Effect of Pulsed Electromagnetic Field Stimulation on the Growth Plate of the Tibia Bone of Rats: An In Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
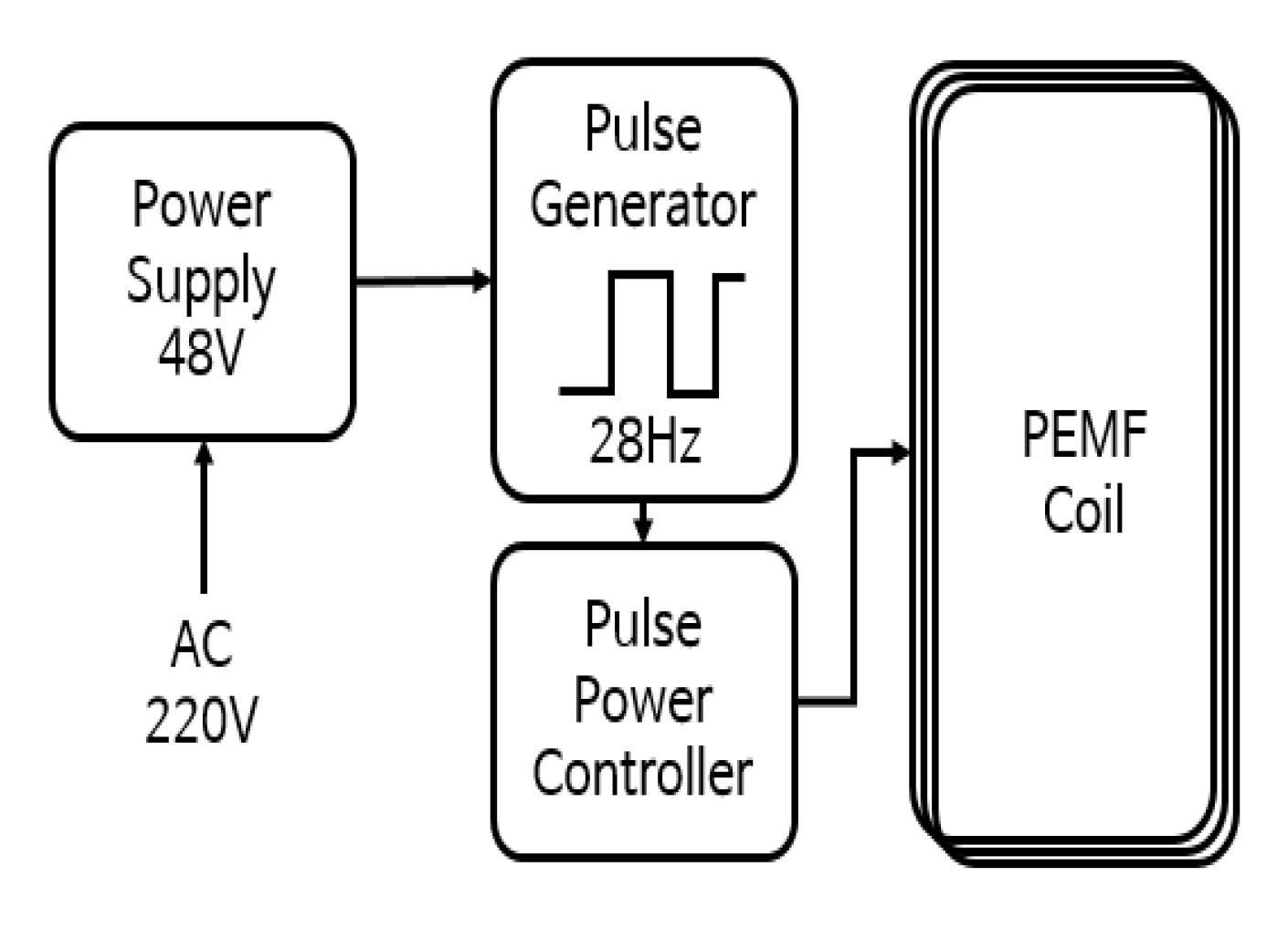
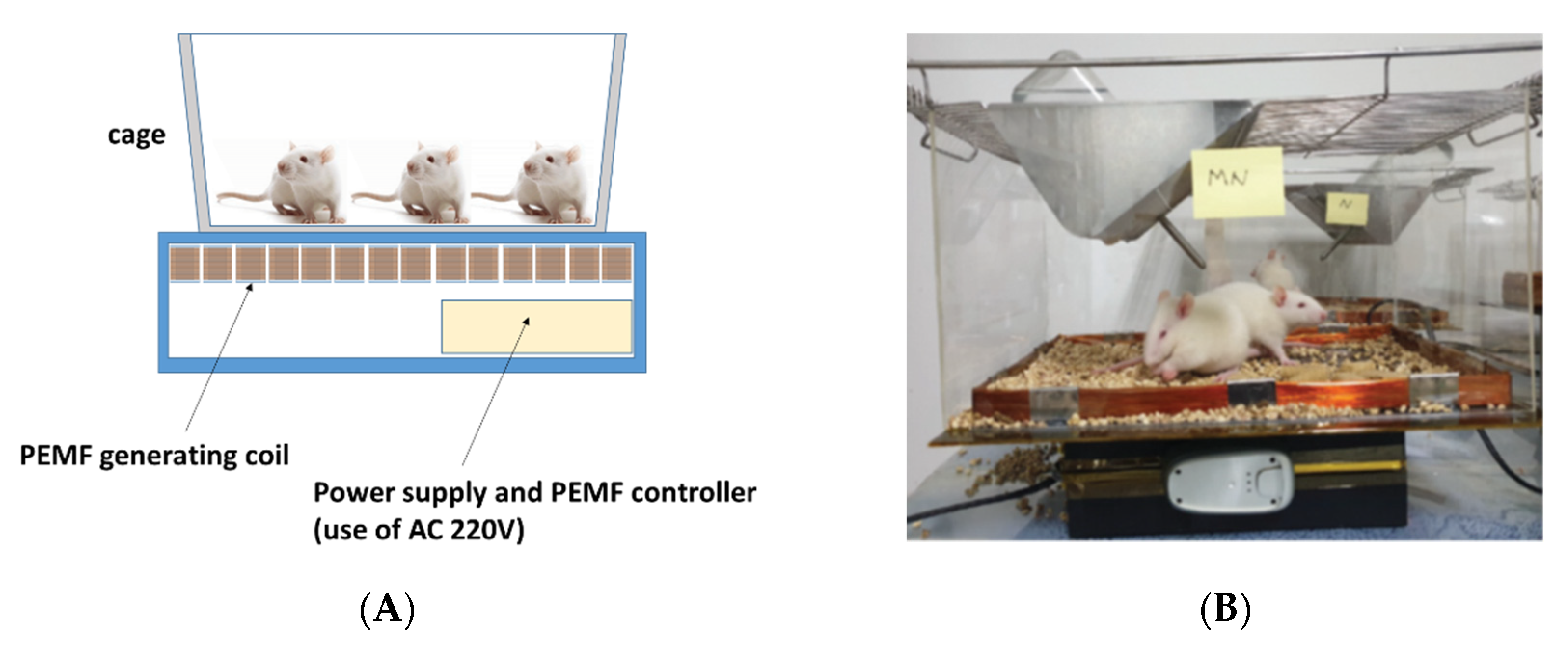
2.1. PEMF Product
2.2. Animals
2.3. Experiment
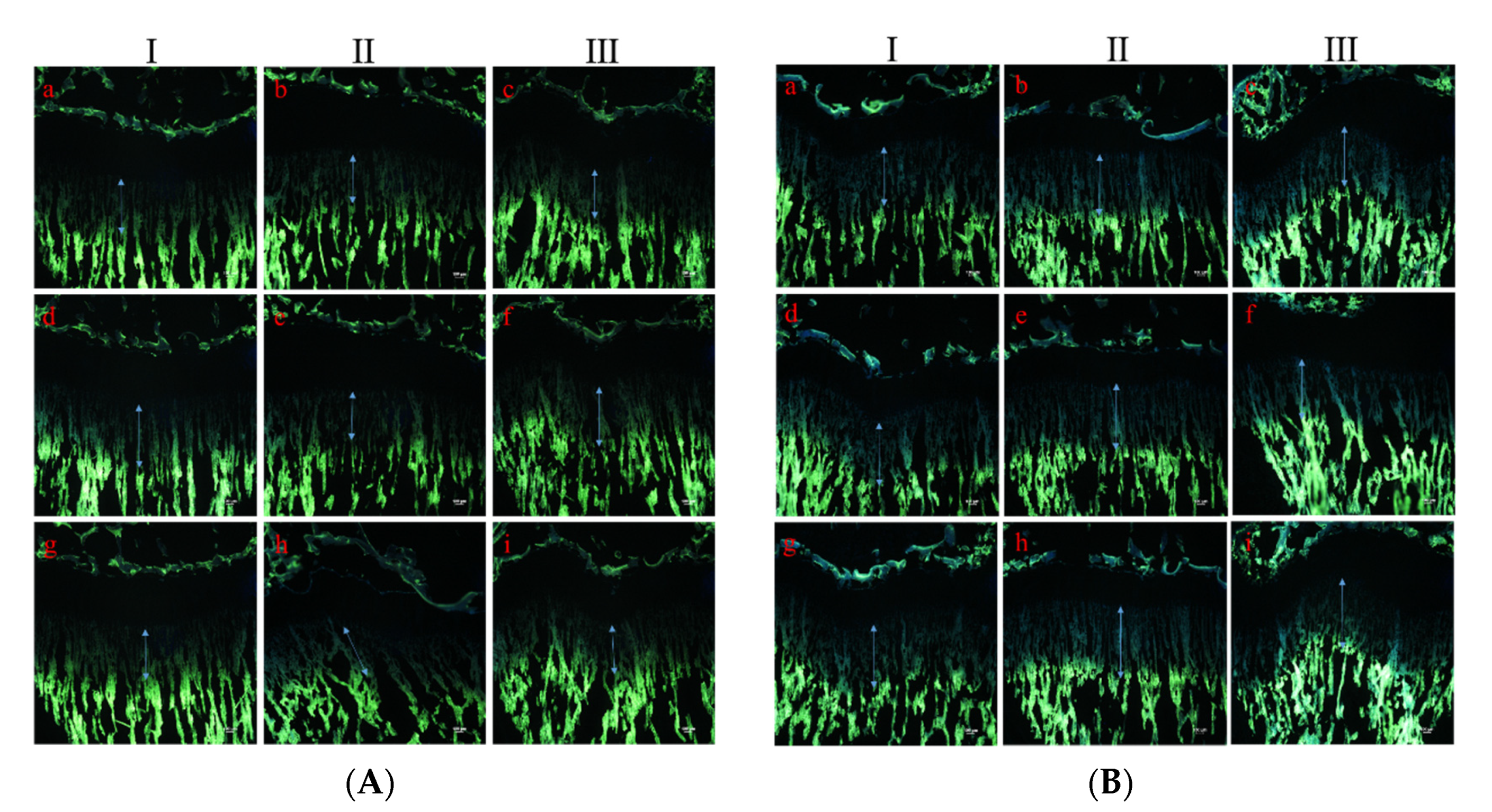
2.4. Measurement of Growth Rate of the Tibia Growth Plate
2.5. Measurement of Levels of Insulin-Like Growth Factor-1 and Nitric Oxide
2.6. Blood Biochemical Analysis
2.7. Statistical Analysis
3. Results
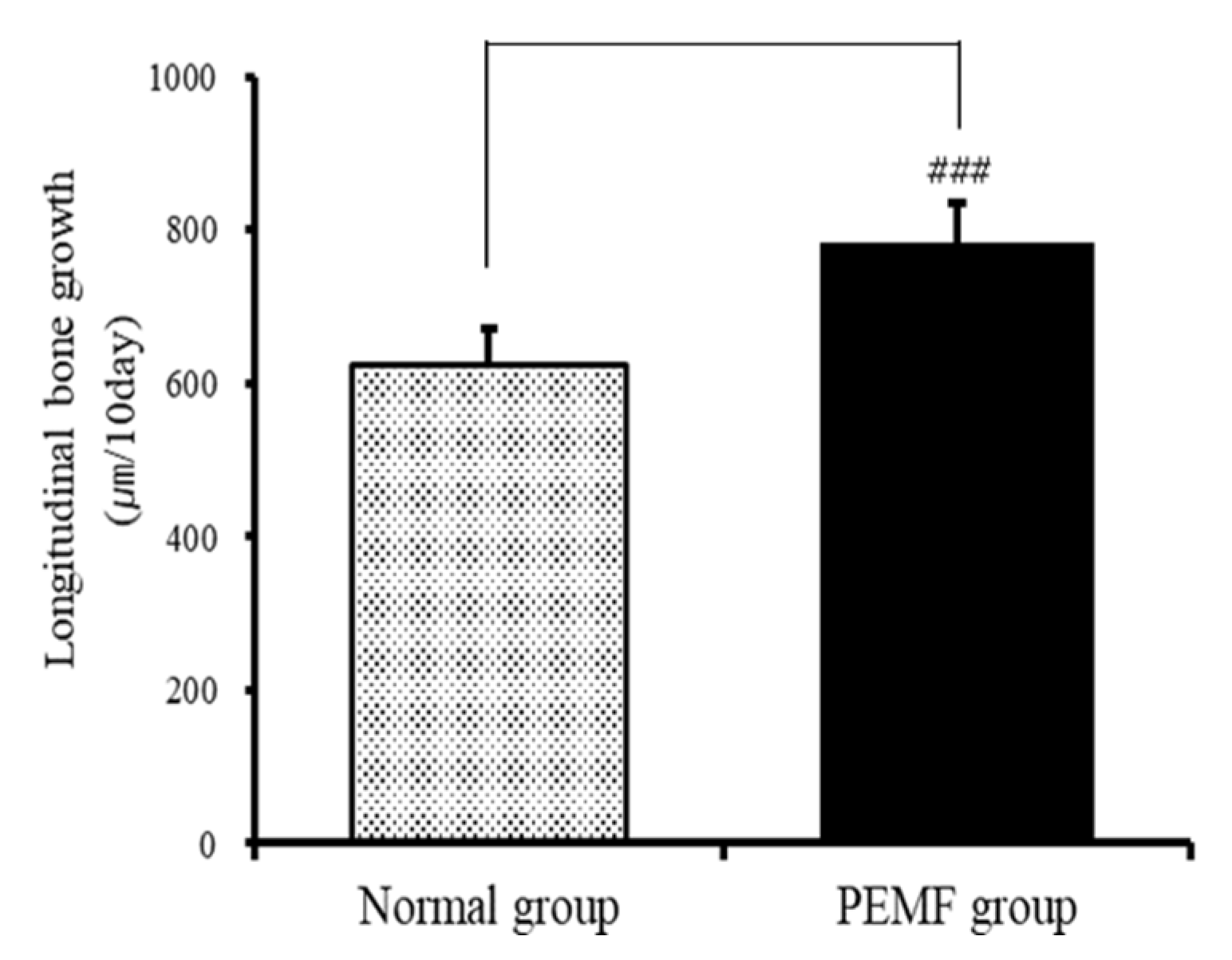
3.1. Effect on Longitudinal Bone Growth Rate
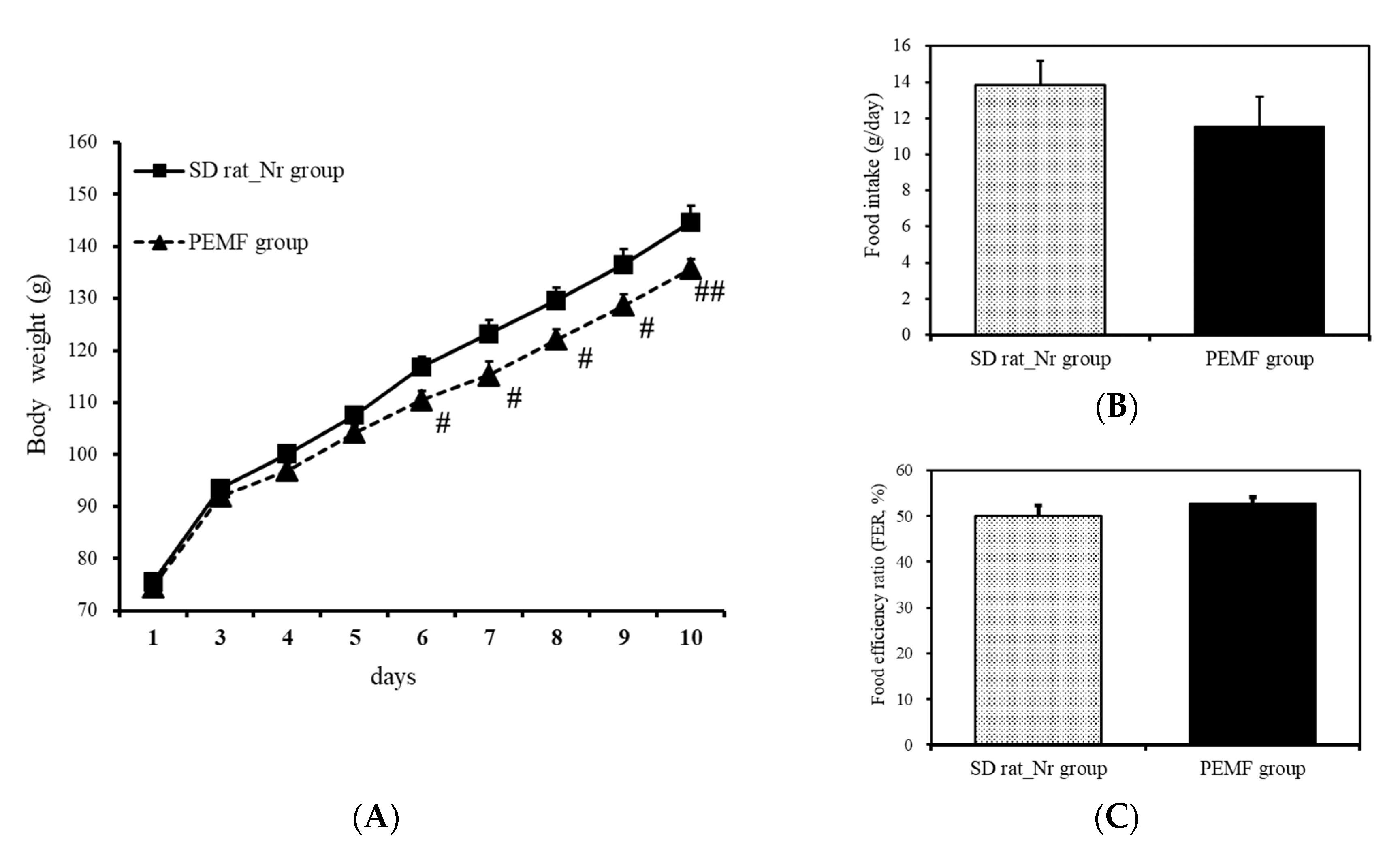
3.2. Changes in Weight and Food Efficiency
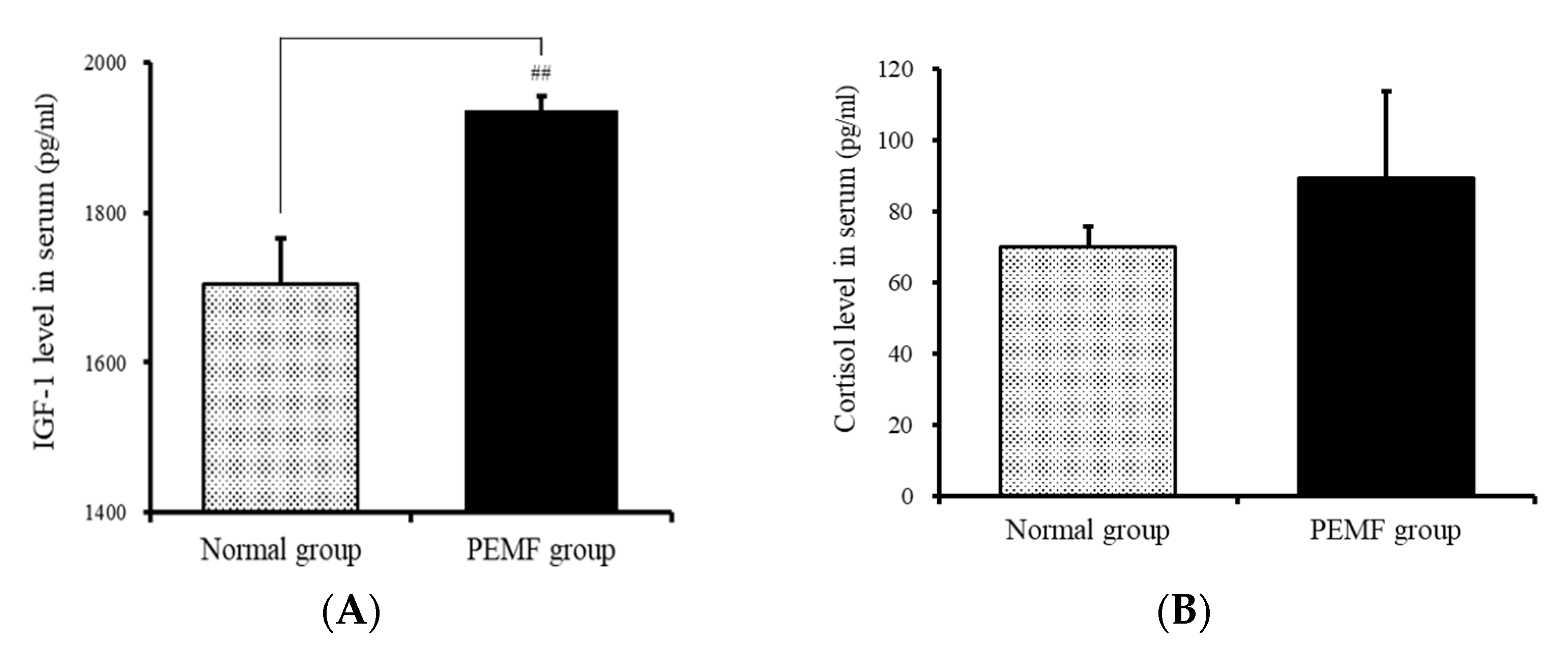
3.3. Insulin-Like Growth Factor 1 Levels in the Serum
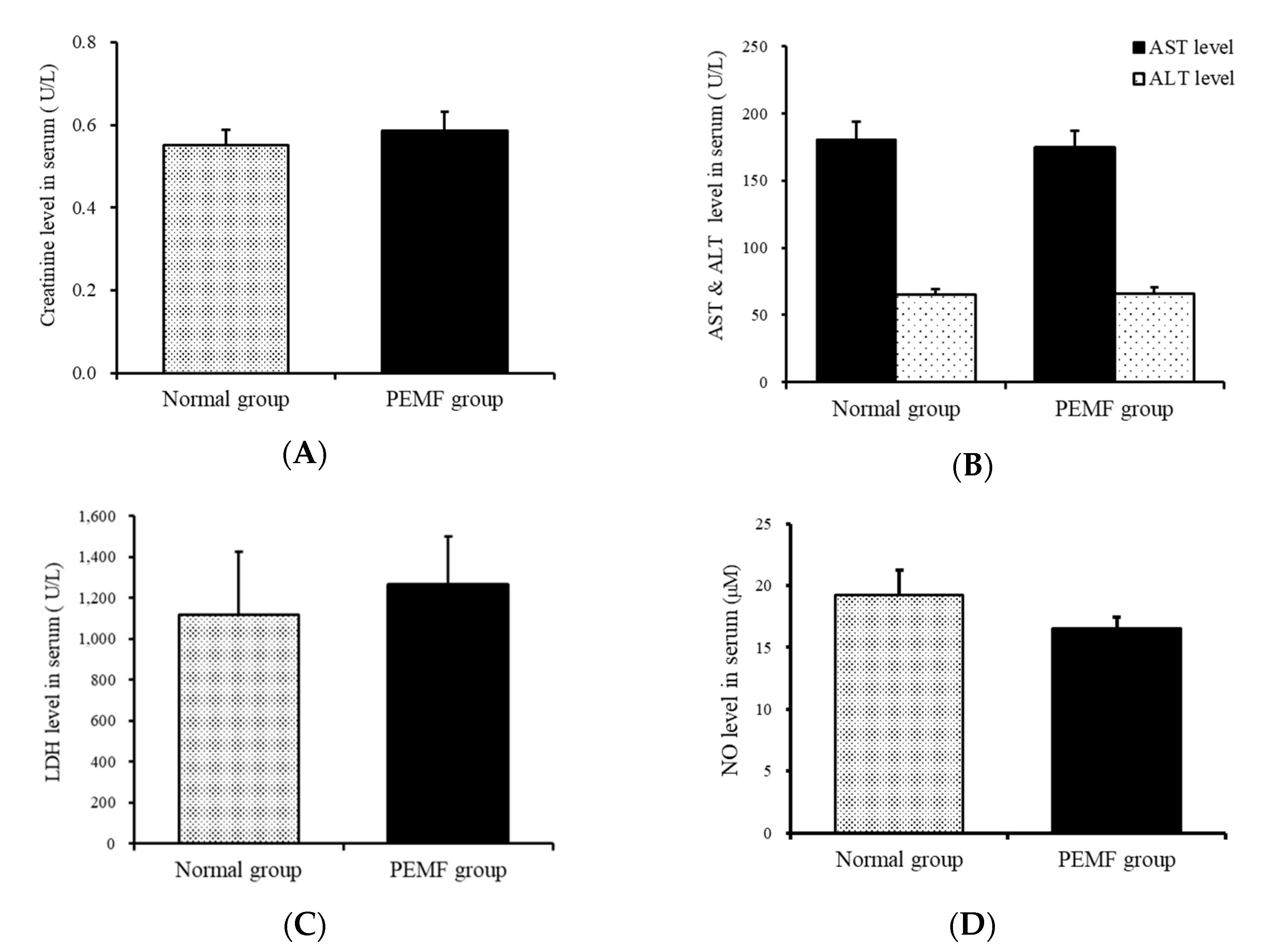
3.4. Aspartate Aminotransferase Levels in the Serum
3.5. LDH Levels in the Serum
3.6. NO Levels in the Serum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peltier, L.F. A brief historical note on the use of electricity in the treatment of fracture. Clin. Orthop. 1981, 161, 4–7. [Google Scholar] [CrossRef]
- Garratt, A.C. Electrophysiology and Electrotherapeutics; Ticker and Fields: Boston, MA, USA, 1861; p. 657. [Google Scholar]
- Wang, T.; Xie, W.; Ye, W.; He, C. Effects of electromagnetic fields on osteoarthritis. Biomed. Pharmacother. 2019, 118, 109282. [Google Scholar] [CrossRef] [PubMed]
- Elshiwi, A.M.; Hamada, H.A.; Mosaad, D.; Ragab, I.M.A.; Koura, G.M.; Alrawaili, S.M. Effect of pulsed electromagnetic field on nonspecific low back pain patients: A randomized controlled trial. Braz. J. Phys. Ther. 2019, 23, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, M.; Morales-Medina, J.C.; Vallelunga, A.; Palmieri, B.; Laurino, C.; Iannitti, T. Mechanisms and therapeutic effectiveness of pulsed electromagnetic field therapy in oncology. Cancer Med. 2016, 5, 3128–3139. [Google Scholar] [CrossRef]
- van der Eerden, B.C.; Karperien, M.; Wit, J.M. Systemic and local regulation of the growth plate. Endocr. Rev. 2003, 24, 782–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Roan, E.; Williams, J.L. Regional variations in growth plate chondrocyte deformation as predicted by three-dimensional multi-scale simulations. PLoS ONE 2015, 10, e0124862. [Google Scholar] [CrossRef]
- Chagin, A.S.; Newton, P.T. Postnatal skeletal growth is driven by the epiphyseal stem cell niche: Potential implications to pediatrics. Pediatr. Res. 2020, 87, 986–990. [Google Scholar] [CrossRef]
- Späth, S.S.; Andrade, A.C.; Chau, M.; Nilsson, O. Local regulation of growth plate cartilage. Endocr. Rev. 2011, 21, 12–22. [Google Scholar]
- Newton, P.T.; Li, L.; Zhou, B.; Schweingruber, C.; Hovorakova, M.; Xie, M.; Sun, X.; Sandhow, L.; Artemov, A.V.; Ivashkin, E.; et al. A radical switch in clonality reveals a stem cell niche in the epiphyseal growth plate. Nature 2019, 567, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Hallett, S.A.; Ono, W.; Ono, N. Growth Plate Chondrocytes: Skeletal Development, Growth and Beyond. Int. J. Mol. Sci. 2019, 20, 6009. [Google Scholar] [CrossRef] [Green Version]
- Lui, J.C. Home for a rest: Stem cell niche of the postnatal growth plate. J. Endocrinol. 2020, 246, R1–R11. [Google Scholar] [CrossRef]
- De Mattei, M.; Caruso, A.; Pezzetti, F.; Pellati, A.; Stabellini, V.; Sollazzo, V.; Traina, G.C. Effects of pulsed electromagnetic fields on human articular chondrocyte proliferation. Connect. Tissue Res. 2001, 42, 269–279. [Google Scholar] [CrossRef]
- Pezzetti, F.; De Mattei, M.; Caruso, A.; Cadossi, R.; Zucchini, P.; Carinci, F.; Traina, G.C.; Sollazzo, V. Effects of Pulsed Electromagnetic Fields on Human Chondrocytes: An in vitro Study. Calcif. Tissue Int. 1999, 65, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Stolfa, S.; Skorvánek, M.; Stolfa, P.; Rosocha, J.; Vasko, G.; Sabo, J. Effects of static magnetic field and pulsed electromagnetic field on viability of human chondrocytes in vitro. Physiol. Res. 2007, 56, S45–S49. [Google Scholar]
- Gierse, H.; Breul, R.; Faensen, M.; Markoll, R. Pulsed signal therapy (PST) stimulates mitosis of human chondrocytes in culture. In Proceedings of the Tenth International Conference on Biomedical Engineering; Singapore Humanitas Press: Singapore, 2000; pp. 473–474. [Google Scholar]
- Lee, D.; Kim, Y.S.; Song, J.; Kim, H.S.; Lee, H.J.; Guo, H.; Kim, H. Effects of phlomis umbrosa root on longitudinal bone growth rate in adolescent female rats. Molecules 2016, 21, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- L Racine, H.; A Serrat, M. The actions of IGF-1 in the growth plate and its role in postnatal bone elongation. Curr. Osteoporos. Rep. 2020, 18, 210–227. [Google Scholar] [CrossRef]
- Gat-Yablonski, G.; Phillip, M. Nutritionally-induced catch-up growth. Nutrients 2015, 7, 517–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Lee, S.H.; Lee, Y.H.; Song, J.; Kim, H. Astragalus Extract Mixture HT042 Increases Longitudinal Bone Growth Rate by Upregulating Circulatory IGF-1 in Rats. Evid. Based Complement. Altern. Med. 2017, 2017, 6935802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakar, S.; Rosen, C.J.; Beamer, W.G.; Ackert-Bicknell, C.L.; Wu, Y.; Liu, J.L.; Ooi, G.T.; Setser, J.; Frystyk, J.; Boisclair, Y.R.; et al. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Investig. 2002, 110, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Ochs, R.L.; Schwarz, H.; Lotz, M. Chondrocyte apoptosis induced by nitric oxide. Am. J. Pathol. 1995, 146, 75–85. [Google Scholar] [PubMed]
- van’t Hof, R.J.; Ralston, S.H. Nitric oxide and bone. Immunology 2001, 103, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, C.C.; Ischiropoulos, H.; Leboy, P.S.; Adams, S.L.; Shapiro, I.M. Nitric oxide-nitric oxide synthase regulates key maturational events during chondrocyte terminal differentiation. Bone 2005, 37, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Diniz, P.; Soejima, K.; Ito, G. Nitric oxide mediates the effects of pulsed electromagnetic field stimulation on the osteoblast proliferation and differentiation. Nitric Oxide 2002, 7, 18–23. [Google Scholar] [CrossRef]
- Touitou, Y.; Selmaoui, B. The effects of extremely low-frequency magnetic fields on melatonin and cortisol, two marker rhythms of the circadian system. Dialogues Clin. Neurosci. 2012, 14, 381–399. [Google Scholar] [PubMed]
- Mann, K.; Wagner, P.; Brunn, G.; Hassan, F.; Hiemke, C.; Röschke, J. Effects of pulsed high-frequency electromagnetic fields on the neuroendocrine system. Neuroendocrinology 1998, 67, 139–144. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, Y.-Y.; Shin, J.-W.; Yang, W.-K.; Kim, M.-J.; Koo, J.-I.; Noh, E.-M.; Min, K.-S.; Yun, M.-Y.; Kim, S.-H. Effect of Pulsed Electromagnetic Field Stimulation on the Growth Plate of the Tibia Bone of Rats: An In Vivo Study. Appl. Sci. 2021, 11, 7571. https://doi.org/10.3390/app11167571
Sung Y-Y, Shin J-W, Yang W-K, Kim M-J, Koo J-I, Noh E-M, Min K-S, Yun M-Y, Kim S-H. Effect of Pulsed Electromagnetic Field Stimulation on the Growth Plate of the Tibia Bone of Rats: An In Vivo Study. Applied Sciences. 2021; 11(16):7571. https://doi.org/10.3390/app11167571
Chicago/Turabian StyleSung, Yoon-Young, Jae-Woo Shin, Won-Kyung Yang, Min-Jin Kim, Ja-Ik Koo, Eun-Mi Noh, Kyoung-Soo Min, Mi-Young Yun, and Seung-Hyung Kim. 2021. "Effect of Pulsed Electromagnetic Field Stimulation on the Growth Plate of the Tibia Bone of Rats: An In Vivo Study" Applied Sciences 11, no. 16: 7571. https://doi.org/10.3390/app11167571
APA StyleSung, Y.-Y., Shin, J.-W., Yang, W.-K., Kim, M.-J., Koo, J.-I., Noh, E.-M., Min, K.-S., Yun, M.-Y., & Kim, S.-H. (2021). Effect of Pulsed Electromagnetic Field Stimulation on the Growth Plate of the Tibia Bone of Rats: An In Vivo Study. Applied Sciences, 11(16), 7571. https://doi.org/10.3390/app11167571






