on bacterial cellulose and silver nanoparticles. J. Mater. Chem. 2010, 20, 6948–6955. [Google Scholar] [CrossRef]
- Pal, S.; Nisi, R.; Stoppa, M.; Licciulli, A. Silver-Functionalized Bacterial Cellulose as Antibacterial Membrane for Wound-Healing Applications. ACS Omega 2017, 2, 3632–3639. [Google Scholar] [CrossRef] [Green Version]
- Azarniya, A.; Eslahi, N.; Mahmoudi, N.; Simchi, A. Effect of graphene oxide nanosheets on the physico-mechanical properties of chitosan/bacterial cellulose nanofibrous composites. Compos. A Appl. Sci. Manuf. 2016, 85, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Yin, L.; Yu, Q.; Zhong, C.; Zhang, J. Bacterial cellulose nanofibrous membrane as thermal stable separator for lithium-ion batteries. J. Power Sources 2015, 279, 21–27. [Google Scholar] [CrossRef]
- Morena, A.G.; Roncero, M.B.; Valenzuela, S.V.; Valls, C.; Vidal, T.; Pastor, F.I.J.; Diaz, P.; Martínez, J. Laccase/TEMPO-mediated bacterial cellulose functionalization: Production of paper-silver nanoparticles composite with antimicrobial activity. Cellulose 2019, 26, 8655–8668. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Li, X.; Shi, S.; Shen, W.; Zhang, X.; Wang, H. In situ synthesis of silver chloride nanoparticles into bacterial cellulose membranes. Mater. Sci. Eng. C 2009, 29, 1216–1219. [Google Scholar] [CrossRef]
- Khalid, A.; Khan, R.; Ul-Islam, M.; Khan, T.; Wahid, F. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr. Polym. 2017, 164, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.P.; Kung, H.N.; Tsai, Y.S.; Tseng, T.N.; Hsu, K.D.; Cheng, K.C. Novel dextran modified bacterial cellulose hydrogel accelerating cutaneous wound healing. Cellulose 2017, 24, 4927–4937. [Google Scholar] [CrossRef]
- Favi, P.M.; Benson, R.S.; Neilsen, N.R.; Hammonds, R.L.; Bates, C.C.; Stephens, C.P.; Dhar, M.S. Cell proliferation, viability, and in vitro differentiation of equine mesenchymal stem cells seeded on bacterial cellulose hydrogel scaffolds. Mater. Sci. Eng. C 2013, 33, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Fricain, J.C.; Granja, P.L.; Barbosa, M.A.; de Jéso, B.; Barthe, N.; Baquey, C. Cellulose phosphates as biomaterials. In vivo biocompatibility studies. Biomaterials 2002, 23, 971–980. [Google Scholar] [CrossRef]
- Loh, E.Y.X.; Mohamad, N.; Fauzi, M.B.; Ng, M.H.; Ng, S.F.; Mohd Amin, M.C.I. Development of a bacterial cellulose-based hydrogel cell carrier containing keratinocytes and fibroblasts for full-thickness wound healing. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahid, F.; Hu, X.H.; Chu, L.Q.; Jia, S.R.; Xie, Y.Y.; Zhong, C. Development of bacterial cellulose/chitosan based semi-interpenetrating hydrogels with improved mechanical and antibacterial properties. Int. J. Biol. Macromol. 2019, 122, 380–387. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Zheng, W.; Gong, N.; Chen, L.; Jiang, X.; Yang, G. Bacterial cellulose-hyaluronan nanocomposite biomaterials as wound dressings for severe skin injury repair. J. Mater. Chem. B 2015, 3, 3498–3507. [Google Scholar] [CrossRef]
- Zmejkoski, D.; Spasojević, D.; Orlovska, I.; Kozyrovska, N.; Soković, M.; Glamočlija, J.; Dmitrović, S.; Matović, B.; Tasić, N.; Maksimović, V. Bacterial cellulose-lignin composite hydrogel as a promising agent in chronic wound healing. Int. J. Biol. Macromol. 2018, 118, 494–503. [Google Scholar] [CrossRef] [Green Version]
- Cam, M.E.; Crabbe-Mann, M.; Alenezi, H.; Hazar-Yavuz, A.N.; Ertas, B.; Ekentok, C.; Ozcan, G.S.; Topal, F.; Guler, E.; Yazir, Y. The comparision of glybenclamide and metformin-loaded bacterial cellulose/gelatin nanofibres produced by a portable electrohydrodynamic gun for diabetic wound healing. Eur. Polym. J. 2020, 134, 109844. [Google Scholar] [CrossRef]
- Azarniya, A.; Tamjid, E.; Eslahi, N.; Simchi, A. Modification of bacterial cellulose/keratin nanofibrous mats by a tragacanth gum-conjugated hydrogel for wound healing. Int. J. Biol. Macromol. 2019, 134, 280–289. [Google Scholar] [CrossRef]
- Aydogdu, M.O.; Altun, E.; Crabbe-Mann, M.; Brako, F.; Koc, F.; Ozen, G.; Kuruca, S.E.; Edirisinghe, U.; Luo, C.; Gunduz, O. Cellular interactions with bacterial cellulose: Polycaprolactone nanofibrous scaffolds produced by a portable electrohydrodynamic gun for point-of-need wound dressing. Int. Wound J. 2018, 15, 789–797. [Google Scholar] [CrossRef]
- Qiu, Y.; Qiu, L.; Cui, J.; Wei, Q. Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater. Sci. Eng. C 2016, 59, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Tsuji, M.; Morimoto, M.; Saimoto, H.; Yano, H. Synthesis of silver nanoparticles templated by TEMPO-mediated oxidized bacterial cellulose nanofibers. Biomacromolecules 2009, 10, 2714–2717. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Yang, Y.-N.; Ho, Y.-C.; Tsai, M.-L.; Mi, F.-L. Drug release and antioxidant/antibacterial activities of silymarin-zein nanoparticle/bacterial cellulose nanofiber composite films. Carbohydr. Polym. 2018, 180, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Ardila, N.; Medina, N.; Arkoun, M.; Heuzey, M.-C.; Ajji, A.; Panchal, C.J. Chitosan–bacterial nanocellulose nanofibrous structures for potential wound dressing applications. Cellulose 2016, 23, 3089–3104. [Google Scholar] [CrossRef]
- Chuah, C.; Wang, J.; Tavakoli, J.; Tang, Y. Novel bacterial cellulose-poly (acrylic acid) hybrid hydrogels with controllable antimicrobial ability as dressings for chronic wounds. Polymers 2018, 10, 1323. [Google Scholar] [CrossRef] [Green Version]
- Volova, T.; Shumilova, A.; Nikolaeva, E.; Kirichenko, A.; Shishatskaya, E. Biotechnological wound dressings based on bacterial cellulose and degradable copolymer P (3HB/4HB). Int. J. Biol. Macromol. 2019, 131, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Spaic, M.; Small, D.P.; Cook, J.R.; Wan, W. Characterization of anionic and cationic functionalized bacterial cellulose nanofibres for controlled release applications. Cellulose 2014, 21, 1529–1540. [Google Scholar] [CrossRef]
- Ciechanska, D. Multifunctional bacterial cellulose/chitosan composite materials for medical applications. Fibres Text. East. Eur. 2004, 12, 69–72. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).