Effects of Resonant Electromagnetic Fields on Biofilm Formation in Pseudomonas aeruginosa
Abstract
:Featured Application
Abstract
1. Introduction
The Relationship between Resonant Electromagnetic Fields, Coherence in Water, and Biological Effects
2. Materials and Methods
2.1. Bioresonance Laboratory
2.2. Exposure System and Procedure
2.3. Microbiological Growth and Biofilm Assay
2.3.1. Inoculum Standardization
2.3.2. Assay for Planktonic Bacterial Growth
2.3.3. Assay for Biofilm Adhesion to Polystyrene Pegs
2.3.4. Calculation of the Relative Biofilm Adhesion Units
3. Results
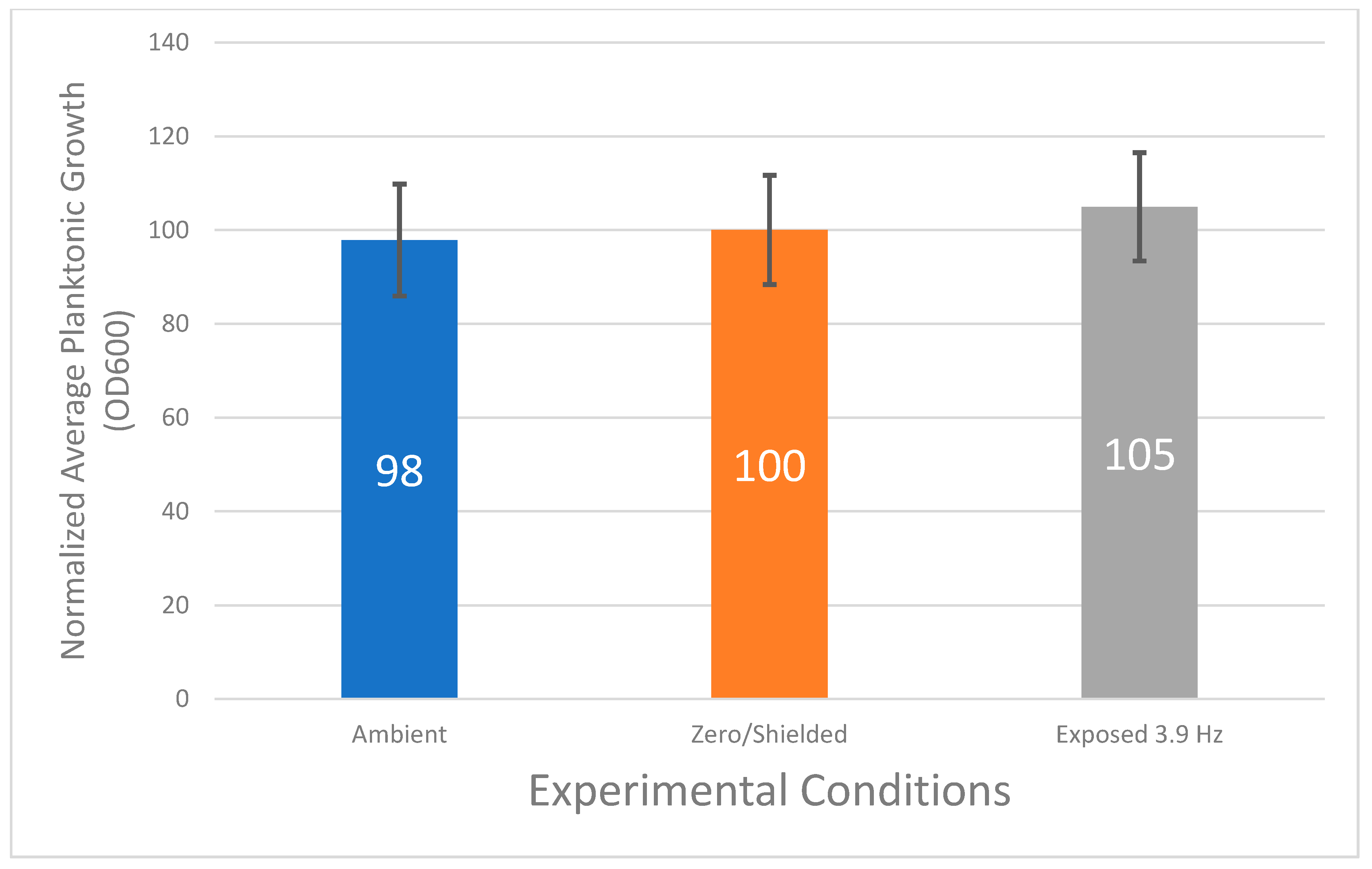
3.1. Planktonic Bacterial Growth Does Not Vary Statistically under Various Magnetic Field Conditions
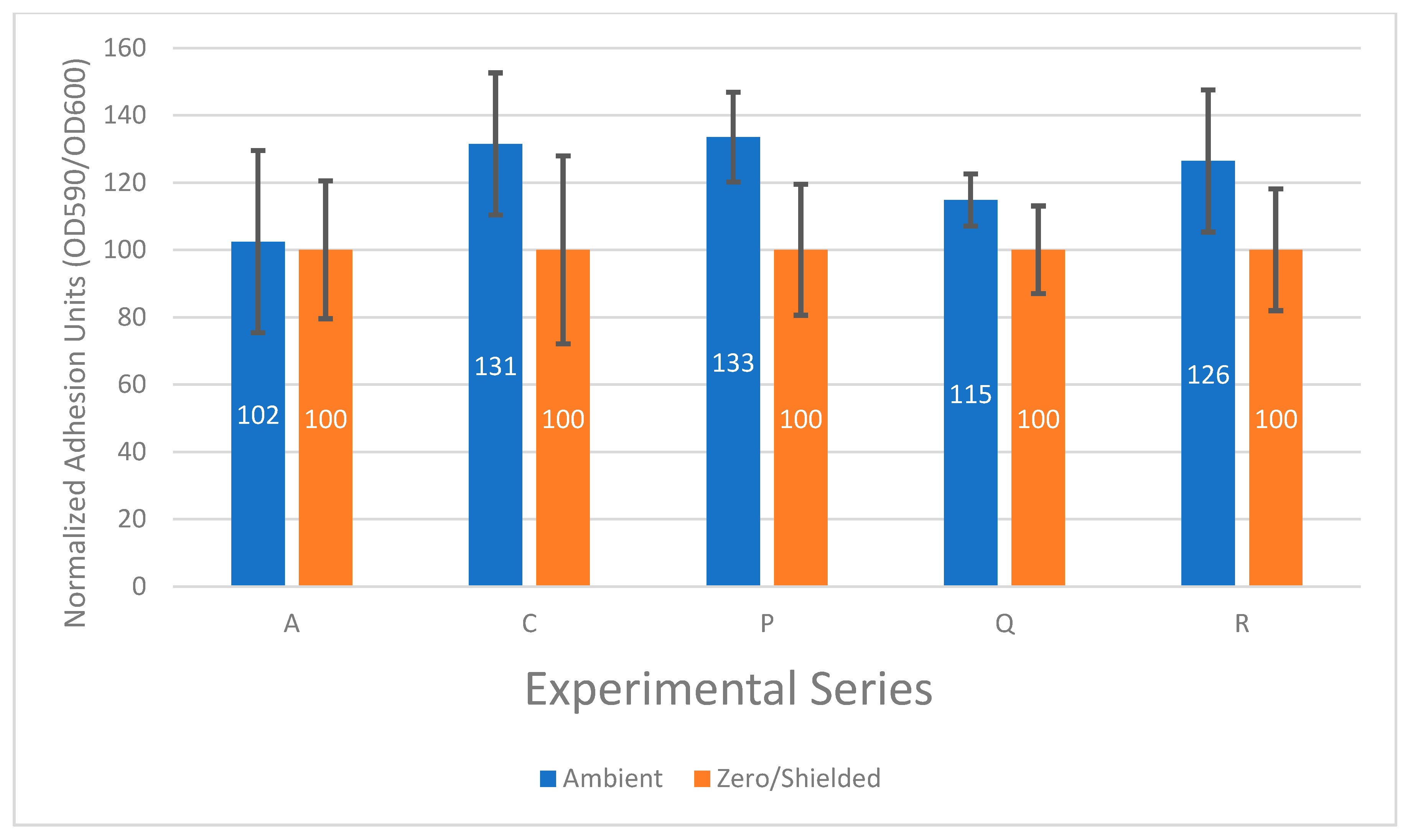
3.2. The Effect of the Ambient Electromagnetic Field on Bacterial Biofilm Formation Is Not Constant
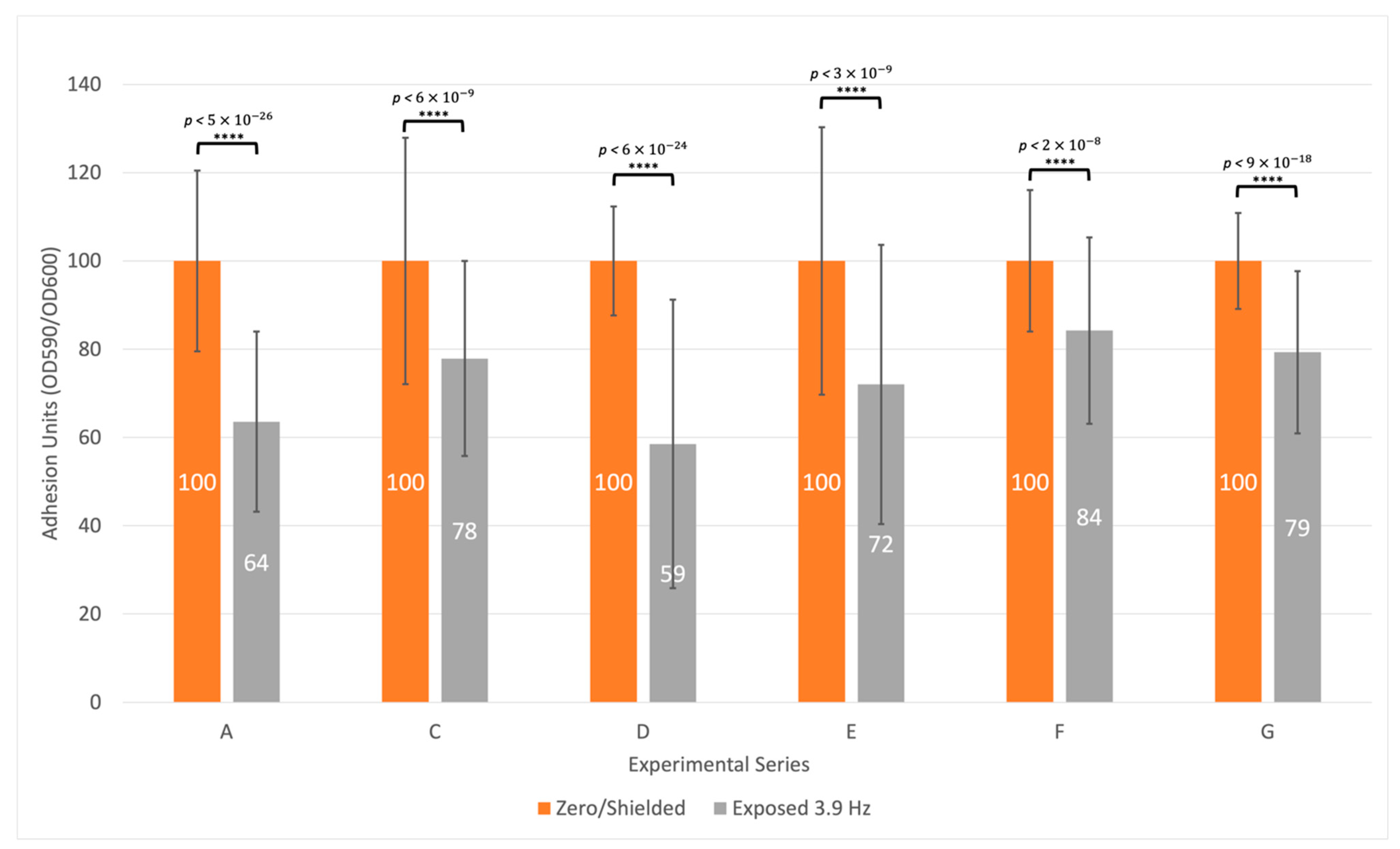
3.3. Biofilm Formation Is Consistently Inhibited at a Specific Frequency
3.4. Other Frequencies Tested Showed Variable and Inconclusive Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toner, E.; Adalja, A.; Gronvall, G.K.; Cicero, A.; Inglesby, T.V. Antimicrobial Resistance Is a Global Health Emergency. Health Secur. 2015, 13, 153–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muehsam, D.; Ventura, C. Life Rhythm as a Symphony of Oscillatory Patterns: Electromagnetic Energy and Sound Vibration Modulates Gene Expression for Biological Signaling and Healing. Glob. Adv. Health Med. 2014, 3, 40–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisi, A.; Ledda, M.; de Carlo, F.; Pozzi, D.; Messina, E.; Gaetani, R.; Chimenti, I.; Barile, L.; Giacomello, A.; D’Emilia, E.; et al. Ion Cyclotron Resonance as a Tool in Regenerative Medicine. Electromagn. Biol. Med. 2008, 27, 127–133. [Google Scholar] [CrossRef]
- Giuliani, L.; D’Emilia, E.; Grimaldi, S.; Lisi, A.; Bobkova, N.; Zhadin, M.N. Investigating the Icr Effect in a Zhadin’s Cell. Int. J. Biomed. Sci. 2009, 5, 181–186. [Google Scholar] [PubMed]
- Fabian, D.; Guillermo Prieto Eibl, M.D.P.; Alnahhas, I.; Sebastian, N.; Giglio, P.; Puduvalli, V.; Gonzalez, J.; Palmer, J.D. Treatment of Glioblastoma (GBM) with the Addition of Tumor-Treating Fields (TTF): A Review. Cancers 2019, 11, 174. [Google Scholar] [CrossRef] [Green Version]
- Liboff, A.R. Cyclotron Resonance in Membrane Transport. In Interactions between Electromagnetic Fields and Cells; Chiabrera, A., Nicolini, C., Schwan, H.P., Eds.; Plenum Press: New York, NY, USA, 1985; pp. 281–290. [Google Scholar]
- Zhadin, M.N.; Novikov, V.V.; Barnes, F.S.; Pergola, N.F. Combined Action of Static and Alternating Magnetic Fields on Ionic Current in Aqueous Glutamic Acid Solution. Bioelectromagnetics 1998, 19, 41–45. [Google Scholar] [CrossRef]
- Adair, R.K. Constraints on Biological Effects of Weak Extremely-Low-Frequency Electromagnetic Fields. Phys. Rev. A 1991, 43, 1039–1048. [Google Scholar] [CrossRef]
- Del Giudice, E.; Preparata, G.; Vitiello, G. Water as a Free Electric Dipole Laser. Phys. Rev. Lett. 1988, 61, 1085–1088. [Google Scholar] [CrossRef]
- Arani, R.; Bono, I.; Giudice, E.D.; Preparata, G. Qed Coherence and the Thermodynamics of Water. Int. J. Mod. Phys. B 1995, 9, 1813–1841. [Google Scholar] [CrossRef]
- Comisso, N.; Giudice, E.D.; Ninno, A.D.; Fleischmann, M.; Giuliani, L.; Mengoli, G.; Merlo, F.; Talpo, G. Dynamics of the Ion Cyclotron Resonance Effect on Amino Acids Adsorbed at the Interfaces. Bioelectromagnetics 2006, 27, 16–25. [Google Scholar] [CrossRef]
- Huang, C.; Wikfeldt, K.T.; Tokushima, T.; Nordlund, D.; Harada, Y.; Bergmann, U.; Niebuhr, M.; Weiss, T.M.; Horikawa, Y.; Leetmaa, M.; et al. The Inhomogeneous Structure of Water at Ambient Conditions. Proc. Natl. Acad. Sci. USA 2009, 106, 15214–15218. [Google Scholar] [CrossRef] [Green Version]
- Taschin, A.; Bartolini, P.; Eramo, R.; Righini, R.; Torre, R. Evidence of Two Distinct Local Structures of Water from Ambient to Supercooled Conditions. Nat. Commun. 2013, 4, 2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renati, P.; Kovacs, Z.; De Ninno, A.; Tsenkova, R. Temperature Dependence Analysis of the NIR Spectra of Liquid Water Confirms the Existence of Two Phases, One of Which Is in a Coherent State. J. Mol. Liq. 2019, 292, 111449. [Google Scholar] [CrossRef]
- Kim, K.H.; Späh, A.; Pathak, H.; Perakis, F.; Mariedahl, D.; Amann-Winkel, K.; Sellberg, J.A.; Lee, J.H.; Kim, S.; Park, J.; et al. Maxima in the Thermodynamic Response and Correlation Functions of Deeply Supercooled Water. Science 2017, 358, 1589–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preparata, G. QED Coherence in Matter; World Scientific: Singapore, 1995; ISBN 978-981-02-2249-9. [Google Scholar]
- Schrödinger, E. What Is Life? With Mind and Matter and Autobiographical Sketches; Canto; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Del Giudice, E.; Fleischmann, M.; Preparata, G.; Talpo, G. On the “Unreasonable” Effects of ELF Magnetic Fields upon a System of Ions. Bioelectromagnetics 2002, 23, 522–530. [Google Scholar] [CrossRef]
- Zhadin, M.; Giuliani, L. Some Problems in Modern Bioelectromagnetics. Electromagn. Biol. Med. 2006, 25, 227–243. [Google Scholar] [CrossRef]
- De Ninno, A.; Congiu Castellano, A. Deprotonation of Glutamic Acid Induced by Weak Magnetic Field: An FTIR-ATR Study. Bioelectromagnetics 2011, 32, 218–225. [Google Scholar] [CrossRef]
- Giuliani, L.; Grimaldi, S.; Lisi, A.; D’Emilia, E.; Bobkova, N.; Zhadin, M. Action of Combined Magnetic Fields on Aqueous Solution of Glutamic Acid: The Further Development of Investigations. Biomagn. Res. Technol. 2008, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Browne, M.; Foley, S.; Babck, N.; Olshefsky, S.; Holland, A.; Ahern, H. Non-Thermal Inhibition of Antibiotic Resistant Bacteria by Oscillating Pulsed Electric Fields (OPEF); The American Society of Microbiology: Denver, CO, USA, 2013. [Google Scholar]
- Di Bonaventura, G.; Pompilio, A.; Crocetta, V.; De Nicola, S.; Barbaro, F.; Giuliani, L.; D’Emilia, E.; Fiscarelli, E.; Bellomo, R.G.; Saggini, R. Exposure to Extremely Low-Frequency Magnetic Field Affects Biofilm Formation by Cystic Fibrosis Pathogens. Future Microbiol. 2014, 9, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Ferrara, S.; Rossi, E.; Johansen, H.K.; Molin, S.; Bertoni, G. The Small RNA ErsA of Pseudomonas Aeruginosa Contributes to Biofilm Development and Motility through Post-Transcriptional Modulation of AmrZ. Front. Microbiol. 2018, 9, 238. [Google Scholar] [CrossRef]
- Harrison, J.J.; Stremick, C.A.; Turner, R.J.; Allan, N.D.; Olson, M.E.; Ceri, H. Microtiter Susceptibility Testing of Microbes Growing on Peg Lids: A Miniaturized Biofilm Model for High-Throughput Screening. Nat. Protoc. 2010, 5, 1236–1254. [Google Scholar] [CrossRef]
- Bartell, J.A.; Cameron, D.R.; Mojsoska, B.; Haagensen, J.A.J.; Pressler, T.; Sommer, L.M.; Lewis, K.; Molin, S.; Johansen, H.K. Bacterial Persisters in Long-Term Infection: Emergence and Fitness in a Complex Host Environment. PLoS Pathog. 2020, 16, e1009112. [Google Scholar] [CrossRef] [PubMed]
- Freebairn, D.; Linton, D.; Harkin-Jones, E.; Jones, D.S.; Gilmore, B.F.; Gorman, S.P. Electrical Methods of Controlling Bacterial Adhesion and Biofilm on Device Surfaces. Expert Rev. Med. Devices 2013, 10, 85–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [Green Version]
- D’Emilia, E.; Giuliani, L.; Lisi, A.; Ledda, M.; Grimaldi, S.; Montagnier, L.; Liboff, A.R. Lorentz Force in Water: Evidence That Hydronium Cyclotron Resonance Enhances Polymorphism. Electromagn. Biol. Med. 2015, 34, 370–375. [Google Scholar] [CrossRef]
- D’Emilia, E.; Ledda, M.; Foletti, A.; Lisi, A.; Giuliani, L.; Grimaldi, S.; Liboff, A.R. Weak-Field H3O+ Ion Cyclotron Resonance Alters Water Refractive Index. Electromagn. Biol. Med. 2017, 36, 55–62. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haagensen, J.A.J.; Bache, M.; Giuliani, L.; Blom, N.S. Effects of Resonant Electromagnetic Fields on Biofilm Formation in Pseudomonas aeruginosa. Appl. Sci. 2021, 11, 7760. https://doi.org/10.3390/app11167760
Haagensen JAJ, Bache M, Giuliani L, Blom NS. Effects of Resonant Electromagnetic Fields on Biofilm Formation in Pseudomonas aeruginosa. Applied Sciences. 2021; 11(16):7760. https://doi.org/10.3390/app11167760
Chicago/Turabian StyleHaagensen, Janus A. J., Michael Bache, Livio Giuliani, and Nikolaj S. Blom. 2021. "Effects of Resonant Electromagnetic Fields on Biofilm Formation in Pseudomonas aeruginosa" Applied Sciences 11, no. 16: 7760. https://doi.org/10.3390/app11167760
APA StyleHaagensen, J. A. J., Bache, M., Giuliani, L., & Blom, N. S. (2021). Effects of Resonant Electromagnetic Fields on Biofilm Formation in Pseudomonas aeruginosa. Applied Sciences, 11(16), 7760. https://doi.org/10.3390/app11167760





