Immediate Implants with Early Loading Accompanying Autogenous Bone Grafting in a Maxilla with Periodontal Destruction: A Case Report
Abstract
:1. Introduction
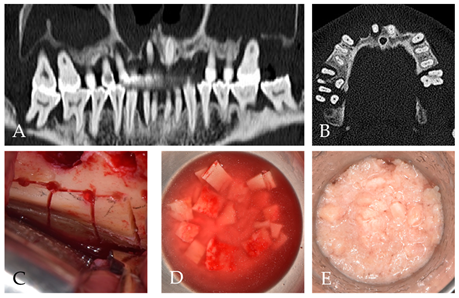
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology-is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant Dent. 2017, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, L.; Amadè, D.S.; Cordaro, M. Clinical results of alveolar ridge augmentation with mandibular block bone grafts in partially edentulous patients prior to implant placement. Clin. Oral Implant. Res. 2002, 13, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zins, J.E.; Whitaker, L.A. Membranous versus endochondral bone: Implications for craniofacial reconstruction. Plast. Reconstr. Surg. 1983, 72, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Reininger, D.; Cobo-Vázquez, C.; Monteserín-Matesanz, M.; López-Quiles, J. Complications in the use of the mandibular body, ramus and symphysis as donor sites in bone graft surgery. A systematic review. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e241–e249. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Jung, R.E.; Avila-Ortiz, G.; Blanco, J.; Cosyn, J.; Fickl, S.; Figuero, E.; Goldstein, M.; Graziani, F.; Madianos, P.; et al. Management of the extraction socket and timing of implant placement: Consensus report and clinical recommendations of group 3 of the xv european workshop in periodontology. J. Clin. Periodontol. 2019, 46, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Brånemark, P.-I.; Breine, U.; Adell, R.; Hansson, B.; Lindström, J.; Ohlsson, Å. Intra-osseous anchorage of dental prostheses: I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Ku, J.-K. Guided bone regeneration. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gultekin, B.A.; Sirali, A.; Gultekin, P.; Ersanli, S. Clinical evaluation of the stability of implants placed at different supracrestal levels. J. Istanb. Univ. Fac. Dent. 2016, 50, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Müller, R.; Ruffoni, D. Bone remodeling and mechanobiology around implants: Insights from small animal imaging. J. Orthop. Res. 2018, 36, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Birkhold, A.I.; Razi, H.; Duda, G.N.; Weinkamer, R.; Checa, S.; Willie, B.M. Mineralizing surface is the main target of mechanical stimulation independent of age: 3d dynamic in vivo morphometry. Bone 2014, 66, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Schulte, F.A.; Ruffoni, D.; Lambers, F.M.; Christen, D.; Webster, D.J.; Kuhn, G.; Müller, R. Local mechanical stimuli regulate bone formation and resorption in mice at the tissue level. PLoS ONE 2013, 8, e62172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Vandamme, K.; Torcasio, A.; Ogawa, T.; Van Lenthe, G.H.; Naert, I.; Duyck, J. In vivo assessment of the effect of controlled high- and low-frequency mechanical loading on peri-implant bone healing. J. R. Soc. Interface 2012, 9, 1697–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leucht, P.; Kim, J.-B.; Wazen, R.; Currey, J.A.; Nanci, A.; Brunski, J.B.; Helms, J.A. Effect of mechanical stimuli on skeletal regeneration around implants. Bone 2007, 40, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Badillo-Perona, V.; Cano-Sánchez, J.; Campo-Trapero, J.; Bascones-Martinez, A. Peri-implant bone mechanobiology. Review of the literature. Med. Oral Patologia Oral Cir. Bucal 2011, 16, e677–e681. [Google Scholar] [CrossRef]
- Ku, J.-K.; Jeong, Y.K.; Choi, Y.-S.; Kim, T.; Cho, I.-W.; Leem, D.H. Conservative technique using oral dressing material for wound dehiscence after ridge augmentation: A technical report with a case series. Appl. Sci. 2021, 11, 6115. [Google Scholar] [CrossRef]
- Gordh, M.; Alberius, P. Some basic factors essential to autogeneic nonvascularized onlay bone grafting to the craniofacial skeleton. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1999, 33, 129–146. [Google Scholar]
- Oppenheimer, A.J.; Tong, L.; Buchman, S.R. Craniofacial bone grafting: Wolff’s law revisited. Craniomaxillofac. Trauma Reconstr. 2008, 1, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Um, I.-W.; Ku, J.-K.; Lee, B.K.; Yun, P.-Y.; Lee, J.K.; Nam, J.-H. Postulated release profile of recombinant human bone morphogenetic protein-2 (rhbmp-2) from demineralized dentin matrix. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Um, I.-W.; Ku, J.-K.; Kim, Y.-K.; Lee, B.-K.; Leem, D.H. Histological review of demineralized dentin matrix as a carrier of rhbmp-2. Tissue Eng. Part B Rev. 2020, 26, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Nkenke, E.; Neukam, F.W. Autogenous bone harvesting and grafting in advanced jaw resorption: Morbidity, resorption and implant survival. Eur. J. Oral Implantol. 2014, 7 (Suppl. S2), S203–S217. [Google Scholar] [PubMed]
- Libertucci, M.; Cosola, S.; Covani, U. A single overturning of ridge for horizontal bone augmentation in maxilla with immediate implant placement: 18-years follow-up. Oral Maxillofac. Surg. Cases 2021, 7, 100213. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, Y.M.; Kim, J.Y.; Kim, M.R.; Kim, S.J. The retrospective study of marginal bone loss around dental implants according to different autogenous bone grafts. J. Korean Assoc. Oral Maxillofac. Surg. 2011, 37, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Matarese, G.; Ramaglia, L.; Fiorillo, L.; Cervino, G.; Lauritano, F.; Isola, G. Implantology and periodontal disease: The panacea to problem solving? Open Dent. J. 2017, 11, 460–465. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, J.-K.; Kim, J.-Y.; Huh, J.-K. Immediate Implants with Early Loading Accompanying Autogenous Bone Grafting in a Maxilla with Periodontal Destruction: A Case Report. Appl. Sci. 2021, 11, 7560. https://doi.org/10.3390/app11167560
Ku J-K, Kim J-Y, Huh J-K. Immediate Implants with Early Loading Accompanying Autogenous Bone Grafting in a Maxilla with Periodontal Destruction: A Case Report. Applied Sciences. 2021; 11(16):7560. https://doi.org/10.3390/app11167560
Chicago/Turabian StyleKu, Jeong-Kui, Jae-Young Kim, and Jong-Ki Huh. 2021. "Immediate Implants with Early Loading Accompanying Autogenous Bone Grafting in a Maxilla with Periodontal Destruction: A Case Report" Applied Sciences 11, no. 16: 7560. https://doi.org/10.3390/app11167560
APA StyleKu, J.-K., Kim, J.-Y., & Huh, J.-K. (2021). Immediate Implants with Early Loading Accompanying Autogenous Bone Grafting in a Maxilla with Periodontal Destruction: A Case Report. Applied Sciences, 11(16), 7560. https://doi.org/10.3390/app11167560






