Biomechanical Analysis of Posterior Ligaments of Cervical Spine and Laminoplasty
Abstract
:1. Introduction
2. Material and Methods
2.1. Model Development
2.2. Model Validation
2.3. Cervical Laminoplasty
2.4. Loads and Boundary Conditions
2.5. Data Analyses
3. Results
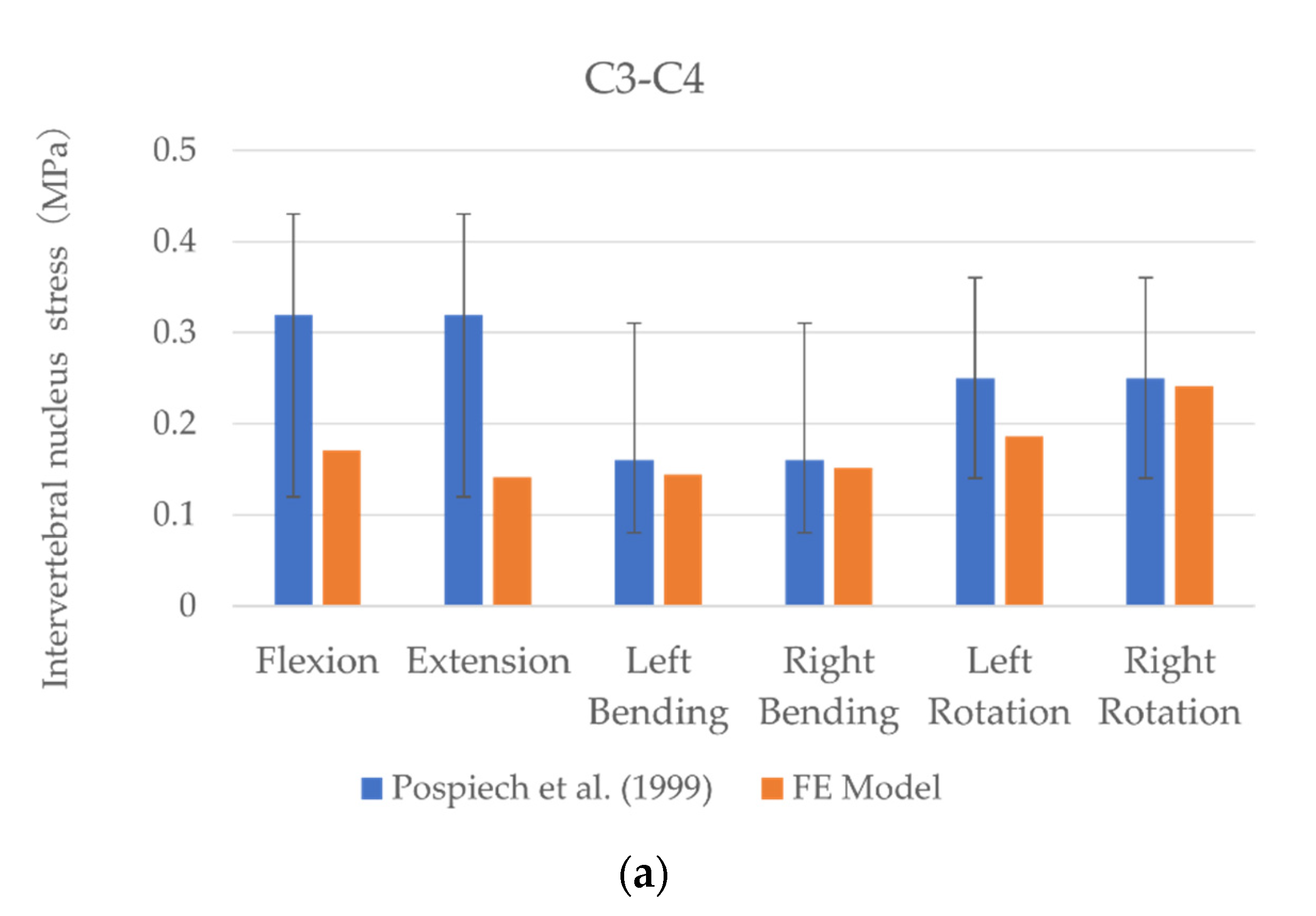
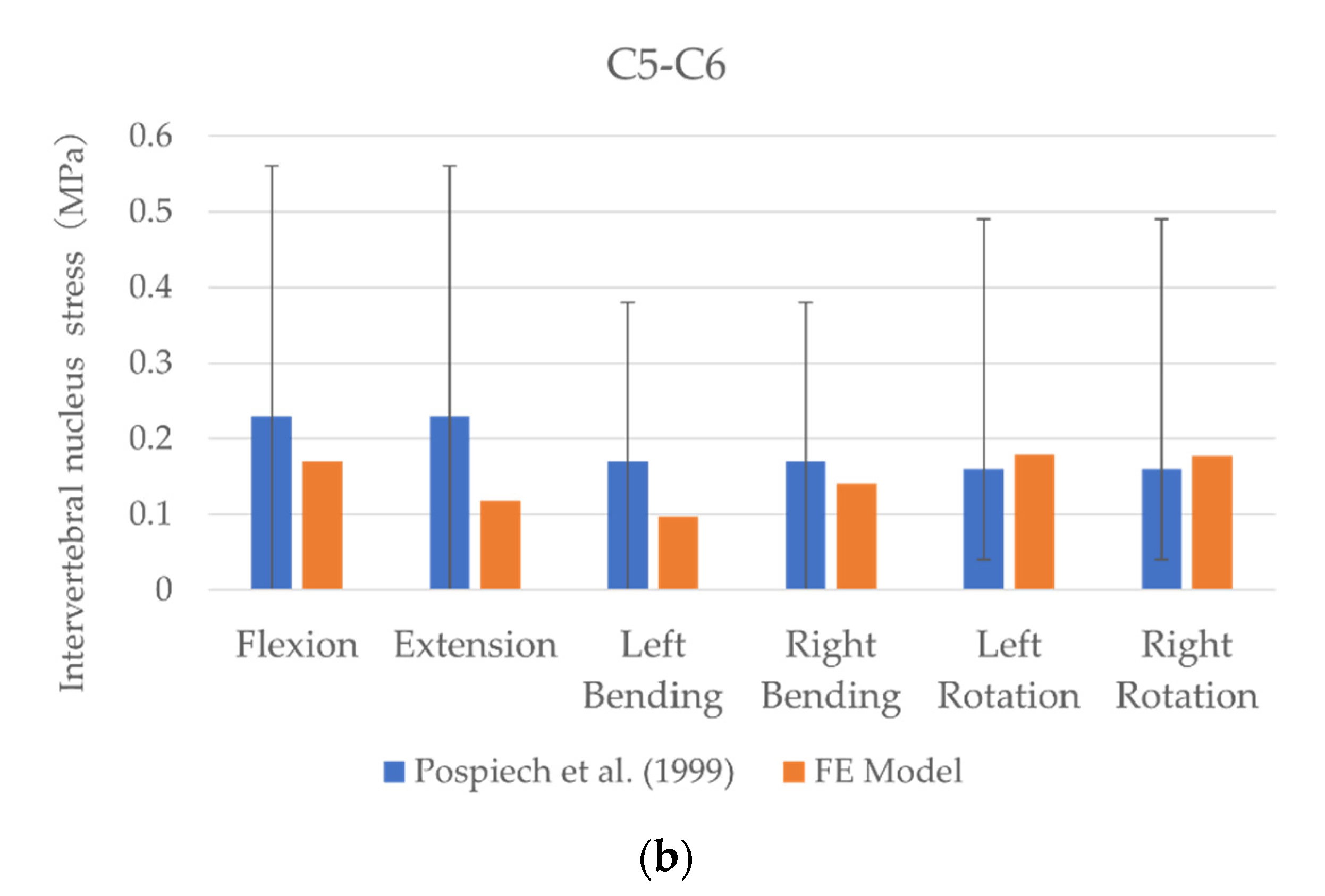
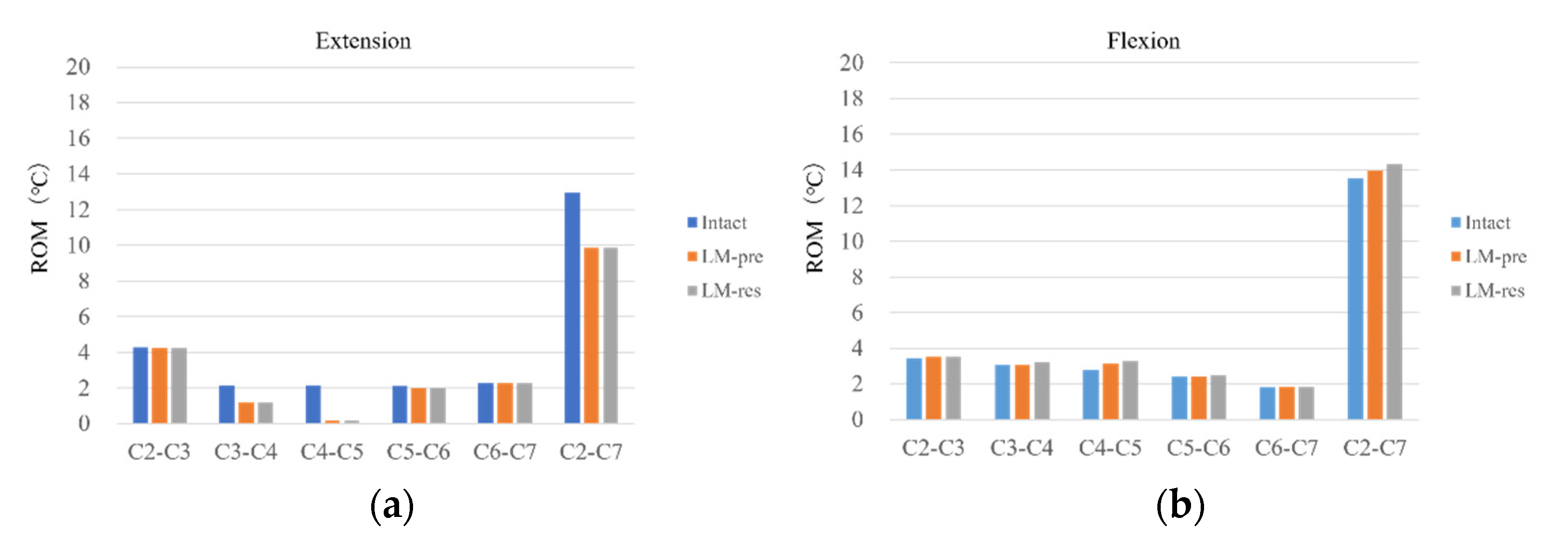
3.1. Model Validation
ROM
3.2. Intervertebral Nucleus Stress
3.3. Facet Contact Force
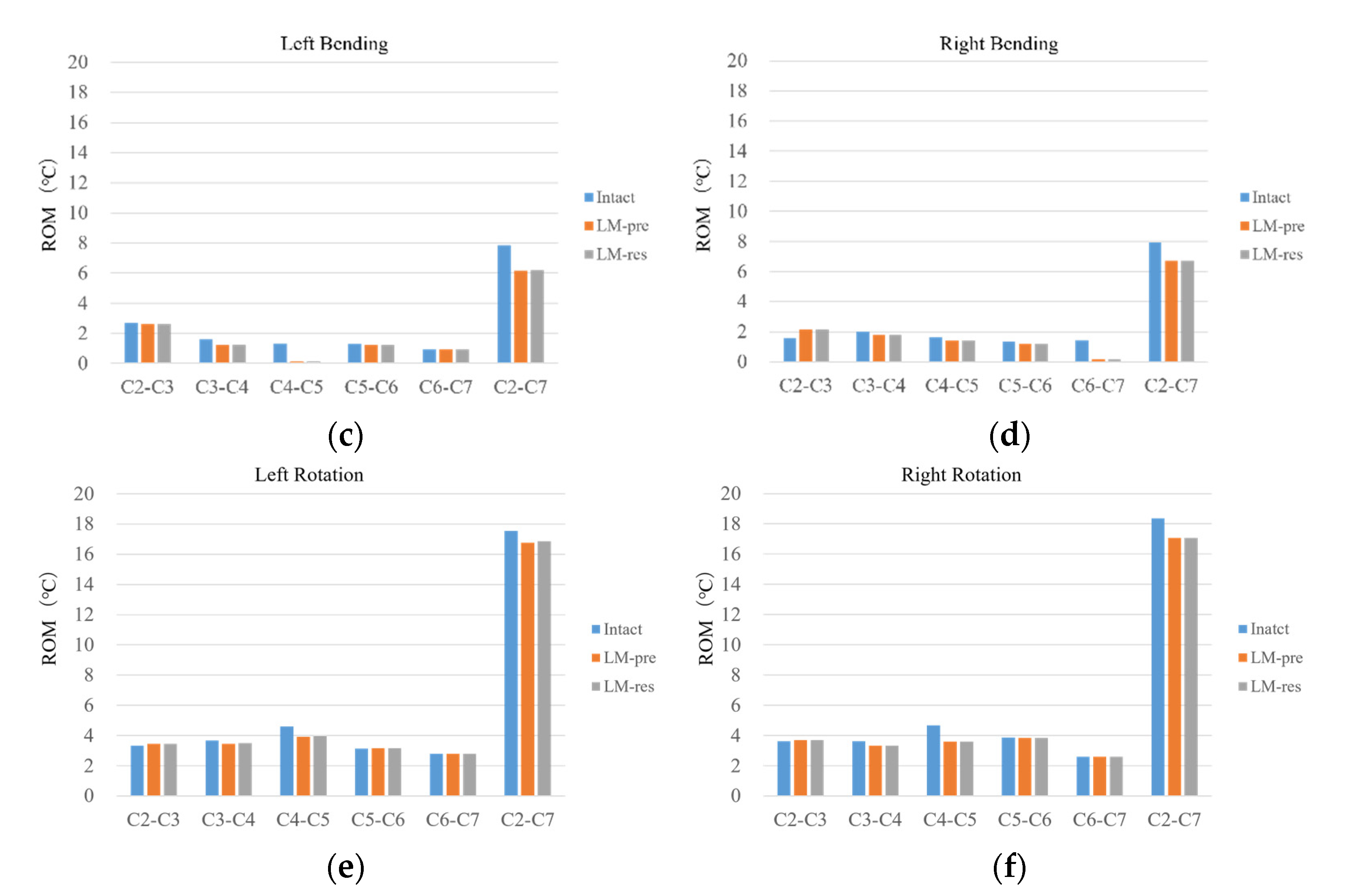
3.4. Comparison of Intact and the Laminoplasty Models
ROM
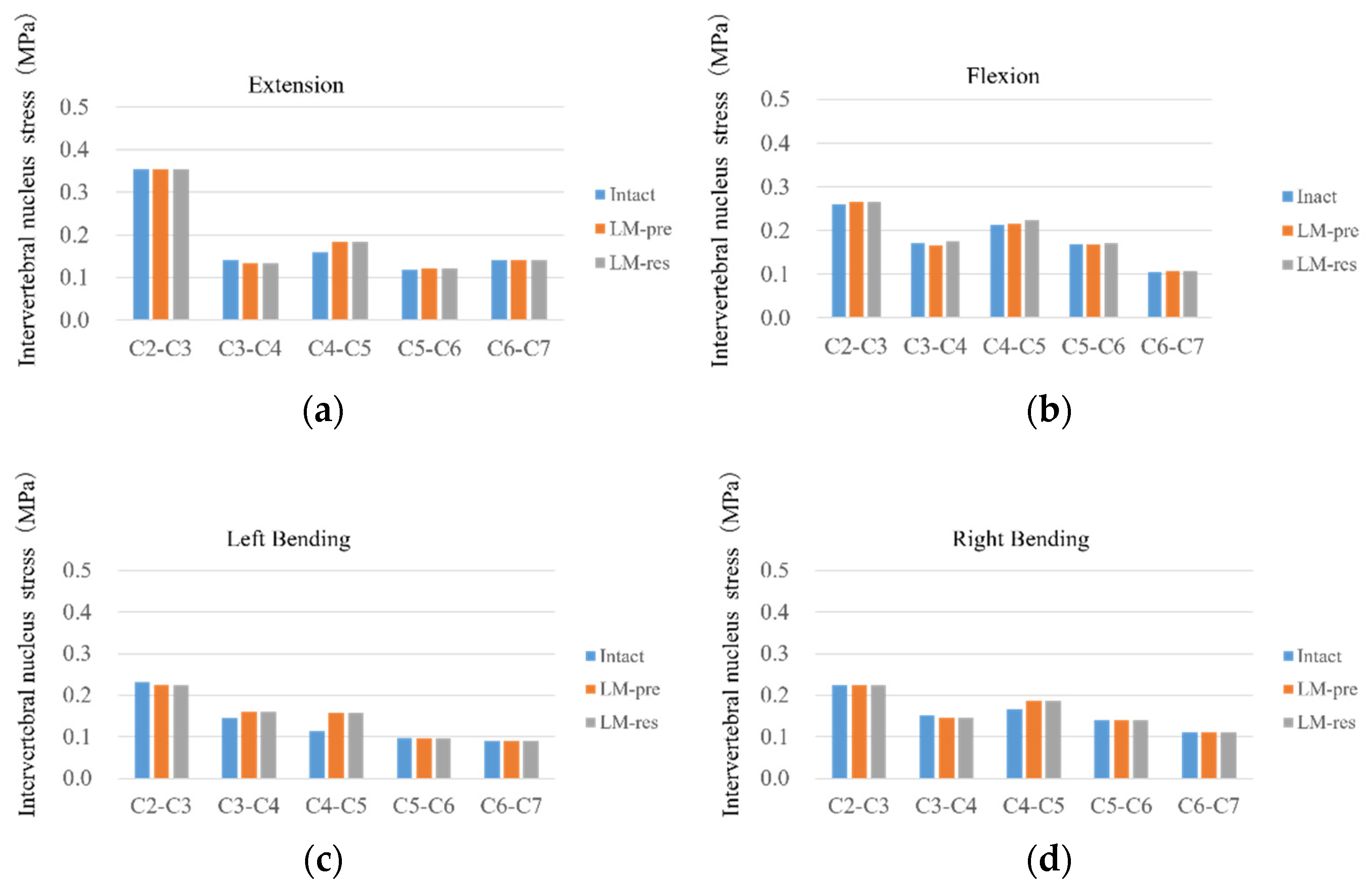
3.5. Intervertebral Nucleus Stress
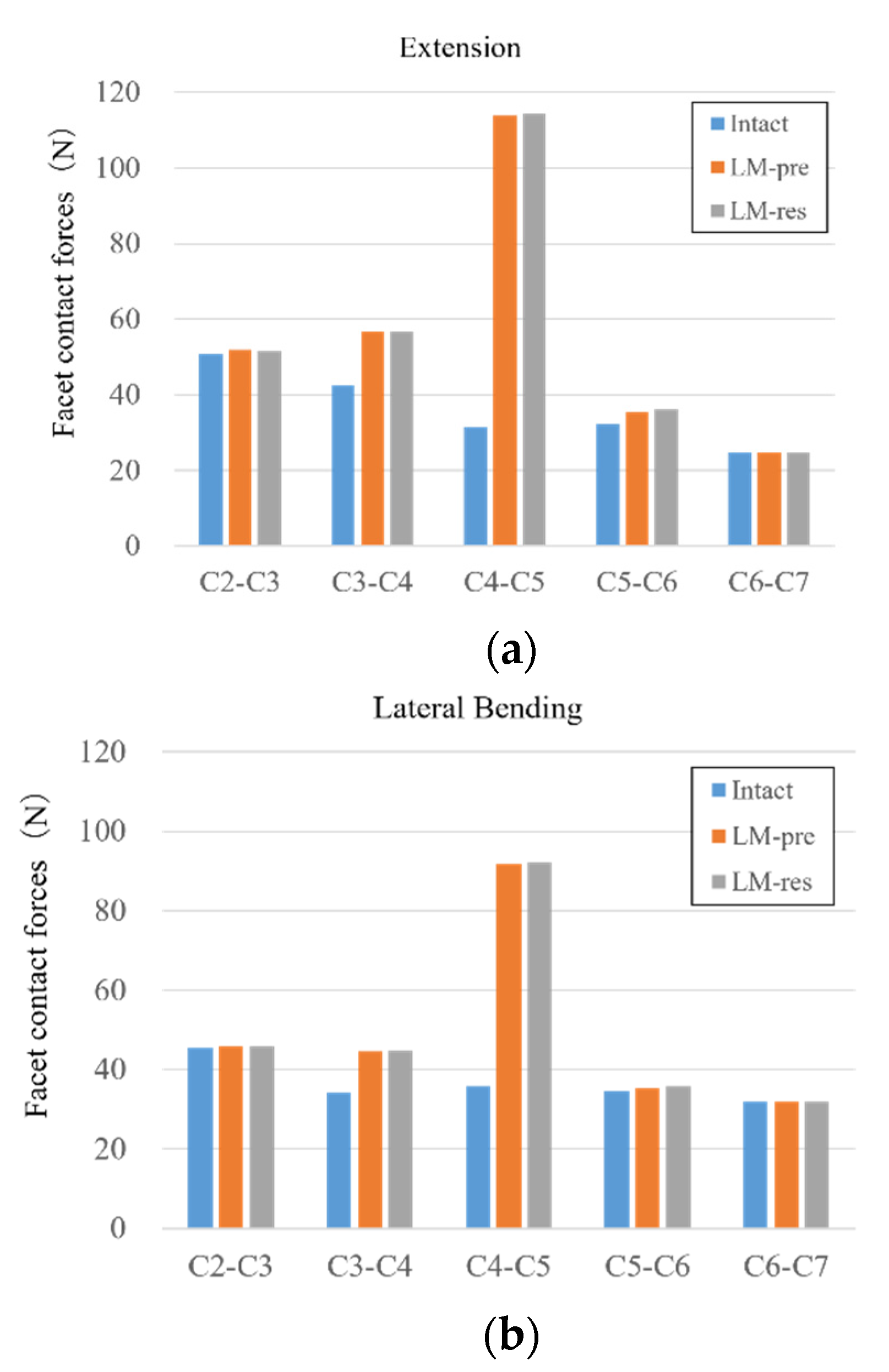
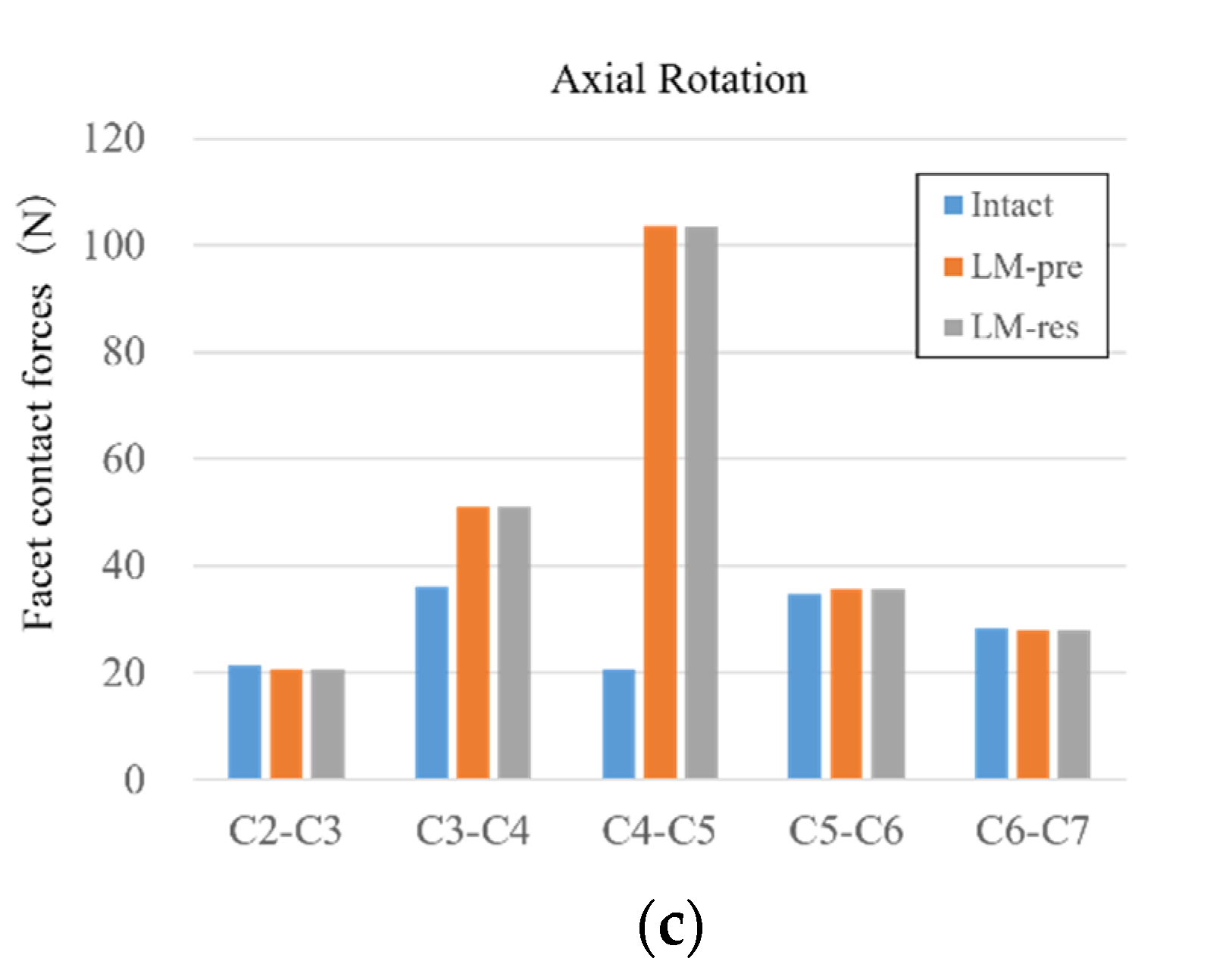
3.6. Facet Contact Forces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirabayashi, S.; Kitagawa, T.; Yamamoto, I.; Yamada, K.; Kawano, H. Development and Achievement of Cervical Laminoplasty and Related Studies on Cervical Myelopathy. Spine Surg. Relat. Res. 2020, 4, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Boody, B.S.; Lendner, M.; Vaccaro, A.R. Ossification of the posterior longitudinal ligament in the cervical spine: A review. Int. Orthop. 2019, 43, 797–805. [Google Scholar] [CrossRef]
- Iyer, A.; Azad, T.D.; Tharin, S. Cervical Spondylotic Myelopathy. Clin. Spine Surg. 2016, 29, 408–414. [Google Scholar] [CrossRef]
- Mazas, S.; Benzakour, A.; Castelain, J.E.; Damade, C.; Ghailane, S.; Gille, O. Cervical disc herniation: Which surgery? Int. Orthop. 2019, 43, 761–766. [Google Scholar] [CrossRef]
- Itoh, T.; Tsuji, H. Technical improvements and results of laminoplasty for compressive myelopathy in the cervical spine. Spine 1985, 10, 729–736. [Google Scholar] [CrossRef]
- Miyazaki, K.; Kirita, Y. Extensive simultaneous multisegment laminectomy for myelopathy due to the ossification of the posterior longitudinal ligament in the cervical region. Spine 1986, 11, 531–542. [Google Scholar] [CrossRef]
- Nagoshi, N.; Yoshii, T.; Egawa, S.; Sakai, K.; Kusano, K.; Nakagawa, Y.; Hirai, T.; Wada, K.; Katsumi, K.; Fujii, K.; et al. Comparison of Surgical Outcomes After Open- and Double-Door Laminoplasties for Patients with Cervical Ossification of the Posterior Longitudinal Ligament: A Prospective Multicenter Study. Spine 2021. [Google Scholar] [CrossRef]
- Chen, Q.; Qin, M.; Chen, F.; Ni, B.; Guo, Q.; Han, Z. Comparison of Outcomes Between Anterior Cervical Decompression and Fusion and Posterior Laminoplasty in the Treatment of 4-Level Cervical Spondylotic Myelopathy. World Neurosurg. 2019, 125, e341–e347. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Bou, E.H.; Jiang, W.; Zhou, F.; He, F.; Yang, H.; Liu, T. Comparative Study Between Anterior Cervical Discectomy and Fusion with ROI-C Cage and Laminoplasty for Multilevel Cervical Spondylotic Myelopathy without Spinal Stenosis. World Neurosurg. 2019, 121, e917–e924. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Endo, K.; Nishimura, H.; Suzuki, H.; Sawaji, Y.; Takamatsu, T.; Seki, T.; Murata, K.; Konishi, T.; Yamamoto, K. Cervical Kyphotic Deformity after Laminoplasty in Patients with Cervical Ossification of Posterior Longitudinal Ligament with Normal Sagittal Spinal Alignment. Spine Surg. Relat. Res. 2018, 2, 210–214. [Google Scholar] [CrossRef] [Green Version]
- Duetzmann, S.; Cole, T.; Ratliff, J.K. Cervical laminoplasty developments and trends, 2003–2013: A systematic review. J. Neurosurg. Spine 2015, 23, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Ebraheim, N.A.; Sanford, C.G., Jr.; Patil, V.; Haman, S.P.; Ren, L.; Yang, H. Preservation of the spinous process-ligament-muscle complex to prevent kyphotic deformity following laminoplasty. Spine J. 2007, 7, 159–164. [Google Scholar] [CrossRef]
- Takeshita, K.; Peterson, E.T.; Bylski-Austrow, D.; Crawford, A.H.; Nakamura, K. The nuchal ligament restrains cervical spine flexion. Spine 2004, 29, E388–E393. [Google Scholar] [CrossRef]
- Goel, V.K.; Clark, C.R.; McGowan, D.; Goyal, S. An in-vitro study of the kinematics of the normal, injured and stabilized cervical spine. J. Biomech. 1984, 17, 363–376. [Google Scholar] [CrossRef]
- Oxland, T.R.; Panjabi, M.M.; Southern, E.P.; Duranceau, J.S. An anatomic basis for spinal instability: A porcine trauma model. J. Orthop. Res. 1991, 9, 452–462. [Google Scholar] [CrossRef]
- Hirabayashi, S. Recent Surgical Methods of Double-door Laminoplasty of the Cervical Spine (Kurokawa’s Method). Spine Surg. Relat. Res. 2018, 2, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Minamide, A.; Yoshida, M.; Simpson, A.K.; Yamada, H.; Hashizume, H.; Nakagawa, Y.; Iwasaki, H.; Tsutsui, S.; Okada, M.; Takami, M.; et al. Microendoscopic laminotomy versus conventional laminoplasty for cervical spondylotic myelopathy: 5-year follow-up study. J. Neurosurg. Spine 2017, 27, 403–409. [Google Scholar] [CrossRef]
- Shiraishi, T.; Fukuda, K.; Yato, Y.; Nakamura, M.; Ikegami, T. Results of skip laminectomy-minimum 2-year follow-up study compared with open-door laminoplasty. Spine 2003, 28, 2667–2672. [Google Scholar] [CrossRef] [PubMed]
- Kanchiku, T.; Imajo, Y.; Suzuki, H.; Yoshida, Y.; Nishida, N.; Taguchi, T. Results of surgical treatment of cervical spondylotic myelopathy in patients aged 75 years or more: A comparative study of operative methods. Arch. Orthop. Trauma. Surg. 2014, 134, 1045–1050. [Google Scholar] [CrossRef]
- Kallemeyn, N.; Gandhi, A.; Kode, S.; Shivanna, K.; Smucker, J.; Grosland, N. Validation of a C2–C7 cervical spine finite element model using specimen-specific flexibility data. Med. Eng. Phys. 2010, 32, 482–489. [Google Scholar] [CrossRef]
- Venkataramana, M.P.; Hans, S.A.; Bawab, S.Y.; Keifer, O.P.; Woodhouse, M.L.; Layson, P.D. Effects of initial seated position in low speed rear-end impacts: A comparison with the TNO rear impact dummy (TRID) model. Traffic Inj. Prev. 2005, 6, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Adam, C.J.; Evans, J.H.; Pettet, G.J.; Pearcy, M.J. Nonlinear finite element analysis of anular lesions in the L4/5 intervertebral disc. J. Biomech. 2007, 40, 2744–2751. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.K.; Clausen, J.D. Prediction of load sharing among spinal components of a C5-C6 motion segment using the finite element approach. Spine 1998, 23, 684–691. [Google Scholar] [CrossRef]
- Pospiech, J.; Stolke, D.; Wilke, H.J.; Claes, L.E. Intradiscal pressure recordings in the cervical spine. Neurosurgery 1999, 44, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Finn, M.A.; Brodke, D.S.; Daubs, M.; Patel, A.; Bachus, K.N. Local and global subaxial cervical spine biomechanics after single-level fusion or cervical arthroplasty. Eur. Spine J. 2009, 18, 1520–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kretzer, R.M.; Hsu, W.; Hu, N.; Umekoji, H.; Jallo, G.I.; McAfee, P.C.; Tortolani, P.J.; Cunningham, B.W. Adjacent-level range of motion and intradiscal pressure after posterior cervical decompression and fixation: An in vitro human cadaveric model. Spine 2012, 37, E778–E785. [Google Scholar] [CrossRef]
- Patel, V.V.; Wuthrich, Z.R.; McGilvray, K.C.; Lafleur, M.C.; Lindley, E.M.; Sun, D.; Puttlitz, C.M. Cervical facet force analysis after disc replacement versus fusion. Clin. Biomech. 2017, 44, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Tsuang, F.Y.; Tsai, J.C.; Lai, D.M. Effect of lordosis on adjacent levels after lumbar interbody fusion, before and after removal of the spinal fixator: A finite element analysis. BMC Musculoskelet. Disord. 2019, 20, 470. [Google Scholar] [CrossRef]
- Arnold, J.G., Jr. The clinical manifestations of spondylochondrosis (spondylosis) of the cervical spine. Ann. Surg. 1955, 141, 872–889. [Google Scholar] [CrossRef]
- Baptiste, D.C.; Fehlings, M.G. Pathophysiology of cervical myelopathy. Spine J. 2006, 6, 190s–197s. [Google Scholar] [CrossRef]
- Morishita, Y.; Falakassa, J.; Naito, M.; Hymanson, H.J.; Taghavi, C.; Wang, J.C. The kinematic relationships of the upper cervical spine. Spine 2009, 34, 2642–2645. [Google Scholar] [CrossRef]
- Taylor, A.R. Mechanism and treatment of spinal-cord disorders associated with cervical spondylosis. Lancet 1953, 1, 717–720. [Google Scholar] [CrossRef]
- Penning, L. Some aspects of plain radiography of the cervical spine in chronic myelopathy. Neurology 1962, 12, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kojima, T.; Kataoka, H.; Imajo, Y.; Yara, T.; Yoshida, Y.; Imagama, T.; Taguchi, T. Selective laminoplasty after the preoperative diagnosis of the responsible level using spinal cord evoked potentials in elderly patients with cervical spondylotic myelopathy: A preliminary report. J. Spinal. Disord. Tech. 2009, 22, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, T. Skip laminectomy—A new treatment for cervical spondylotic myelopathy, preserving bilateral muscular attachments to the spinous processes: A preliminary report. Spine J. 2002, 2, 108–115. [Google Scholar] [CrossRef]
- Dahdaleh, N.S.; Wong, A.P.; Smith, Z.A.; Wong, R.H.; Lam, S.K.; Fessler, R.G. Microendoscopic decompression for cervical spondylotic myelopathy. Neurosurg. Focus 2013, 35, E8. [Google Scholar] [CrossRef] [Green Version]
- Panjabi, M.M.; White, A.A., 3rd; Johnson, R.M. Cervical spine mechanics as a function of transection of components. J. Biomech. 1975, 8, 327–336. [Google Scholar] [CrossRef]
- Stoner, K.E.; Abode-Iyamah, K.O.; Fredericks, D.C.; Viljoen, S.; Howard, M.A.; Grosland, N.M. A comprehensive finite element model of surgical treatment for cervical myelopathy. Clin. Biomech. 2020, 74, 79–86. [Google Scholar] [CrossRef]
- Tejapongvorachai, T.; Tanaviriyachai, T.; Daniel Riew, K.; Limthongkul, W.; Tanavalee, C.; Keeratihattayakorn, S.; Buttongkum, D.; Singhatanadgige, W. Curved versus straight-cut hinges for open-door laminoplasty: A finite element and biomechanical study. J. Clin. Neurosci. 2020, 78, 371–375. [Google Scholar] [CrossRef]
- Khuyagbaatar, B.; Kim, K.; Purevsuren, T.; Lee, S.H.; Kim, Y.H. Biomechanical Effects on Cervical Spinal Cord and Nerve Root Following Laminoplasty for Ossification of the Posterior Longitudinal Ligament in the Cervical Spine: A Comparison Between Open-Door and Double-Door Laminoplasty Using Finite Element Analysis. J. Biomech. Eng. 2018, 140. [Google Scholar] [CrossRef]
- Kubo, S.; Goel, V.K.; Yang, S.J.; Tajima, N. The biomechanical effects of multilevel posterior foraminotomy and foraminotomy with double-door laminoplasty. J. Spinal. Disord. Tech. 2002, 15, 477–485. [Google Scholar] [CrossRef]
- Subramaniam, V.; Chamberlain, R.H.; Theodore, N.; Baek, S.; Safavi-Abbasi, S.; Senoğlu, M.; Sonntag, V.K.; Crawford, N.R. Biomechanical effects of laminoplasty versus laminectomy: Stenosis and stability. Spine 2009, 34, E573–E578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kode, S.; Gandhi, A.A.; Fredericks, D.C.; Grosland, N.M.; Smucker, J.D. Effect of multilevel open-door laminoplasty and laminectomy on flexibility of the cervical spine: An experimental investigation. Spine 2012, 37, E1165–E1170. [Google Scholar] [CrossRef] [PubMed]
- Seichi, A.; Takeshita, K.; Ohishi, I.; Kawaguchi, H.; Akune, T.; Anamizu, Y.; Kitagawa, T.; Nakamura, K. Long-term results of double-door laminoplasty for cervical stenotic myelopathy. Spine 2001, 26, 479–487. [Google Scholar] [CrossRef]
- Ratliff, J.K.; Cooper, P.R. Cervical laminoplasty: A critical review. J. Neurosurg. 2003, 98, 230–238. [Google Scholar] [CrossRef]
- Mihara, H.; Ohnari, K.; Hachiya, M.; Kondo, S.; Yamada, K. Cervical myelopathy caused by C3–C4 spondylosis in elderly patients: A radiographic analysis of pathogenesis. Spine 2000, 25, 796–800. [Google Scholar] [CrossRef]
- Holmes, A.; Wang, C.; Han, Z.H.; Dang, G.T. The range and nature of flexion-extension motion in the cervical spine. Spine 1994, 19, 2505–2510. [Google Scholar] [CrossRef]
- Rahmani, M.S.; Terai, H.; Akhgar, J.; Suzuki, A.; Toyoda, H.; Hoshino, M.; Tamai, K.; Ahmadi, S.A.; Hayashi, K.; Takahashi, S.; et al. Anatomical analysis of human ligamentum flavum in the cervical spine: Special consideration to the attachments, coverage, and lateral extent. J. Orthop. Sci. 2017, 22, 994–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashiguchi, A.; Kanchiku, T.; Nishida, N.; Taguchi, T. Biomechanical Study of Cervical Posterior Decompression. Asian Spine J. 2018, 12, 391–397. [Google Scholar] [CrossRef] [PubMed]

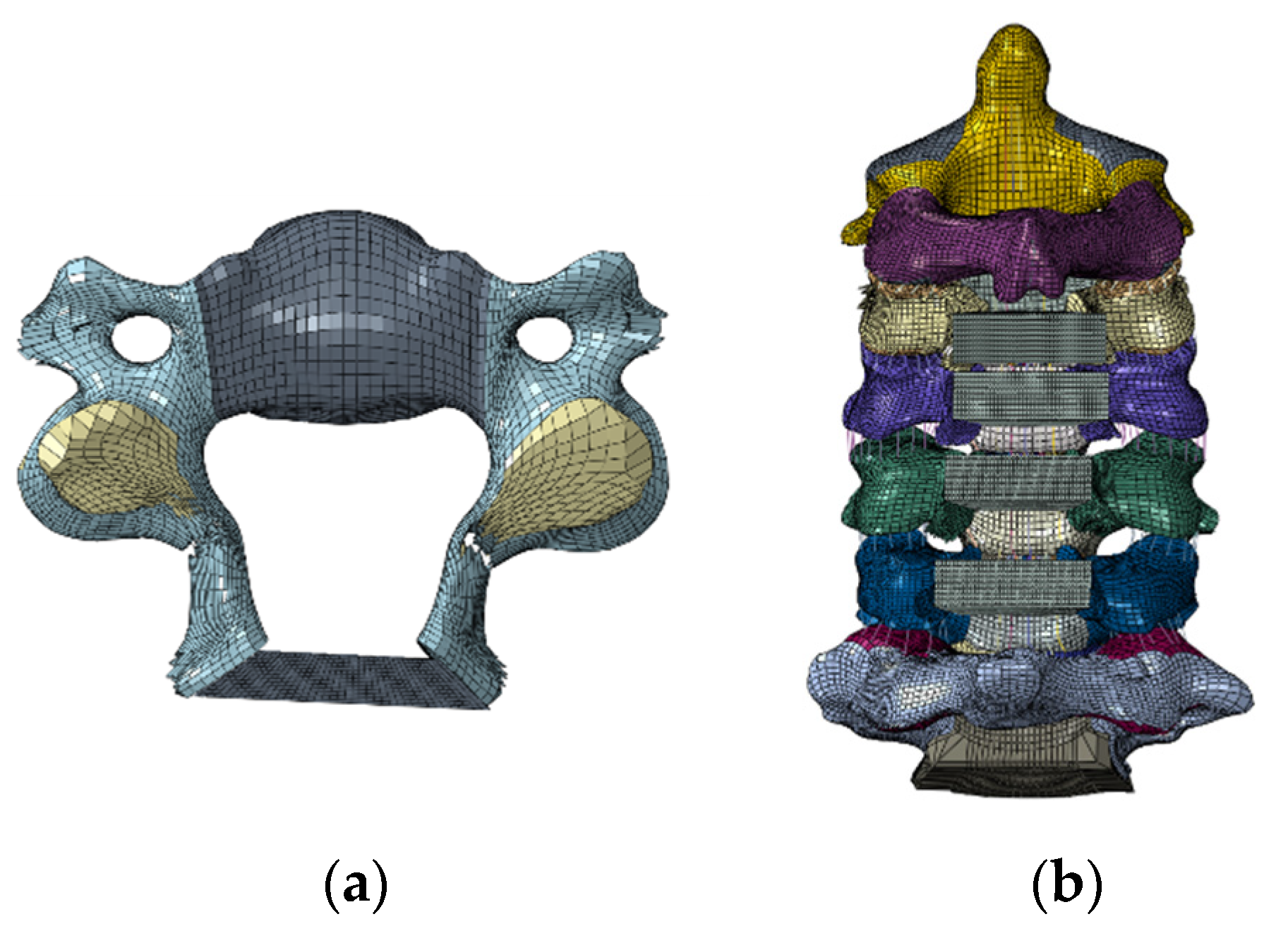




| Component | Material Properties | Constitute Relation | Element Type | Area (mm2) |
|---|---|---|---|---|
| Bone [25] | ||||
| Vertebral cortical bone | E = 10,000 MPa | Isotropic, Elastic | C3D8 | - |
| v = 0.3 | ||||
| Vertebral cancellous bone | E = 450 MPa | Isotropic, Elastic | C3D8 | - |
| v = 0.25 | ||||
| Vertebrae-Posterior | E = 3500 MPa | Isotropic, Elastic | C3D10 | - |
| v = 0.25 | ||||
| Artificial bone | E = 10,000 MPa | Isotropic, Elastic | C3D8 | - |
| v = 0.3 | ||||
| Intervertebral Disc [24] | ||||
| Ground substance of annulus fibrosis | C10 = 0.7 | Hyper-elastic, Mooney-Rivlin | C3D8 | - |
| C01 = 0.2 | ||||
| Nucleus pulposus | C10 = 0.12 | Incompressible Hyper-elastic, Mooney-Rivlin | C3D8 | - |
| C01 = 0.03 | ||||
| D1 = 0 | ||||
| Ligaments [23] | ||||
| Anterior Longitudinal Ligament | 15.0 (<12%), 30.0 (>12%) | Non-linear, Hypo-elastic | T3D2 | 6.1 |
| v = 0.3 | ||||
| Posterior Longitudinal Ligament | 10.0 (<12%), 20.0 (>12%) | Non-linear, Hypo-elastic | T3D3 | 5.4 |
| v = 0.3 | ||||
| Capsular Ligament | 7.0 (<30%), 30 (>12%) | Non-linear, Hypo-elastic | T3D4 | 46.6 |
| v = 0.3 | ||||
| Ligamentum Flavum | 5.0 (<25%), 10.0 (>25%) | Non-linear, Hypo-elastic | T3D5 | 50.1 |
| v = 0.3 | ||||
| Interspinous Ligament | 4.0 (20–40%), 8.0 (>40%) | Non-linear, Hypo-elastic | T3D6 | 13.1 |
| v = 0.3 | ||||
| Facet Joints [21] | ||||
| Apophyseal Joints | Non-linear Soft contact, GAPPUNI elements | - | - | - |
| Segment | Intervertebral Nucleus Stress (MPa)—FE Model | |||||
| Flexion | Extension | Left Bending | Right Bending | Left Rotation | Right Rotation | |
| C2–C3 | 0.26 | 0.35 | 0.23 | 0.22 | 0.27 | 0.26 |
| C3–C4 | 0.17 | 0.14 | 0.14 | 0.15 | 0.19 | 0.24 |
| C4–C5 | 0.21 | 0.16 | 0.11 | 0.17 | 0.22 | 0.21 |
| C5–C6 | 0.17 | 0.12 | 0.1 | 0.14 | 0.18 | 0.18 |
| C6–C7 | 0.11 | 0.14 | 0.09 | 0.11 | 0.1 | 0.13 |
| Segment | Intervertebral Nucleus Stress (MPa)—In Vitro | |||||
| Flexion | Extension | Left Bending | Right Bending | Left Rotation | Right Rotation | |
| C2–C3 | 0.08–0.36 | 0.08–0.36 | 0.12–0.36 | 0.12–0.36 | - | - |
| C3–C4 | 0.12–0.43 | 0.12–0.43 | 0.08–0.31 | 0.08–0.31 | 0.14–0.36 | 0.14–0.36 |
| C4–C5 | - | - | - | - | - | - |
| C5–C6 | 0.01–0.56 | 0.01–0.56 | 0.01–0.38 | 0.01–0.38 | 0.04–0.49 | 0.04–0.49 |
| C6–C7 | 0.01–0.17 | 0.01–0.17 | 0.01–0.11 | 0.01–0.11 | - | - |
| Segment | Facet Contact Forces (N)—FE Model | Facet Contact Forces (N)—In Vitro | ||||
|---|---|---|---|---|---|---|
| Extension | Lateral Bending | Axial Rotation | Extension | Lateral Bending | Axial Rotation | |
| C2–C3 | 50.9 | 45.6 | 21.3 | - | - | - |
| C3–C4 | 42.6 | 34.2 | 36.2 | 12.5–62.5 | 29.5–81.2 | 34.5–88.1 |
| C4–C5 | 31.4 | 35.8 | 20.7 | 13.9–43.9 | 36.2–74.8 | 34.7–88.2 |
| C5–C6 | 32.4 | 34.6 | 34.7 | - | - | - |
| C6–C7 | 24.6 | 31.9 | 28.4 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, N.; Mumtaz, M.; Tripathi, S.; Kelkar, A.; Sakai, T.; Goel, V.K. Biomechanical Analysis of Posterior Ligaments of Cervical Spine and Laminoplasty. Appl. Sci. 2021, 11, 7645. https://doi.org/10.3390/app11167645
Nishida N, Mumtaz M, Tripathi S, Kelkar A, Sakai T, Goel VK. Biomechanical Analysis of Posterior Ligaments of Cervical Spine and Laminoplasty. Applied Sciences. 2021; 11(16):7645. https://doi.org/10.3390/app11167645
Chicago/Turabian StyleNishida, Norihiro, Muzammil Mumtaz, Sudharshan Tripathi, Amey Kelkar, Takashi Sakai, and Vijay K. Goel. 2021. "Biomechanical Analysis of Posterior Ligaments of Cervical Spine and Laminoplasty" Applied Sciences 11, no. 16: 7645. https://doi.org/10.3390/app11167645
APA StyleNishida, N., Mumtaz, M., Tripathi, S., Kelkar, A., Sakai, T., & Goel, V. K. (2021). Biomechanical Analysis of Posterior Ligaments of Cervical Spine and Laminoplasty. Applied Sciences, 11(16), 7645. https://doi.org/10.3390/app11167645







