Compost Quality and Sanitation on Industrial Scale Composting of Municipal Solid Waste and Sewage Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Strategy and Raw Materials
2.2. Analytical Methods
2.2.1. Physico-Chemical Parameters
2.2.2. Indicator Parameters of Biological Stability and Maturity
2.2.3. Fecal Contamination and Pathogens
2.3. Data Analysis
3. Results and Discussion
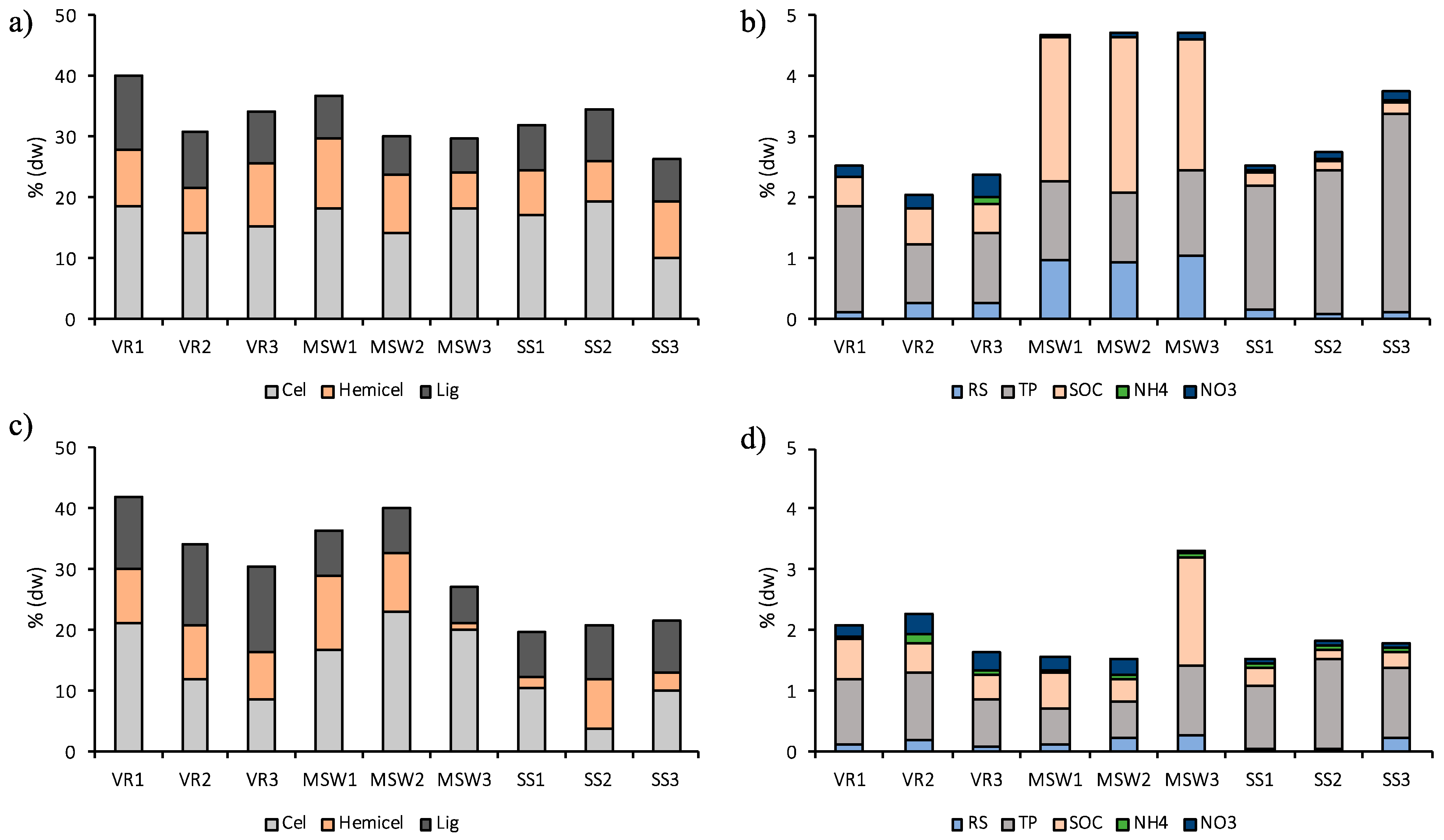
3.1. Bio-Materials Characterization
3.2. Evolution of Soluble and Polymeric Fractions
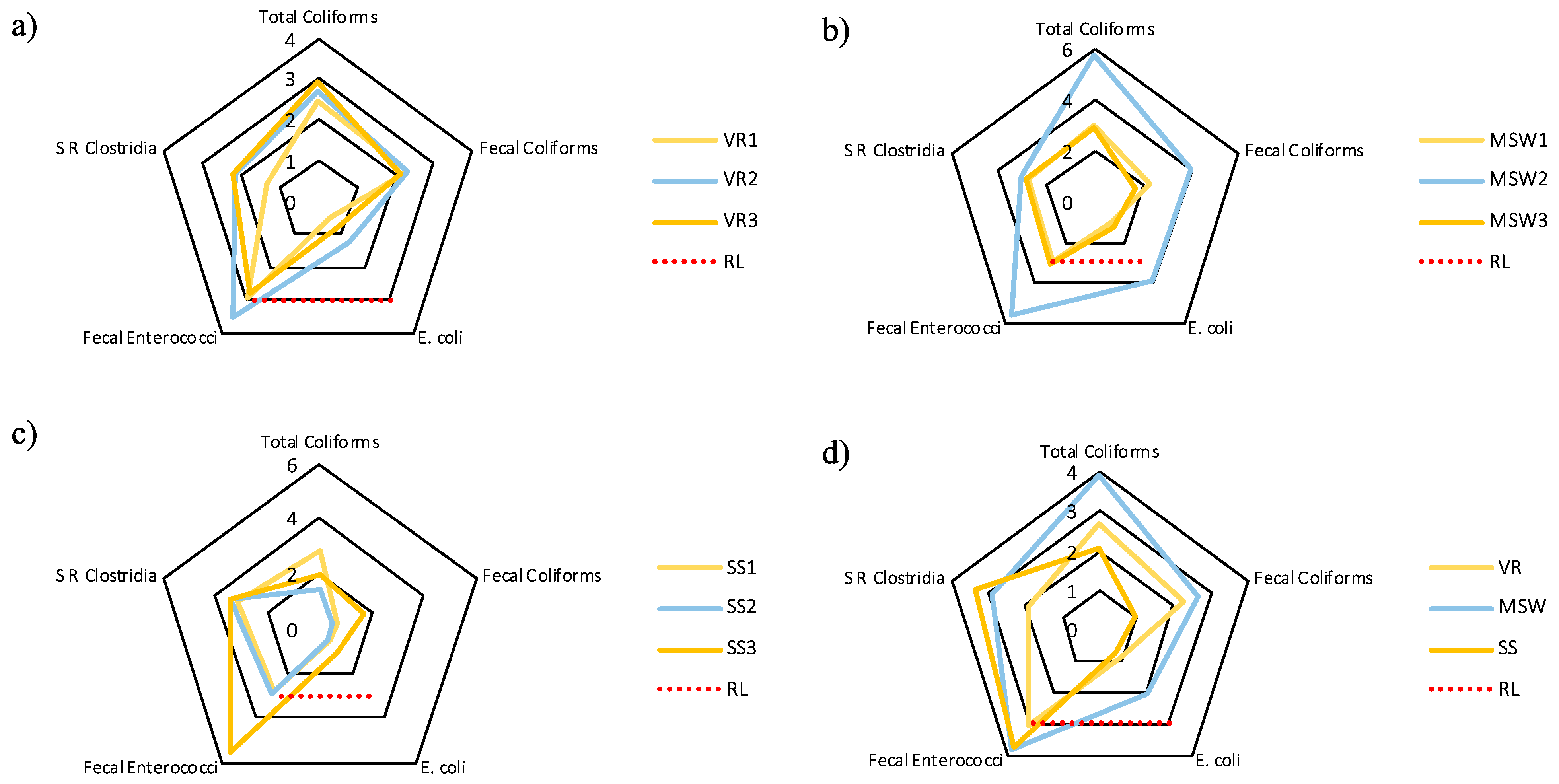
3.3. Fecal Contamination in Composts
3.4. Composts Characterization and Discriminant and Correlations Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Van-Ewijk, S.; Stegemann, J.A. Recognising waste use potential to achieve a circular economy. Waste Manag. 2020, 105, 1–7. [Google Scholar] [CrossRef]
- Pergola, M.; Persiani, A.; Palese, A.M.; Di Meo, V.; Pastore, V.; D’Adamo, C.; Celano, G. Composting: The way for sustainable agriculture. Appl. Soil Ecol. 2018, 123, 744–750. [Google Scholar] [CrossRef]
- Alvarenga, P.; Palma, P.; Gonçalves, A.P.; Fernandes, R.M.; Cunha-Queda, A.C.; Duarte, E.; Vallini, G. Evaluation of chemical and ecotoxicological characteristics of biodegradable organic residues for application to agricultural land. Environ. Int. 2007, 33, 505–513. [Google Scholar] [CrossRef]
- Alvarenga, P.; Mourinha, C.; Farto, M.; Santos, T.; Palma, P.; Sengo, J.; Morais, M.C.; Cunha-Queda, C. Sewage sludge, compost and other representative organic wastes as agricultural soil amendments: Benefits versus limiting factors. Waste Manag. 2015, 40, 44–52. [Google Scholar] [CrossRef]
- Barrena, R.; Font, X.; Gabarrell, X.; Sánchez, A. Home composting versus industrial composting: Influence of composting system on compost quality with focus on compost stability. Waste Manag. 2014, 34, 1109–1116. [Google Scholar] [CrossRef] [Green Version]
- Ayilara, M.S.; Olanrewaju, O.S.; Babalola, O.O.; Odeyemi, O. Waste management through composting: Challenges and potentials. Sustainability 2020, 12, 4456. [Google Scholar] [CrossRef]
- Siles-Castellano, A.B.; López, M.J.; López-González, J.A.; Suárez-Estrella, F.; Jurado, M.M.; Estrella-González, M.J.; Moreno, J. Comparative analysis of phytotoxicity and compost quality in industrial composting facilities processing different organic wastes. J. Clean. Prod. 2020, 252, 119820. [Google Scholar] [CrossRef]
- Majbar, Z.; Lahlou, K.; Abbou, M.B.; Ammar, E.; Triki, A.; Abid, W.; Nawdali, M.; Bouka, H.; Taleb, M.; El Haji, M.; et al. Co-composting of olive mill waste and wine-processing waste: An application of compost as soil amendment. J. Chem. 2018, 2018, 7918583. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, W.; Li, Y.; Wang, G.; Li, G. Performance of co-composting sewage sludge and organic fraction of municipal solid waste at different proportions. Bioresour. Technol. 2018, 250, 853–859. [Google Scholar] [CrossRef]
- Troschinetz, A.M.; Mihelcic, J.R. Sustainable recycling of municipal solid waste in developing countries. Waste Manag. 2009, 29, 915–923. [Google Scholar] [CrossRef]
- Wilson, D.C.; Rodic, L.; Schieinberg, A.; Velis, C.A.; Alabaster, G. Comparative analysis of solid waste management in 20 cities. Waste Manag. Res. 2012, 30, 237–254. [Google Scholar] [CrossRef] [Green Version]
- Lohri, C.R.; Diener, S.; Zabaleta, I. Treatment technologies for urban solid biowaste to create value products: A review with focus on low- and middle-income setting. Rev Environ. Sci. Biotechnol. 2017, 16, 81–130. [Google Scholar] [CrossRef] [Green Version]
- Pascual, J.A.; García, C.; Hernández, T. Lasting microbiological and biochemical effects of the addition of municipal solid waste to an arid soil. Biol. Fertil. Soils. 1999, 30, 1–6. [Google Scholar] [CrossRef]
- Soumaré, M.; Tack, F.M.G.; Verloo, M.G. Characterization of Malian and Belgian solid waste composts with respect to fertility and suitability for land application. Waste Manag. 2003, 23, 517–522. [Google Scholar] [CrossRef]
- Hargraves, J.C.; Adl, M.S.; Warman, P.R. A review of the use of composted municipal solid waste in agriculture. Agric. Ecosys. Environ. 2008, 123, 1–14. [Google Scholar] [CrossRef]
- Zaharioiu, A.; Bucura, F.; Ionete, E.I.; Ionete, R.E.; Ebrasu, D.; Sandru, C.; Marin, F.; Oancea, S.; Nicolescu, V.; Miricioiu, M.G.; et al. Thermochemical decomposition of sewage sludge: An eco-friendly solution for a sustainable energy future by using wastes. Rev. Chim. 2020, 71, 171–181. [Google Scholar] [CrossRef]
- Nafez, A.H.; Nikaeen, M.; Kadkhodaie, S.; Hatamzadeh, M.; Moghim, S. Sewage sludge composting: Quality assessment for agricultural application. Environ. Monit. Assess. 2015, 187, 709. [Google Scholar] [CrossRef]
- Iordache, M.; Iordache, A.M.; Sandru, C.; Voica, C.; Zgavarogea, R.; Miricioiu, M.G.; Ionete, R.E. Assessment of heavy metals pollution in sediments from reservoirs of the Olt River as tool for environmental risk management. Rev. Chim. 2019, 70, 4153–4162. [Google Scholar] [CrossRef]
- Smith, S.R. A critical review of the bioavailability and impacts of heavy metals in municipal solid waste composts compared to sewage sludge. Environ. Int. 2009, 35, 142–156. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.W.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619–620, 815–826. [Google Scholar] [CrossRef]
- Almeira, N.; Komilis, D.; Barrena, R.; Gea, T.; Sánchez, A. The importance of aeration mode and flow rate in the determination of the biological activity and stability of organic wastes by respiration indices. Bioresour. Technol. 2015, 196, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Cesaro, A.; Belgiorno, V.; Guida, M. Compost from organic solid waste: Quality assessment and European regulations for its sustainable use. Resour. Conserv. Recy. 2015, 94, 72–79. [Google Scholar] [CrossRef]
- Tønner-Klank, L.; Møller, J.; Forslund, A.; Dalsgaard, A. Microbiological assessments of compost toilets: In situ measurements and laboratory studies on the survival of fecal microbial indicators using sentinel chambers. Waste Manag. 2007, 27, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- López-González, J.A.; López, M.J.; Vargas-García, M.C.; Suárez-Estrella, F.; Jurado, M.; Moreno, J. Tracking organic matter and microbiota dynamics during the stages of lignocellulosic waste composting. Bioresour. Technol. 2013, 146, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for the determination of reducing sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Herber, D.; Phipps, P.; Stronoe, R. Chapter III. Chemical Analysis of Microbial Cells. In Methods in Microbiology; Norris, J., Ribbons, D.W., Eds.; Academy Press: New York, NY, USA, 1971; Volume 5B, pp. 210–344. [Google Scholar]
- Zucconi, F.; Monaco, A.; de Bertoldi, M. Biological evaluation of compost maturity. Biocycle 1981, 22, 27–29. [Google Scholar]
- Ponsá, S.; Gea, T.; Sánchez, A. Different indices to express biodegradability in organic solid wastes. J. Environ. Qual. 2010, 39, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Das, K.C.; McClendon, R.W. The influence of temperature and moisture contents regimes on the aerobic microbial activity of a biosolids composting blend. Bioresour. Technol. 2003, 86, 131–137. [Google Scholar] [CrossRef]
- Bonito, G.; Isikhuemhen, O.S.; Vilgalys, R. Identification of fungi associated with municipal compost using DNA-based techniques. Bioresour. Technol. 2010, 101, 1021–1027. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Siles, J.A.; Diz, J.; Chica, A.F.; Martín, M.A. Modelling of composting process of different organic waste at pilot scale: Biodegradability and odor emissions. Waste Manag. 2017, 59, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.; Li, S.; Tang, X.; Wei, Z. The “quality” and “quantity” of microbial species drive the degradation of cellulose during composting. Bioresour. Technol. 2021, 320, 124425. [Google Scholar] [CrossRef]
- Bożym, M.; Siemiątkowski, G. Characterization of composted sewage sludge during the maturation process: A pilot scale study. Environ. Sci. Pollut. Res. 2018, 25, 34332–34342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lashermes, G.; Houot, S.; Doublet, J.; Steyer, J.P.; Zhu, Y.G.; Barriuso, E.; Garnier, P. Modelling of organic matter dynamics during the composting process. Waste Manag. 2012, 32, 19–30. [Google Scholar] [CrossRef]
- Varma, V.S.; Das, S.; Sastri, C.V.; Kalambhad, A.S. Microbial degradation of lignocellulosic fractions during drum composting of mixed organic waste. Sustain. Environ. Res. 2017, 27, 265–272. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Liu, B.; Wang, K.; Su, C.; Wu, C. Ammonia emissions and biodegradation of organic carbon during sewage sludge composting with different extra carbon sources. Int. Biodeterior. Biodegrad. 2013, 85, 624–630. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R1009&from=ES (accessed on 10 June 2021).
- Christensen, K.K.; Carlsbæk, M.; Kron, E. Strategies for evaluating the sanitary quality of composting. J. Appl. Microbiol. 2002, 92, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Heinonen-Tanski, H.; Mohaibes, M.; Karinen, P.; Koivunen, J. Methods to reduce pathogen microorganisms in manure. Livest. Sci. 2006, 102, 248–255. [Google Scholar] [CrossRef]
- Gurusamy, N.N.; Puffer, N.; de Jongh, C.; Rodríguez Gil, C.; Aspray, T.J. Effect of initial moisture content and sample storage duration on compost stability using the ORG0020 dynamic respiration test. Waste Manag. 2021, 125, 215–219. [Google Scholar] [CrossRef]
- Estrella-González, M.J.; Jurado, M.M.; Suárez-Estrella, F.; López, M.J.; López-González, J.A.; Siles-Castellano, A.; Moreno, J. Enzymatic profiles associated with the evolution of the lignocellulosic fraction during industrial-scale composting of anthropogenic waste: Comparative analysis. J. Environ. Manag. 2019, 248, 109312. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, Y.; Zhang, W.; Zhou, H.; Chen, X.; Li, Y.; Wei, D.; Wei, Z. Roles of bacterial community in the transformation of organic nitrogen toward enhanced bioavailability during composting with different wastes. Bioresour. Technol. 2019, 285, 121326. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef] [PubMed]
- BernBernal, M.P.; Soomer, S.G.; Chadwick, D.; Qing, C.; Guoxue, L.; Michel, F.C., Jr. Chapter Three—Current Approaches and Future Trends in Compost Quality Criteria for Agronomic, Environmental, and Human Health Benefits. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 144, pp. 143–233. [Google Scholar] [CrossRef]
- Brinton, W.F., Jr.; Storms, P.; Blewett, T.C. Occurrence and levels of fecal indicators and pathogenic bacteria in market-ready recycled organic matter composts. J. Food Prot. 2009, 72, 332–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Facility a | Waste Mixture | Method of Composting | Time of Composting Process (Months) |
|---|---|---|---|
| VR1 | Cucumber and zucchini crop residues: stalks, leaves | Open air, turned wind-rows | 4 |
| VR2 | Cucumber and zucchini crop residues: stalks, leaves | Open air, turned wind-rows | 4 |
| VR3 | Pepper crop residues: stalks, leaves | Open air, turned wind-rows | 3 |
| MSW1 | Municipal solid waste b | In-vessel turned wind-rows in bays | 3.5 |
| MSW2 | Municipal solid waste b | In-vessel turned wind-rows in bays | 4.5 |
| MSW3 | Municipal solid waste b | In-vessel tunnel composting (turning by augers) | 3 |
| SS1 | Sewage sludge + straw 1:1 v/v | Open air, turned wind-rows | 3.5 |
| SS2 | Sewage sludge + pruning wastes 1:1 v/v | Open air, turned wind-rows | 3 |
| SS3 | Dried sewage sludge + pruning wastes 1:2 v/v | In-vessel tunnel composting (turning by augers) | 3 |
| Facility ** | M (%) | pH | EC (mS cm−1) | OM (%) | C/N | AT4 (gO2 kg−1 OM) | DRI (gO2 kg−1 OM h−1) | GI (%) |
|---|---|---|---|---|---|---|---|---|
| VR1 | 85.94 d | 8.10 ef | 16.78 c | 74.06 de | 12.63 d | 52.38 a | 1.07 ab | 7.70 ab |
| VR2 | 66.62 b | 8.04 ef | 14.73 cd | 73.48 d | 13.30 d | 67.03 a | 1.70 b | 0.00 a |
| VR3 | 29.27 a | 6.78 c | 13.53 d | 77.13 f | 10.61 c | 38.98 a | 0.46 a | 1.91 b |
| MSW1 | 78.19 c | 5.16 b | 3.98 ab | 74.73 e | 28.47 f | 196.56 b | 3.01 c | 0.00 a |
| MSW2 | 84.83 cd | 3.94 a | 2.65 a | 75.15 e | 28.05 f | 160.95 b | 2.73 c | 0.00 a |
| MSW3 | 61.06 b | 7.22 d | 5.37 b | 71.73 c | 20.10 e | 49.65 a | 0.74 a | 0.00 a |
| SS1 | 81.87 cd | 7.95 e | 3.02 a | 71.97 c | 9.29 b | 23.27 a | 0.62 a | 40.42 c |
| SS2 | 66.37 b | 8.63 g | 2.56 a | 64.91 a | 9.50 ab | 57.83 a | 0.95 a | 18.70 b |
| SS3 | 67.67 b | 8.40 fg | 3.06 a | 66.14 b | 6.44 a | 31.86 a | 0.61 a | 38.34 c |
| Facility ** | M (%) | pH | EC (mS cm−1) | OM (%) | C/N | AT4 (gO2 kg−1 OM) | DRI (gO2 kg−1 OM h−1) | GI (%) |
|---|---|---|---|---|---|---|---|---|
| VR1 | 41.05 g | 9.18 f | 8.48 c | 48.43 c | 11.73 bc | 23.20 b | 0.47 cd | 45.79 bc |
| VR2 | 20.46 c | 8.08 d | 17.36 e | 63.30 f | 14.09 d | 30.05 b | 0.44 cb | 2.66 a |
| VR3 | 24.72 d | 9.68 g | 9.97 d | 39.64 b | 10.91 b | 25.72 b | 0.38 bc | 46.43 bc |
| MSW1 | 11.31 b | 8.66 e | 4.97 b | 53.91 e | 11.79 c | 34.23 c | 0.47 cd | 32.73 b |
| MSW2 | 5.66 a | 7.50 b | 5.58 b | 38.05 b | 15.63 e | 75.78 d | 0.73 d | 45.31 bc |
| MSW3 | 50.95 h | 6.00 a | 10.29 d | 63.65 f | 22.44 f | 30.23 b | 0.50 cd | 0.00 a |
| SS1 | 30.80 e | 7.72 c | 4.67 b | 47.19 c | 8.92 a | 1.32 a | 0.04 a | 91.08 d |
| SS2 | 32.33 f | 8.26 d | 2.72 a | 26.09 a | 9.63 a | 9.04 a | 0.20 acb | 99.80 d |
| SS3 | 32.39 f | 8.52 e | 5.52 b | 50.18 d | 14.21 d | 7.99 a | 0.09 ab | 52.68 c |
| %Degr S F | S R Clostridia | %Degr SOC | Fecal Enterococci | E. coli | Fecal Coliform | Total Coliforms | AT4 | DRI | GI | C/N | OM | pH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %Degr Holocellulose | X | 0.42 | −0.53 | −0.42 | −0.41 | −0.76 | −0.43 | −0.70 | −0.62 | 0.56 | −0.61 | X | X |
| %Degr S F | 0.48 | X | X | X | X | X | X | X | X | X | X | X | |
| S R Clostridia | X | X | X | −0.48 | X | X | −0.56 | 0.47 | X | X | X | ||
| %Degr SOC | X | 0.58 | 0.58 | 0.71 | 0.77 | 0.51 | −0.47 | 0.44 | X | X | |||
| Fecal Enterococci | 0.57 | X | X | X | X | X | 0.61 | X | X | ||||
| E. coli | 0.55 | 0.65 | 0.60 | 0.40 | −0.50 | 0.66 | X | −0.44 | |||||
| Fecal Coliforms | 0.52 | 0.77 | 0.64 | −0.40 | 0.47 | X | X | ||||||
| Total Coliforms | 0.56 | X | −0.41 | X | X | X | |||||||
| AT4 | 0.83 | −0.59 | 0.60 | X | X | ||||||||
| DRI | −0.56 | 0.48 | X | X | |||||||||
| GI | −0.71 | −0.73 | X | ||||||||||
| C/N | 0.50 | −0.45 | |||||||||||
| OM | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siles-Castellano, A.B.; López-González, J.A.; Jurado, M.M.; Estrella-González, M.J.; Suárez-Estrella, F.; López, M.J. Compost Quality and Sanitation on Industrial Scale Composting of Municipal Solid Waste and Sewage Sludge. Appl. Sci. 2021, 11, 7525. https://doi.org/10.3390/app11167525
Siles-Castellano AB, López-González JA, Jurado MM, Estrella-González MJ, Suárez-Estrella F, López MJ. Compost Quality and Sanitation on Industrial Scale Composting of Municipal Solid Waste and Sewage Sludge. Applied Sciences. 2021; 11(16):7525. https://doi.org/10.3390/app11167525
Chicago/Turabian StyleSiles-Castellano, Ana B., Juan A. López-González, Macarena M. Jurado, María J. Estrella-González, Francisca Suárez-Estrella, and María J. López. 2021. "Compost Quality and Sanitation on Industrial Scale Composting of Municipal Solid Waste and Sewage Sludge" Applied Sciences 11, no. 16: 7525. https://doi.org/10.3390/app11167525
APA StyleSiles-Castellano, A. B., López-González, J. A., Jurado, M. M., Estrella-González, M. J., Suárez-Estrella, F., & López, M. J. (2021). Compost Quality and Sanitation on Industrial Scale Composting of Municipal Solid Waste and Sewage Sludge. Applied Sciences, 11(16), 7525. https://doi.org/10.3390/app11167525








