Abstract
Olive oil production represents an agro-industrial activity of vital economic importance for many Mediterranean countries. However, it is associated with the generation of a huge amount of by-products, both in solid and liquid forms, mainly constituted by olive mill wastewater, olive pomace, wood, leaves, and stones. Although for many years olive by-products have only been considered as a relevant environmental issue, in the last decades, numerous studies have deeply described their antioxidant, anti-inflammatory, immunomodulatory, analgesic, antimicrobial, antihypertensive, anticancer, anti-hyperglycemic activities. Therefore, the increasing interest in natural bioactive compounds represents a new challenge for olive mills. Studies have focused on optimizing methods to extract phenols from olive oil by-products for pharmaceutical or cosmetic applications and attempts have been made to describe microorganisms and metabolic activity involved in the treatment of such complex and variable by-products. However, few studies have investigated olive oil by-products in order to produce added-value ingredients and/or preservatives for food industries. This review provides an overview of the prospective of liquid olive oil by-products as a source of high nutritional value compounds to produce new functional additives or ingredients and to explore potential and future research opportunities.
1. Introduction
Olive growing is spread over 10 million and 800 thousand hectares across the world, 97% of which are concentrated in the Mediterranean area, where the olive tree (Olea europaea L.) has always occupied a central role in among its population. Olive oil is one of the oldest foods and, among European countries, Spain produces about 826 thousand tons of oil, corresponding to more than 52% of world production, and Italy holds 33% of the EU production [1]. However, olive oil extraction represents a serious environmental issue due to the generation of a high quantity of waste in a very short time. The olive mill waste, both in liquid and solid forms, includes: olive mill wastewaters (OMWW), wood and leaves, olive pomace (OP), and stones [2].
The worldwide production of OMWW is estimated around 6 × 106 m3 and 98% is produced in the Mediterranean basin. The ratio of olive oil production to OMWW is 1.0:2.5 L, reaching, in Italy, a total of 1.4 million m3 of OMWW and 30 million m3 in the Mediterranean basin [3,4].
In recent years, technological innovations in olive oil extraction have affected the whole supply chain, impacting the composition of OMWW, which is primarily composed of vegetation water, and water added both during malaxation and during pressing. Specifically, three different extraction processes are commonly applied: (1) the traditional press process; (2) the two- and; (3) the three-phase decanter process. In the traditional process, olives are washed, crushed, mixed, and malaxed with the addition of a small quantity of water which can easily separate the oil from the other fractions. The resulting paste is then pressed to drain the residual oil and the liquid waste from the presses. It consists of a mixture of olive juice and added water and residual oil. Finally, the olive oil is separated from the water by vertical centrifugation or decanting. The traditional process is applied almost exclusively in small olive mills, with larger mills having been replaced by continuous systems. Through the use of an industrial decanter to separate all the phases, the discontinuous pressing process has been replaced by the continuous centrifugation, using a three-phase system, and later on a two-phase system [5,6]. The two-phase system, adopted in Spain and widespread in most countries, does not require the addition of water, other than during horizontal centrifugation, and results in olive oil and semi-solid olive cake [2]. The three-phase decanter process requires the addition of hot water, in 0.6–1.3 m3/1000 kg of processed olives [2] and results in olive oil, OMWW, and olive cake (residual solids). As a result of these differences, the three-phase extraction process presents a slightly higher yield, leading to a lower amount of olive cake but a significantly higher production of OMWW.
The management of liquid wastes in olive mills has always been challenging, and extensive efforts have been carried out to find an effective strategy. Nevertheless, the disposal of OMWW in soil or waterways continues to represent a serious issue for Mediterranean countries due to its severe phytotoxicity and antimicrobial properties that can compromise the balance of ecological systems, with detrimental long-term environmental effects. In many cases, direct disposal of OMWW into lakes, rivers, and water streams has resulted in disastrous environmental consequences due to their high content of phenolic compounds, organic and long-chain fatty acids, and tannins.
In addition to traditional decantation, various systems of purification and disposal have been proposed, such as chemical, agronomic, and biotechnological interventions. However, such approaches underestimate “waste” as a possible primary resource of high nutritional value compounds.
According to EU Directive 2018/851 [7], “waste management in the Union should be improved and transformed into sustainable materials management in order to safeguard, protect and improve the quality of the environment, protect human health, ensure the prudent, efficient and rational use of natural resources, promote the principles of the circular economy, intensify the use of renewable energies, increase energy efficiency, reduce the Union’s dependence on imported resources, provide new economic opportunities and contribute to long-term competitiveness”.
The Italian legislation, in addition to the definition of waste, identifies the conditions under which a substance or object is not to be considered waste, introducing the concept of by-product, which is described in Article 183-bis of the Legislative Decree n. 152/06 [8] as “the substance or object originates from a production process, of which it is an integral part, and whose primary purpose is not the production of such substance or object; it is certain that the substance or object will be used, during the same or a subsequent production or use process, by the producer or third parties; the substance or object can be used directly without any further treatment other than normal industrial practice; the further use is legal, i.e. the substance or object fulfils, for the specific use, all relevant product and health and environmental protection requirements and will not lead to overall negative impacts on the environment or human health”.
As matter of fact, olive oil by-products contain a high amount of bioactive compounds, namely phenols (as reported in Table 1). The most of the phenolic fraction present in olives is found in OMWW (up to 53%) and OP (approximately 45%), with only 2% of the initial content remaining in virgin olive oil [9]. The phenolic compounds present in OMWW are hydroxytyrosol, tyrosol, verbascoside, acids (such as caffeic, gallic, vanillic, and syringic) and polymeric substances [10,11]. Recently, the use of OMWW has been successfully proposed for different applications, and many studies have focused on obtaining compounds with high added value, i.e., phenolic extracts, through different approaches, including enzymatic and chromatographic techniques, solvent extraction methods, and membrane processes, such as microfiltration, ultrafiltration, nanofiltration, and reverse osmosis [6]. Therefore, OMWW could be considered as a potential low-cost starting matrix for extraction of antioxidants to be applied in several fields, including the food industry, where they could be used for both fortifying and prolonging the shelf life of final products [12,13,14].
In the present work, a literature survey was carried out taking into account a fixed timeline, between 1996 and 2020, and the keywords “olive mill wastewater”.
Searching on ScienceDirect, 794 records were found. Most of them fell within the scope of environmental science, such as chemical engineering, energy fuels, and agriculture, with quite constant increasing numbers in recent years, from two papers published in 1992 to 54 papers in the last five years (Figure 1a). To confirm the increasing interest in biotechnological approaches to OMWW treatment, 298 records were identified in the field of biotechnology and microbiology (Figure 1b) [15].
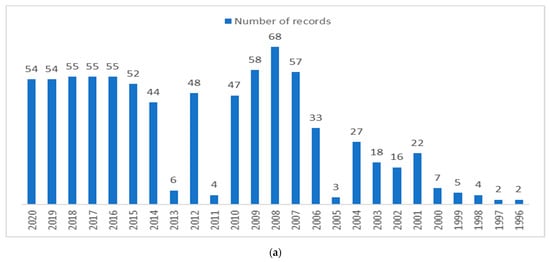
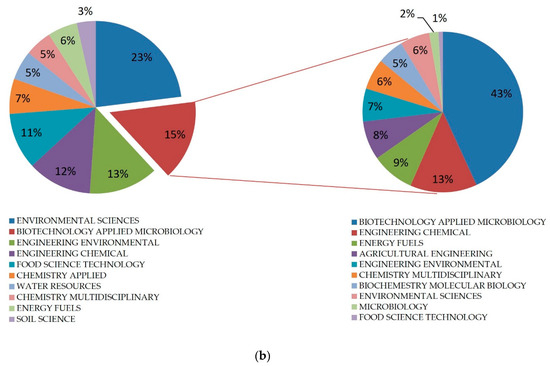
Figure 1.
(a) Records on olive mill wastewater found on PubMed; (b) distribution in different application areas of general records on OMWW, and specific records focusing on applied biotechnology and microbiology.
The aim of this review is to provide a summary of updated information on research that has been conducted using OMWW as a renewable raw material to generate high added-value ingredients/products for agro-food industries, including the functional food sector.
2. Characteristics of OMWW
2.1. Physicochemical Traits of OMWW
The OMWW is a mixture of vegetation water and soft tissues (mucilage, pectin) of olive fruits and water used in various stages of the extraction process, i.e., water added during centrifugation, and water from equipment washing [16]. The physicochemical traits of OMWW are strongly influenced by soil and climate conditions of the growing area, olive cultivar, ripeness state and, above all, by the oil extraction system. The OMWW is dark, almost black, and characterized by a typical, rather intense, odor. Due to the content of organic acids, namely malic and citric acids, OMWW presents pH values between 2.0 and 6.0 (Table 1). Reducing sugars, essentially glucose (90%) and fructose (10%), tannins, phenolic compounds, polyalcohols, minerals, pectins, and lipids are also present. Compared with other organic wastes, OMWW presents a higher concentration of potassium and considerable levels of nitrogen, phosphorus, calcium, magnesium, and iron [17], derived from contact with oil during the extraction phase, and due to the high hydrophilic nature of phenols [18].

Table 1.
Physicochemical characteristics of OMWW, adapted by Demerche et al. [6].
Table 1.
Physicochemical characteristics of OMWW, adapted by Demerche et al. [6].
| Parameters | Values | Reference |
|---|---|---|
| pH | 2.2–5.9 | [19,20] |
| Water (%) | 80–96 | [21] |
| Chemical oxygen demand (g/L) | 30–320 | [22,23,24,25] |
| Biological oxygen demand (g/L) | 35–132 | [23,24,25] |
| Dry matter (%) | 6.3–7.2 | [26,27] |
| Ash (%) | 1.0 | [26,28,29] |
| Electrical conductivity (ds/m) | 5.5–10 | [16,20] |
| Organic matter (%) | 57–62 | |
| Total carbon (%) | 2.0–3.3 | [26,30,31] |
| Total nitrogen (g/L) | 2.0–2.4 | [21] |
| Total sugar (g/L) | 5.0–12.0 | [16,21,26,32,33,34] |
| Total fat (%) | 1.0–23 | [35] |
| Total suspended solids (g/L) | 25–30 | [36,37] |
| Polyalcohol (%) | 9.0–15 | [21,35,38] |
| Total phenols (g/L) | 0.5–6.1 | [6,26,29,33,39,40,41] |
2.2. Microbiological Traits of OMWW
The microbial community present in OMWW is strongly influenced by several parameters, among which the ripeness state and the olive variety are the most influential [42,43]. The microbial density in OMWW varies between 105 and 106 CFU/mL (CFU: colony-forming unit) and is mainly composed of yeasts, bacteria, and molds [42,43,44,45]. The yeast population includes species belonging to Pichia, Candida, and Saccharomyces genera [44,45]. A survey carried out on OMWW revealed the presence of over 100 identified fungi, mainly belonging to the genera Acremonium, Alternaria, Aspergillus, Bionectria, Byssochlamys, Chalara, Cerrena, Fusarium, Lasiodiplodia, Lecythophora, Paecilomyces, Penicillium, Phycomyces, Phoma, Rhinocladiella, and Scopulariopsis [46].
Although many studies report that the culturable microbial population is represented by only a few bacterial communities, such as: Firmicutes, Actinobacteria, Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria, recently, microarray analyses have revealed a high-density of a larger microbial population, including Proteobacteria, Bacteroidetes, Chloroflexi, Cyanobacteria, and Actinobacteria. However, the most commonly reported microbial communities, representing 50% of the 16S rRNA gene sequences deposited in GenBank, include Gammaproteobacteria (Enterobacteriaceae, Moraxellaceae, Xanthomonadaceae, and Pseudomonadaceae) with a percentage of almost 30%, and Betaproteobacteria (Oxalobacteraceae and Comamonadaceae) with a percentage of 21.5% [46]. Alphaproteobacteria and Actinobacteria (Micrococcaceae, Microbacteriaceae, and Propionibacteriaceae) together comprised 20%, whereas Firmicutes (Bacillaceae, Clostridiaceae, Lactobacillaceae, and Paenibacillaceae) and Bacterioides (Prevotellaceae, Porphyromonadaceae, and Sphingobatteriaceae) phyla accounted for approximately 6.8%, respectively. Furthermore, differences in microbial population have been detected, highlighting that only 15% of operational taxonomic units (OTUs) are commonly detected [47]. In addition, high densities of enteric bacteria belonging to Porphyromonadaceae, Prevotellaceae, Lachnospiraceae, Eubacteriaceae, Peptococcaceae, Peptostreptococcaceae, and Ruminococcaceae spp. or to genera Acinetobacter, Enterobacter spp., Pseudomonas, Citrobacter, Escherichia, Klebsiella, and Serratia spp. have been reported [48].
3. Reuse of OMWW
3.1. OMWW Management and Bioremediation
The implementation of any treatment based on the circular economy approach and “waste” reuse concept represents a competitive and innovative choice for agro-food companies for achieving a reduction in cost management and environmental impact.
According to Tsagaraki and co-workers [39], 1 m3 of OMWW corresponds to 100–200 m3 of domestic wastewater. The COD and BOD5 values of OMWW are very consistent and even higher when obtained by conventional systems (150 g O2/L COD and 90 g O2/L BOD5 vs. 90 g O2/L COD and 30 g O2/L BOD5 for conventional and two-phase extraction systems, respectively).
According to the European Directive 2000/60/CE [49], the OMWW requires specific treatment prior to direct discharge to ensure environmental protection and for regenerated wastewater. Indeed, the disposal of untreated OMWW on agricultural soil causes severe environmental damages, such as altering the color of natural water sources and exercising toxic effects on aquatic life and soil quality. OMWW is characterized by a high content of components with low biodegradability (e.g., long-chain fatty acids, lipids, and simple and complex sugars). Therefore, the most common applied systems for OMWW reuse are concerned with lowering the pollutant load and/or extracting bioactive compounds for different applications [25].
A plethora of physicochemical treatments has been developed in order to remove the phenolic compounds. However, in the majority of the studies no ecotoxicological evaluation has been reported and the success of treatment is mainly based on the reduction of color, COD, phenol content, etc. The most relevant parameter used to evaluate compost phytotoxicity is the germination index (GI). Low GI values could be attributed to the fact that at the starting stage, substrates have high concentrations of water-soluble organic substances, toxic constituents such as alcohols, organic fatty acids and phenolic compounds, high C/N ratios due to the presence of ammonia and other toxic nitrogen-based products, as well as high heavy metal and mineral salt contents [50,51].
In addition to traditional settling (conducted in tanks called “hell”), various treatments have been proposed: physicochemical, biological, or a combination.
Physicochemical systems include different methods based on the use of flocculants, coagulants, membrane filtration and reverse osmosis [52], or applying oxidation cryogenesis, electrocoagulation [53,54], or a photochemical system [55]. Generally, after these treatments, the resulting products can be spread on agricultural soil as an organic fertilizer or simply subjected to evaporation in open tanks [56]. However, these practices are expensive as they produce matrices, such as sludge, that must either undergo further treatments or be disposed of.
Several reports confirm that microorganisms can be proposed as a promising alternative for bioremediation of OMWW [51]. Biological methods, involving anaerobic or aerobic digestion and composting, have been applied to break complex organic compounds into simpler molecules and may lead to the production of proteins, exopolysaccharides, or energy [57,58]. The main interest in anaerobic digestion is the production of energy and reuse of the effluent for irrigation purposes [59]. However, the leading limitation is the inhibition of methanogenic bacteria by both phenolic and organic acids compounds [60]. According to Azbar and co-workers [36], anaerobic filters or up flow anaerobic sludge bed reactors are suitable systems to remove unwanted compounds from OMWW. Filidei et al. [61] proposed sedimentation–filtration treatment of OMWW prior to anaerobic digestion as a useful method for its disposal. On the other hand, aerobic treatment is used to reduce the polluting load, responsible for certain biostatic and phytotoxic effects. Aerobic treatment has also been applied to reduce the polluting effect of municipal wastewater, focusing on the degradation of phenolic compounds. Several microorganisms, such as Pleurotus ostreatus, Bacillus pumilus, Yarrowia lipolytica, etc., have been tested [62,63,64]. Furthermore, a pool of Candida boidinii and Pichia holstii strains has been selected for its ability to reduce (up to 40%) the phenolic content of OMWW combined with 6.0 g/L of (NH4)2SO4 at 10 °C [65].
OMWW has been proposed [66,67] as a growth substrate for Azotobacter vinelandii and the resultant effluents applied to cropland as fertilizer. Therefore, recent studies have shown that the biotechnological potential of indigenous microbiota should be further exploited with respect to bioremediation of OMWW and inactivation of plant and human pathogens.
3.2. OMWW Phenolic Compounds for Agricultural Use
Phenolic compounds from OMWW might be used for integrated pest management programs. Several studies have reported the use of microorganisms (as single or consortia) to degrade organic compounds in effluents [68,69]. Although OMWWs do not contain toxic substances, they are characterized by high COD values and a high concentration of compounds with biostatic activity. Recently, increasing attention has been focused on the degrading properties of microorganisms and biological aerobic treatments using yeasts and filamentous fungi, which have emerged as suitable biofertilization methods for conducting residues with lower toxicity, COD, and phenolic contents. Aissam et al. [70] treated OMWW with microorganisms isolated from the same source, such as Candida boidinii, Geotrichum candidum, Penicillium sp. and Aspergillus niger, obtaining a 40–73% reduction in phenols and a 45–78% reduction in COD value. Bleve et al. [45] identified several strains belonging to the genera Geotrichum, Saccharomyces, Pichia, Rhodotorula, and Candida that showed strain-dependent phenol removal efficiency, decreasing phenolic and COD values, regardless of initial phenolic concentrations. In particular, G. candidum, both as free and Ca-alginate immobilized cells, showed the best degradation performance, and when immobilized showed a double reduction rate ability. Indeed, Ca-alginate improved the proteolytic stability of the enzymes responsible for the degradation process. Maza-Márquez et al. [69] demonstrated that the use of a microalgal–bacterial consortium, in a photo-bioreactor, induces a decrease in pollutant load by affecting COD, BOD5, phenolic compounds, color, and turbidity values of OMWW. The dominant green microalgae Scenedesmus obliquus, Chlorella vulgaris along with cyanobacteria Anabaena sp., showed a synergistic effect on resistance to toxic pollutants, leading to their decomposition. In addition, the effect of Lactiplantibacillus plantarum strains on decolorization and biodegradation of phenolic compounds was evaluated [69], highlighting strains able to decrease the OMWW pH within 6 days. Growth of L. plantarum induced the depolymerization of high molecular weight phenols, resulting in discoloration of fresh OMWW and a significant reduction in total phenols [71]. Approximately 58% of the color, 55% of the COD, and 46% of the phenolic compounds were removed when OMWW was diluted tenfold before L. plantarum addition.
Furthermore, OMWW has also been proposed for biopesticide and compost production. The OMWW application on soil and crops resulted in a growth suppression of most phytopathogenic bacteria and fungi and weed species without any effect on crop growth. However, certain measures should be adhered to when OMWW is used as a biopesticide, especially regarding dose and timing of use [47].
4. OMWW as a Source of Biopolymers and Bio-Energy Production
4.1. Enzyme and Exopolysaccharide Production
OMWW represents a suitable substrate for the production of enzymes by fungi. Fungi are microorganisms known for their ability to synthesize different biological catalysts that can be used in different areas. In particular, Ntougias and co-workers [46] demonstrated that ligninolytic fungi are a useful source of phenoloxidase, polyphenoloxidase, and peroxidase useful for removing recalcitrant compounds in OMWW.
Several yeast strains have been characterized as highly pectolytic, xylanolytic, provided with cellulase, β-glucanase, β-glucosidase, peroxidase, and polygalacturonase activities, which could effectively degrade the complex compounds responsible for OMWW toxicity [65,72].
Several yeasts have been described as able to reduce phenolics and sugars present in OMWW, although white-rot fungi appear to contribute more to discoloration [73]. Moreover, Giannoutsou and co-workers [74] isolated six phenotypically distinct groups of yeasts and three selected isolates were identified through biochemical tests and partial 18S rDNA gene sequence analysis as most closely related to Saccharomyces spp., Candida boidinii, and G. candidum. These fungal genera have been reported as able to degrade the phenolic content present in OMWW [75,76].
Several reports also propose strains belonging to different species, such as Panus trigrinus, Hericium erinaceus, and Pleurotus citrinopileatus for laccase (Lac) and manganese peroxidase (Mnp) production [77,78,79]. Filamentous fungi, such as Aspergillus oryzae, A. niger, Aspergillus ibericus, Aspergillus uvarum, G. candidum, Rizhopus oryzae, Rhizopus arrhizus, and Penicillium citrinum, have been described as lipolytic reservoirs due to their ability to produce lipase [80]. These enzymes have been used in different industries, such as dairy and pharmaceutical [81]. Moreover, OMWW has been confirmed as a suitable substrate for production of pectinase, with Cryptococcus albidus var. albidus IMAT 473 showing the best biotechnological aptitude. This enzyme, compared with other products on the market, showed a broad spectrum endopolygalacturonase activity [82,83,84].
Besides enzyme production, OMWWs have also been evaluated as a source of polysaccharides, especially exopolysaccharides (ESP) [85] with glucose as the main monosaccharide, followed by galactose, arabinose, rhamnose, and galacturonic acid. Xanthan, a glucose-mannose and glucuronic acid repeating unit compound, is the main ESP used in different products, such as in cosmetic formulations or as a supplement and thickening compound [86]. However, the EPS production through a fermentation process depends on the type of microorganism. The first production of EPS in OMWW (used at 30% v/v) was obtained through a strain of Xanthomonas campestris that showed a productive capacity of 4 g/L [87]. Similarly, Paenibacillus jamilae sp. highlighted, on OMWW, the production of an EPS consisting of fucose, xylose, rhamnose, arabinose, mannose, galactose, and glucose. Morillo et al. [88] reported that P. jamilae CECT 5266 strain (in a 80% v/v of OMWW) produced an EPS consisting of glucose, galactose, mannose, arabinose, rhamnose, hexosamine, and uronic acid, in agreement with results previously reported by Ruiz-Bravo et al. [89] using the strain P. jamilae CP-7.
4.2. Production of Bio-Energy and Biofuels
The need to reduce dependence on conventional fossil fuels in favor of new alternative energy resources is a top global priority. Green energies could contribute to the reduction of greenhouse gas emissions and their consequent unfavorable impacts on global warming and climate change [90]. The high content of organic matter and the low content of nitrogen, volatile acid sugars, polyalcohols, and fats, make OMWW an attractive resource for the production of bioenergy and alternative biofuels, such as methane or ethanol [6,51]. Several microorganisms are used for biohydrogen production, through single or combined catabolic pathways (e.g., Rhodobacter sphaeroides, Rhodopseudomonas palustris, and Chlamydomonas reinhardtii). The production of these substances takes place through a process of anaerobic digestion, which consists of two phases. During the first phase, macromolecules, such as carbohydrates, proteins, and lipids, are transformed by hydrolytic and acidogenic fermentative bacteria into simple or intermediate organic compounds, volatile organic acids (acetic, propionic, and butyric acids), alcohols (ethanol), ketones, CO2, and hydrogen. In the second step, through interactions between methanogenic and acetogenic microorganisms, these metabolites are transformed into CH4 and CO2 [91]. However due to the presence of oily residues or phenols responsible for antimicrobial activity, OMWW must be first treated or diluted [92]. As already known, before implementing an anaerobic digestion process, the treatment of OMWW with some fungi, such as A. niger, Aspergillus terreus, and Pleurotus sajor-caju play a key role in order to increase the final production of the reference bioenergy compound. Hamdi et al. [57] and Borja and co-workers [93], through a comparative kinetic study, demonstrated that the pretreatment of OMWW with A. niger and A. terreus increased the methane yield. Massadeh and Modallal [94] evaluated the ability of a P. sajor-caju strain to degrade the phenols of OMWW producing ethanol. For this purpose, the authors examined the effects of dilution with water (in a 1:1 ratio), heat treatment (at 100 °C), and treatment with H2O2. The results showed that the degradation of phenols by P. sajor-caju reached a level of 50% in heat-treated OMWW, 53% in heat-treated OMWW pretreated with H2O2, and 58% in undiluted heat-treated OMWW. The highest ethanol yield was obtained in samples pretreated with P. sajor-caju and after 48 h of fermentation with 50% diluted and heat-treated OMWW. Further biological treatment was carried out with Saccharomyces cerevisiae. Sarris et al. [95] and Nikolaou et al. [96] confirmed the aptitude of S. cerevisiae to produce ethanol and optimal fermentation parameters were detected using the 1:1 OMWW/water mixture ratio. The fermentation kinetics of molasses mixed with OMWW where S. cerevisiae was immobilized affected the ethanol yield, reaching values up to 67.8 g/L per day. Moreover, Zanichelli et al. [97] proposed a multiphase treatment using S. cerevisiae added to OMWW with glucose, to a final sugar concentration of 200 g/L, with A. niger extract to hydrolyze the present polysaccharides. Although S. cerevisiae showed low fermentative performance, indigenous strains belonging to Pichia fermentans and Candida spp. reduced phenolic content up to 15% and 18%, respectively, without any addition or pretreatment [98]. Furthermore, Sarris et al. [99] demonstrated the ability of Y. lipolytica strain ACA-DC 5029 to grow on media containing a low concentration of crude glycerol and OMWW, producing a significant amount of citric acid and erythritol. In the presence of high glycerol concentration, a shift towards erythritol production was observed, simultaneously with high amounts of citric acid production. The strain showed promising characteristics to be used in the biotransformation of biodiesel derived from the combination of crude glycerol and OMWW and the subsequent production of added-value chemical compounds.
4.3. Use of OMWW in Feed Formulation
The use of agro-industrial by-products in animal feed can represent an economically and environmentally advantageous solution for the livestock sector, increasing its profitability and sustainability [100]. Olive oil by-products have been tested for the formulation of feed for lambs, pigs, and chickens by evaluating the antioxidant activity on animals and on final products. Makri et al. [101] evaluated the effect of OMWW addition in a silage formulation for lambs, containing 52.5% of solids, 7.5% of OMWW, and 40% of water. The administration of OMWW-containing silage was found effective in improving animal welfare and productivity. Furthermore, several authors tested the effectiveness of a reduction in oxidative stress and in the stimulation of the immune response of the same extract for pigs. Gerasopoulos et al. [102] studied the antioxidant effect of the addition of 4% of OMWW (representing the retentate obtained by microfiltration) in silage. Piglets fed with the fortified formulation showed an increase in tested biomarkers (as total antioxidant capacity: TAC; glutathione: GSH; catalase activity: CAT; protein carbonyls: CARB; and reactive thiobarbituric acids: TBARS) in blood and tissues and a decrease in oxidative stress, with an overall increase in productivity. In addition, Varricchio et al. [103] evaluated the antioxidant activity in piglets fed with phenol extracts, and results highlighted an increase in leukocytes and cyclooxygenase-2 (COX-2), known as markers of inflammation. Gerasopoulos et al. [104] repeated the test in chickens, highlighting markers of antioxidant activity with the same silage formulation proposed for piglet feeding. The results confirmed that such supplementation lowers the levels of lipid peroxidation and protein oxidation by increasing the total antioxidant capacity in plasma confirming that both OMWW and oil by-products (leaves and olive pomace) can be a viable alternatives to fortify animal feeds.
5. Bioactive Properties of OMWW
Olive oil by-products are rich in bioactive compounds with potential health benefits [41]. Ciriminna et al. [105] investigated the relationship between phenolics and health benefits on food, pharmaceutical, and cosmetic applications. Regarding the food sector, the addition of phenols from OMWW seems very interesting not only to strengthen the beneficial effects of foods themselves, but also to extend their shelf life. In the U.S., olive pulp extracts have been approved by the Food and Drug Administration (FDA) with GRAS (Generally Recognized as Safe) (GRN No. 459) status as antioxidants in baked goods, beverages, cereals, sauces and dressings, condiments, and snacks, at a final concentration of up to 3 g/kg [106,107]. Commercial OMWW implementation in food and recovery of phenols is of great interest [108,109] and at least five companies worldwide recover phenols from OMWWs [110] to sell them as natural preservatives or bioactive additives in food products [111].
5.1. Antioxidant Properties
Many reports, both in humans and animals, confirmed that most degenerative diseases, such as cancer and cardiovascular diseases [112] are related to oxidative stress, which has also been identified as a causative agent for declining immune function and atherosclerosis [113]. Several nutraceuticals aimed to reduce the oxidative stress are currently available on the market [114]. Phenols are recognized as the main compounds responsible for the health effects of the Mediterranean diet in prevention of chronic diseases and diet-associated diseases (DRDs), such as obesity, metabolic syndrome, type 2 diabetes (T2D), cardiovascular disease (CVD), hypertension, and some cancers. Their role has been clearly recognized by the European Food Safety Authority [115] with the health claim: “Olive oil polyphenols contribute to the protection of blood lipids from oxidative stress.”
In recent years, an increased interest in the extraction of phenols from OMWW has been registered and different extraction techniques have been proposed [116]. Phenols are active ingredients of many medicinal plants and the mechanisms of their pharmacological activity are not yet fully understood. Beyond the mechanism of protection, based on antioxidant activities, phenols have highlighted: scavenger property against free radicals and reactive oxygen forms (ROS); ability to act as chelators of heavy metals (especially iron) and capability to inhibit lipoxygenase, involved in inflammatory processes. The main radical species, involved in diseases, responsible of cytotoxic effect and in damaging membranes’ lipids, are superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (OH−) [117].
5.2. Antimicrobial Properties
The main phenolic compounds present in OMWW are those derived by oleuropein hydrolysis, as hydroxytyrosol, tyrosol and elenolic acid, but also other phenols: caffeic acid, p-coumaric acid, vanillic acid, syringic acid, gallic acid, luteolin, quercetin, cyanidin, verbascoside, and other polymeric compounds [10,11]. Marković et al. [118] demonstrated that hydroxytyrosol, tyrosol, oleuropein, and oleocanthal present a wide spectrum of biological effects on physiological processes, being antiatherogenic, cardioprotective, anticancer, neuroprotective, antidiabetic, anti-obesity compounds. Furneri and co-workers [119] revealed that oleuropein was also effective against Mycoplasma fermentans and Mycoplasma hominis, which are naturally resistant to erythromycin and often also to tetracycline. Biocompounds of olive products, such as aliphatic aldehydes [120], have also been shown to inhibit or retard the growth of a range of bacteria and yeasts and could be considered as an alternative for the prevention or treatment of infections. Moreover, they have been evaluated for drug formulations to reduce the spread of antimicrobial resistance bacteria [121]. Bisignano et al. [122] demonstrated that hydroxytyrosol possesses an in vitro antimicrobial property against respiratory and gastrointestinal infectious agents, such as Vibrio parahaemolyticus, Vibrio cholerae, Salmonella Typhi, Haemophilus influenzae, Staphylococcus aureus, and Moraxella catarrhalis, at low concentrations.
6. OMWW as Replacer of Synthetic Additives
The strong demand for adequate nutrition is accompanied by the concern for environmental pollution with a considerable emphasis on the recovery and recycling of food by-products and wastes [9].
Several studies have focused on replacing synthetic additives with natural substances, mainly derived from plants and agro-industry by-products [123,124] with promising results. The addition of such substances not only inhibits the growth of pathogens but also prolongs the shelf life of food products. OMWWs are added as such or as extracts, concentrated and stabilized and, in some cases, microencapsulated. Specifically, encapsulation protects them from degradation due to different factors reducing the amount of compounds required to be efficient and controlling their release into the food matrix [125].
Besides therapeutic benefits, biophenols present in OMWW have been explored for their antimicrobial, antifungal, and antiviral properties. Obied et al. [14] reported that the phenolic fraction of OMWW shows antibacterial activity against several species, particularly S. aureus, Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa. However, the antimicrobial activity was found to be higher when the whole phenolic content is used, compared with the activity of the single phenolic compound [126]. In particular, Serra et al. [127] showed that natural OMWW extracts exhibited a higher antimicrobial activity compared with the three individual biophenols (quercetin, hydroxytyrosol, and oleuropein), suggesting a synergic effect among molecules. In most cases, to inhibit the growth of target strains, the effective tested dose was 1000 μg/mL. In addition, it has been shown that individual phenolic compounds, used at low concentrations, were not able to inhibit the growth of E. coli, Klebsiella pneumoniae, S. aureus, and Staphylococcus pyogenes, while whole OMWW was effective in inhibition of both Gram-positive and Gram-negative bacteria [121]. Other authors, however, reported that the bactericidal and fungicidal activities of OMWW are mainly related to the content of phenolic monomers, such as hydroxytyrosol and tyrosol [128]. Hydroxytyrosol was found to also be active against foodborne pathogens such as Listeria monocytogenes, S. aureus, Salmonella Enterica, and Yersinia spp. [129] and against beneficial microorganisms, such as L. acidophilus and Bifidobacterium bifidum. In addition, Fasolato et al. [130] confirmed the bactericidal effect of phenol extract purified from OMWWs. In particular, S. aureus and L. monocytogenes showed the lowest level of resistance (minimum bactericidal concentration MBC = 1.5-3.0 mg/mL) while Gram-negative bacteria (e.g., Salmonella Typhimurium and Pseudomonas spp.) showed higher resistance, with MBC values ranging from 6 to 12 mg/mL. In the same study, among the tested starter species, the growth of Staphylococcus xylosus and L. curvatus was drastically reduced (at concentrations of 0.75 and 1.5 mg/mL MBC, respectively).
Application of OMWW as Food Supplement
In several studies, olive oil by-products have been added as concentrates or ingredients in the formulation of novel foods in different agro-food supply chains (Table 2). In a review, Galanakis [131] collected data related to the addition of OMWW extracts (but also of other oil industrial by-products) to fortify meat and meat products. The results showed that the obtained antioxidants induce an improvement of hygienic conditions and rheological characteristics of the final products. Olive phenols have shown better performance in raw meat treatment [132] as they were able to hinder lipid oxidation. To evaluate such an effect, an oxidation test with a thiobarbituric acid reaction (TBAR) was applied for a storage period of 72 h at 4 °C. Results in limiting lipid oxidation appear to be dependent on the concentration of phenols (500 mg ascorbic acid or catechin/L and 100 mg olive phenols/L). Lopez et al. [133] and Veneziani et al. [111] recently applied OMWW-extracted polyphenols in fermented sausages and white meat burgers, improving quality parameters and extending their shelf life. In particular, the addition of the extracts inhibited the fungal growth and spore germination in fermented sausages by performing a dose- and species -dependent activity both in vitro and in situ tests. In particular, the treatment with 2.5% of OMWW extract strongly inhibited in situ growth of Cladosporium cladosporioides, Penicillium aurantiogriseum, Penicillium commune, and Eurotium amstelodami. Veneziani et al. [111] evaluated the effect of OMWW-extracted polyphenols in white meat burgers, wrapped in PVC, on improving sensorial and hygienic characteristics. The addition of the phenolic extract at different concentrations (0.75 and 1.50 g/kg) delayed the growth of mesophilic aerobic bacteria, highlighting a dose-dependent behavior, with a 24 h extension of shelf life, compared with both the control and sample treated with the lowest concentration. In addition, Fasolato et al. [130], according to Servili et al. [134], found that a 38.6 g/L concentration of phenolic extract was effective in increasing fresh chicken breast shelf life. Samples were immersed in a solution containing the extract for a few seconds, before packing and storage at 4 °C. The results showed a delay of growth of both Enterobacteriaceae and Pseudomonas spp. with at least a 2 day increase in shelf life, compared with the control. In addition, the treatments were shown to positively affect the odor of meat, decreasing the TBARS value. De Leonardis et al. [135] proposed the addition of lard with olive phenols as a ‘‘novel food’’, showing that the natural antioxidants of OMWW were highly effective in oxidative stabilization of lard. The phenol extract significantly increased the oxidative stability of lard, and the applied doses (100–200 ppm) were not cytotoxic when tested on mouse cell lines (embryonic fibroblasts). In addition, several studies have tested phenol extracts in dairy products to enhance antioxidant activity and better stabilize the products. Troise et al. [136] tested the antioxidant activity of OMWW phenolic extract in UHT milk samples, on inhibition of the Maillard reaction (MR), by adding phenolic extract at 0.1 and 0.05% w/v, revealing the reduction of reactive carbonyl species formation in samples before heat treatment, inducing a greater stability without any detrimental sensorial effects. Phenol extracts (100 and 200 mg/L) from OMWW have also been added in a functional milk drink (similar to yogurt) and fermented with a GABA-producing strain (L. plantarum C48) and a LAB strain of human origin (L. paracasei 15N). The results showed that the addition of phenolic compounds did not interfere with either the fermentation process or the activities of functional LAB [134]. The addition of extracts of both OMWW and olive pomace, at different concentrations (2, 4, 6, and 8 mg/100 g of butter) was tested in a butter formulation [137], revealing that the highest concentration confers resistance to oxidative stress during storage at 25 °C for 3 months, inhibiting the growth of S. aureus, total coliforms, yeast, and molds. Roila et al. [138] added biophenol extract (at 250 μg/mL and 500 μg/mL) to mozzarella cheese retarding the growth of Pseudomonas fluorescens and Enterobacteriaceae. The shelf life was directly proportional to the concentration, increasing by 2 and 4 days, respectively. Galanakis et al. [139] tested the antioxidant effect of OMWW phenolic extracts in combination with other antioxidants, demonstrating a reduction in oxidative deterioration during baking of bread and rusks and showing an antimicrobial effect against S. aureus, B. subtilis, E. coli, and P. aeruginosa (at 200 mg/Kg of flour). Recently, Cedola et al. [140] enriched bakery products by adding OMWW and OP previously subjected to ultrafiltration and evaluated the quality traits of final products from both a chemical and sensory point of view. Ultrafiltered OMWW, was used both in bread dough (1500 g of wheat flour, 900 g of OMWW, 45 g of fresh compressed yeast) and for the formulation of spaghetti at a final concentration of 30% w/w. The results showed that the addition of OMWW into bread and pasta slightly increased the chemical quality of bread and pasta without compromising their sensory traits. Zbakh et al. [141] proposed the exploitation of OMWW for setting up a functional beverage. Commercial products can include different additives, such as ascorbic acid as antioxidants, chelators including ethylenediaminetetraacetic acid (EDTA) and acidifiers, such as citric acid or carbon dioxide. The use of additional antioxidants was not required in beverages when OMWW extract were applied. Recently, a certain interest has developed in new beverages using aqueous extracts obtained with olive leaves, characterized by a high concentration of biophenols. Some of these products are already in the market and sold as integrators for human consumption. Further studies are required to investigate the effects of different formulations on the bioavailability of OMWW phenols and on their beneficial effects. These biological properties can have a significant impact on human health through reducing the incidence of many diseases, especially cardiovascular and chronic degenerative diseases.

Table 2.
Application of OMWW in agro-food chains.
As previously reported by Zbakh et al. [141], which confirmed that OMWW phenolic compounds are highly bioavailable and safe, the potential application of OMWW for setting up functional beverages as a natural concentrate of substances with antioxidant action could be a promising opportunity. To date, on the market there are beverages containing water extracts with different pharmacological indications: antioxidant, blood pressure regulator, and incidence on the metabolism of lipids and carbohydrates, although no reference legislation for the use of olive water for human consumption is currently available.
7. Conclusions and Future Perspectives
According to The future of Food and Agriculture: trends and challenges [142], about one third of all produced food is lost or wasted along the food chain, from production to consumption, highlighting an inefficiency of current food systems. The valorization and reuse of food by-products can create a virtuous recycling system in accordance with the Global Food 2030 objectives.
Although the two-phase extraction system is slowly replacing the traditional olive oil extraction techniques, the disposal of OMWW remains a problem for many small olive oil mills in Italy and in other Mediterranean countries and the valorization of such a by-product appears more important than ever for the agro-food industry.
The chance for agro-food companies to implement a circular economy strategy has offered new choices in by-product valorization. Despite several chemical characterizations of olive by-products, further searches are needed to fully understand the resources of such an interesting valuable raw material. Future olive oil waste management strategies should include a combination of physical and biotechnological processes, followed by further treatments, for producing valuable by-products with high functional activities. In this way, costs of treatments could be compensated by the income from useful by-products.
Author Contributions
Conceptualization, C.C. and C.L.R.; methodology, F.V.R., N.R. and A.P.; software, P.F. and N.R.; validation, C.L.R., C.C. and A.V.; investigation, P.F. and A.V.; resources, C.C. and C.L.R.; data curation, P.F., A.V. and A.P.; writing—original draft preparation, P.F. and F.V.R.; writing—review and editing, C.C. and C.L.R.; supervision, C.C. and F.V.R.; funding acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
PON “RICERCA E INNOVAZIONE” 2014–2020, Azione II—Obiettivo Specifico 1b—Progetto “Miglioramento delle produzioni agroalimentari mediterranee in condizioni di carenza di risorse idriche—WATER4AGRIFOOD. Project number: ARS01_00825. CUP B64I20000160005.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This study was supported by “Miglioramento delle produzioni agroalimentari mediterranee in condizioni di carenza di risorse idriche“—WATER4AGRIFOOD-PON Ricerca e Innovazione 2014–2020 and conducted within a Ph.D. research program in Biotecnologie (XXXV cycle) by Paola Foti who received a grant “Dottorato innovativo con caratterizzazione industriale, PON RI 2014–2010”, titled “Olive oil by-products as a new functional food and source of nutritional food ingredients” from the Department of Agriculture, Food and Environment (Scientific Tutor: C.C. Cinzia Caggia).
Conflicts of Interest
The authors declare no conflict of interest.
References
- FaoStat. Food and Agriculture Organization of the United Nations. Crops 2020. Available online: http://www.fao.org/faostat/en/?#data/QC (accessed on 1 January 2021).
- Roig, A.; Cayuela, M.L.; Sanchez-Monedero, M. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Casa, R.; D’Annibale, A.; Pieruccetti, F.; Stazi, S.; Sermanni, G.G.; Cascio, B.L. Reduction of the phenolic components in olive-mill wastewater by an enzymatic treatment and its impact on durum wheat (Triticum durum Desf.) germinability. Chemosphere 2003, 50, 959–966. [Google Scholar] [CrossRef]
- Rinaldi, M.; Rana, G.; Introna, M. Olive-mill wastewater spreading in southern Italy: Effects on a durum wheat crop. Field Crop. Res. 2003, 84, 319–326. [Google Scholar] [CrossRef]
- Fernandez-Bolaños, J.; Rodríguez, G.; Rodríguez, R.; Guillén, R.; Jimenez-Araujo, A. Extraction of interesting organic compounds from olive oil waste. Grasas y Aceites 2006, 57, 95–106. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process. Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- EU Directive 2018/851 of the European Parliament and of the Council of 30 May 2018 Amending Directive 2008/98/EC on Waste —European Environment Agency. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2018.150.01.0109.01.ENG (accessed on 1 January 2021).
- DECRETO LEGISLATIVO 3 aprile 2006, n. 152 Norme in Materia Ambientale. (GU Serie Generale n.88 del 14-04-2006-Suppl. Ordinario n. 96). Available online: https://www.camera.it/parlam/leggi/deleghe/06152dl.htm (accessed on 1 January 2021).
- Di Nunzio, M.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Capozzi, F.; Gianotti, A.; Bordoni, A. Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res. Int. 2019, 131, 108940. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, I.; Kontogianni, V.G.; Kotsiou, K.; Linsalata, V.; Logrieco, A.F.; Tasioula-Margari, M.; Cardinali, A. Polyphenolic characterization of olive mill wastewaters, coming from Italian and Greek olive cultivars, after membrane technology. Food Res. Int. 2014, 65, 301–310. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.; Konczak, I.; Rehman, A.-U.; Robards, K. Chemistry and bioactivity of olive biophenols in some antioxidant and antiproliferative in vitro bioassays. Chem. Res. Toxicol. 2008, 22, 227–234. [Google Scholar] [CrossRef] [PubMed]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and fractionation of phenolic compounds extracted from olive oil mill wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- El Abbassi, A.; Kiai, H.; Hafidi, A. Phenolic profile and antioxidant activities of olive mill wastewater. Food Chem. 2012, 132, 406–412. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.; Robards, K. Potent antioxidant biophenols from olive mill waste. Food Chem. 2008, 111, 171–178. [Google Scholar] [CrossRef]
- WOS. 2021. Available online: https://wcs.webofknowledge.com (accessed on 1 January 2021).
- Paredes, C.; Cegarra, J.; Roig, A.; Sanchez-Monedero, M.; Bernal, M.P. Characterization of olive mill wastewater (alpechin) and its sludge for agricultural purposes. Bioresour. Technol. 1999, 67, 111–115. [Google Scholar] [CrossRef]
- Peri, C. The Extra-Virgin Olive Oil Handbook; Peri, C., Ed.; John Wiley & Sons, Ltd.: Oxford, UK, 2014. [Google Scholar]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of olive oil antioxidants between oil and water phases. J. Agric. Food Chem. 2002, 50, 596–601. [Google Scholar] [CrossRef]
- Akar, T.; Tosun, I.; Kaynak, Z.; Ozkara, E.; Yeni, O.; Sahin, E.N.; Akar, S.T. An attractive agro-industrial byproduct in environmental cleanup: Dye biosorption potential of untreated olive pomance. J. Hazard. Mater. 2009, 166, 1217–1225. [Google Scholar] [CrossRef]
- Baeta-Hall, L.; Sàágua, M.C.; Bartolomeu, M.L.; Anselmo, A.M.; Rosa, M.F. Biodegradation of olive oil husks in composting aerated piles. Bioresour. Technol. 2005, 96, 69–78. [Google Scholar] [CrossRef]
- Pisante, M.; Inglese, P.; Lercker, G. L’ulivo e l’olio; Cultura&Cultura, Ed.; Bayer Crop Science: Bologna, Italy, 2009; pp. 691–692. [Google Scholar]
- Galiatsatou, P.; Metaxas, M.; Arapoglou, D.; Kasselouri-Rigopoulou, V. Treatment of olive mill waste water with activated carbons from agriculturalby-products. Waste Manag. 2002, 22, 803–812. [Google Scholar] [CrossRef]
- Al-Malah, K.; Azzam, M.O.; Abu-Lail, N.I. Olive mills effluent (OME) wastewater post-treatment using activated clay. Sep. Purif. Technol. 2000, 20, 225–234. [Google Scholar] [CrossRef]
- Niaounakis, M.; Halvadakis, C.P. Olive Processing Waste Management: Literature Review and Patent Survey, 2nd ed.; Elsevier Ltd.: Oxford, UK, 2006. [Google Scholar]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Crit. Rev. Food Sci. Nutr. 2017, 58, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.; Martí, E.; Montserrat, G.; Cruañas, R.; Garau, M. Characterisation and evolution of a soil affected by olive oil mill wastewater disposal. Sci. Total. Environ. 2001, 279, 207–214. [Google Scholar] [CrossRef]
- Vlyssides, A.; Loizidou, M.; Zorpas, A.A. Characteristics of solid residues from olive oil processing as bulking material for co?composting with industrial wastewaters. J. Environ. Sci. Health Part A 1999, 34, 737–748. [Google Scholar] [CrossRef]
- Martin-Garcia, A.I.; Moumen, A.; Ruiz, D.Y.; Alcaide, E.M. Chemical composition and nutrients availability for goats and sheep of two-stage olive cake and olive leaves. Anim. Feed Sci. Technol. 2003, 107, 61–74. [Google Scholar] [CrossRef]
- Lafka, T.-I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic and antioxidant potential of olive oil mill wastes. Food Chem. 2011, 125, 92–98. [Google Scholar] [CrossRef]
- Di Giovacchino, L.; Costantini, N.; Serraiocco, A.; Surrichio, G.; Basti, C. Natural antioxidants and volatile compounds of virgin olive oils obtained by the two or three-phase centrifugal decanters. Eur. J. Lipid Sci. Technol. 2001, 103, 279–285. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef]
- García García, I.; Jiménez Peña, P.R.; Bonilla Venceslada, J.L.; Martín Martín, A.; Martín Santos, M.A.; Ramos Gómez, E. Removal of phenol compounds from olive mill wastewater using Phanerochaete chrysosporium, Aspergillus niger, Aspergillus terreus and Geotrichum Candidum. Process Biochem. 2000, 35, 751–758. [Google Scholar] [CrossRef]
- Vlyssides, A.; Loizides, M.; Karlis, P. Integrated strategic approach for reusing olive oil extraction by-products. J. Clean. Prod. 2004, 12, 603–611. [Google Scholar] [CrossRef]
- Caputo, A.C.; Scacchia, F.; Pelagagge, P.M. Disposal of by-products in olive oil industry: Waste-to-energy solutions. Appl. Therm. Eng. 2003, 23, 197–214. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Gonzálvez, J.; García, D.; Cegarra, J. Agrochemical characterisation of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Azbar, N.; Bayram, A.; Filibeli, A.; Müezzinoğlu, A.; Sengul, F.; Ozer, A. A Review of waste management options in olive oil production. Crit. Rev. Environ. Sci. Technol. 2004, 34, 209–247. [Google Scholar] [CrossRef]
- Fiestas, R.; De Ursinos, J.A.; Borja-Padilla, R. Biomethanization. Int. Biodeter. Biodegr. 1996, 38, 145–153. [Google Scholar] [CrossRef]
- Khoufi, S.; Hamza, M.; Sayadi, S. Enzymatic hydrolysis of olive wastewater for hydroxytyrosol enrichment. Bioresour. Technol. 2011, 102, 9050–9058. [Google Scholar] [CrossRef]
- Tsagaraki, E.; Lazarides, H.N.; Petrotos, K. Olive mill wastewater treatment. In Utilization of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, V., Eds.; Springer: Boston, MA, USA, 2007; pp. 133–157. [Google Scholar] [CrossRef]
- Yangui, T.; Dhouib, A.; Rhouma, A.; Sayadi, S. Potential of hydroxytyrosol-rich composition from olive mill wastewater as a natural disinfectant and its effect on seeds vigour response. Food Chem. 2009, 117, 1–8. [Google Scholar] [CrossRef]
- Obied, H.; Allen, M.S.; Bedgood, D.; Prenzler, P.; Robards, K.; Stockmann, R. Bioactivity and analysis of biophenols recovered from olive mill waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef]
- Kavroulakis, N.; Ntougias, S. Bacterial and β-proteobacterial diversity in Olea europaea var. mastoidis- and O. europaea var. koroneiki-generated olive mill wastewaters: Influence of cultivation and harvesting practice on bacterial community structure. World J. Microbiol. Biotechnol. 2010, 27, 57–66. [Google Scholar] [CrossRef]
- Tsiamis, G.; Tzagkaraki, G.; Chamalaki, A.; Xypteras, N.; Andersen, G.; Vayenas, D.; Bourtzis, K. Olive-mill wastewater bacterial communities display a cultivar specific profile. Curr. Microbiol. 2011, 64, 197–203. [Google Scholar] [CrossRef]
- Ben Sassi, A.; Ouazzani, N.; Walker, G.M.; Ibnsouda, S.; El Mzibri, M.; Boussaid, A. Detoxification of olive mill wastewater by Maroccan yeast isolates. Biodegradation 2008, 19, 337–3461. [Google Scholar] [CrossRef]
- Bleve, G.; Lezzi, C.; Chiriatti, M.; D’Ostuni, I.; Tristezza, M.; Di Venere, D.; Sergio, L.; Mita, G.; Grieco, F. Selection of non-conventional yeasts and their use in immobilized form for the bioremediation of olive oil mill wastewaters. Bioresour. Technol. 2011, 102, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Ntougias, S.; Bourtzis, K.; Tsiamis, G. The microbiology of olive mill wastes. BioMed Res. Int. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- El-Abbassi, A.; Saadaoui, N.; Kiai, H.; Raiti, J.; Hafidi, A. Potential applications of olive mill wastewater as biopesticide for crops protection. Sci. Total. Environ. 2017, 576, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Venieri, D.; Rouvalis, A.; Iliopoulou-Georgudaki, J. Microbial and toxic evaluation of raw and treated olive oil mill wastewaters. J. Chem. Technol. Biotechnol. 2010, 85, 1380–1388. [Google Scholar] [CrossRef]
- EC Directive, (2000), Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy, Official Journal, L 327, 22.12.2000. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=LEGISSUM:l28002b (accessed on 1 January 2021).
- Said-Pullicino, D.; Gigliotti, G. Oxidative biodegradation of dissolved organic matter during composting. Chemosphere 2007, 68, 1030–1040. [Google Scholar] [CrossRef]
- Ahmed, P.; Fernández, P.M.; Figueroa, L.I.C.; Pajot, H. Exploitation alternatives of olive mill wastewater: Production of value-added compounds useful for industry and agriculture. Biofuel Res. J. 2019, 6, 980–994. [Google Scholar] [CrossRef]
- Paraskeva, C.; Papadakis, V.; Tsarouchi, E.; Kanellopoulou, D.; Koutsoukos, P. Membrane processing for olive mill wastewater fractionation. Desalination 2007, 213, 218–229. [Google Scholar] [CrossRef]
- Adhoum, N.; Monser, L. Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem. Eng. Process. Process. Intensif. 2004, 43, 1281–1287. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.; Pimentel-Moral, S.; Verardo, V.; Martinez-Ferez, A. A focus on advanced physico-chemical processes for olive mill wastewater treatment. Sep. Purif. Technol. 2017, 179, 161–174. [Google Scholar] [CrossRef]
- Cermola, F.; DellaGreca, M.; Iesce, M.; Montella, S.; Pollio, A.; Temussi, F. A mild photochemical approach to the degradation of phenols from olive oil mill wastewater. Chemosphere 2004, 55, 1035–1041. [Google Scholar] [CrossRef]
- Belaqziz, M.; El-Abbassi, A.; Lakhal, E.K.; Agrafioti, E.; Galanakis, C.M. Agronomic application of olive mill wastewater: Effects on maize production and soil properties. J. Environ. Manag. 2016, 171, 158–165. [Google Scholar] [CrossRef]
- Hamdi, M. Effects of agitation and pretreatment on the batch anaerobic digestion of olive mill wastewater. Bioresour. Technol. 1991, 36, 173–178. [Google Scholar] [CrossRef]
- Hachicha, S.; Cegarra, J.; Sellami, F.; Hachicha, R.; Drira, N.; Medhioub, K.; Ammar, E. Elimination of polyphenols toxicity from olive mill wastewater sludge by its co-composting with sesame bark. J. Hazard. Mater. 2009, 161, 1131–1139. [Google Scholar] [CrossRef]
- Koutsos, T.; Chatzistathis, T.; Balampekou, E. A new framework proposal, towards a common EU agricultural policy, with the best sustainable practices for the re-use of olive mill wastewater. Sci. Total. Environ. 2018, 622–623, 942–953. [Google Scholar] [CrossRef]
- Hamdi, M. Anaerobic digestion of olive mill wastewaters. Process. Biochem. 1996, 31, 105–110. [Google Scholar] [CrossRef]
- Filidei, S.; Masciandaro, G.; Ceccanti, B. Anaerobic digestion of olive oil mill effluents: Evaluation of wastewater organic load and phytotoxicity reduction. Water Air Soil Pollut. 2003, 145, 79–94. [Google Scholar] [CrossRef]
- Tomati, U.; Galli, E.; Di Lena, G.; Buffone, R. Induction of laccase in Pleurotus ostreatus mycelium grown in olive oil waste waters. Agrochimica 1991, 35, 273–279. [Google Scholar]
- Ramos-Cormenzana, A.; Juarez-Jimenez, B.; García-Pareja, M.P. Antimicrobial activity of olive mill wastewaters (alpechin) and biotransformed olive oil mill wastewater. Int. Biodeter. Biodegr. 1996, 38, 283–290. [Google Scholar] [CrossRef]
- Scioli, C.; Vollaro, L. The use of Yarrowia lipolytica to reduce pollution in olive mill wastewaters. Water Res. 1997, 31, 2520–2524. [Google Scholar] [CrossRef]
- Sinigaglia, M.; Di Benedetto, N.; Bevilacqua, A.; Corbo, M.R.; Capece, A.; Romano, P. Yeasts isolated from olive mill wastewaters from southern Italy: Technological characterization and potential use for phenol removal. Appl. Microbiol. Biotechnol. 2010, 87, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Ehaliotis, C.; Papadopoulou, K.; Kotsou, M.; Mari, I.; Balis, C. Adaptation andpopulation dynamics of Azotobacter vinelandii during aerobic biological treatment of olive-mill wastewater. FEMS Microbiol. Ecol. 1999, 30, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Piperidou, C.I.; Chiadou, C.I.; Stalikas, C.D. Bioremediation of olive oil millwastewater: Chemical alterations induced by Azotobacter vinelandii. J. Agric. Food Chem. 2000, 48, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Cerrone, F.; Barghini, P.; Pesciaroli, C.; Fenice, M. Efficient removal of pollutants from olive washing wastewater in bubble-column bioreactor by Trametes versicolor. Chemosphere 2011, 84, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Maza-Márquez, P.; Gonzalez-Martinez, A.; Martínez-Toledo, M.V.; Fenice, M.; Lasserrot, A.; González-López, J. Biotreatment of industrial olive washing water by synergetic association of microalgal-bacterial consortia in a photobioreactor. Environ. Sci. Pollut. Res. 2016, 24, 527–538. [Google Scholar] [CrossRef]
- Aissam, H.; Penninckx, M.J.; Benlemlih, M. Reduction of phenolics content and COD in olive oil mill wastewaters by indigenous yeasts and fungi. World J. Microbiol. Biotechnol. 2007, 23, 1203–1208. [Google Scholar] [CrossRef]
- Lamia, A.; Moktar, H. Fermentative decolorization of olive mill wastewater by Lactobacillus plantarum. Process. Biochem. 2003, 39, 59–65. [Google Scholar] [CrossRef]
- Romo-Sánchez, S.; Alves-Baffi, M.; Arévalo-Villena, M.; Úbeda-Iranzo, J.; Briones-Pérez, A. Yeast biodiversity from oleic ecosystems: Study of their biotechnological properties. Food Microbiol. 2010, 27, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Abdelhadi, B.S.; Benlemilih, M.; Koraichi, S.I.; Ahansal, L.; Hammoumi, A.; Boussaid, A. Suitability of yeasts for the treatment of olive mill wastewater. Aquat. Environ. Toxicol. 2010, 4, 113–117. [Google Scholar]
- Giannoutsou, E.; Meintanis, C.; Karagouni, A. Identification of yeast strains isolated from a two-phase decanter system olive oil waste and investigation of their ability for its fermentation. Bioresour. Technol. 2004, 93, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Markham, J.L.; Peiris, P.; Nair, N.; Spooner-Hart, R.N.; Holford, P. Screening and selection of fungi for bioremediation of olive mill wastewater. World J. Microbiol. Biotechnol. 2009, 26, 567–571. [Google Scholar] [CrossRef]
- Millán, B.; Lucas, R.; Robles, A.; García, T.; De Cienfuegos, G.A.; Gálvez, A. A study on the microbiota from olive-mill wastewater (OMW) disposal lagoons, with emphasis on filamentous fungi and their biodegradative potential. Microbiol. Res. 2000, 155, 143–147. [Google Scholar] [CrossRef]
- Fenice, M.; Giovannozzisermanni, G.; Federici, F.; D’Annibale, A. Submerged and solid-state production of laccase and Mn-peroxidase by Panus tigrinus on olive mill wastewater-based media. J. Biotechnol. 2003, 100, 77–85. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Larou, E.; Mountzouris, K.C.; Zervakis, G.I. Detoxification of olive mill wastewater and bioconversion of olive crop residues into high-value-added biomass by the choice edible mushroom Hericium erinaceus. Appl. Biochem. Biotechnol. 2016, 180, 195–209. [Google Scholar] [CrossRef]
- Zerva, A.; Zervakis, G.; Christakopoulos, P.; Topakas, E. Degradation of olive mill wastewater by the induced extracellular ligninolytic enzymes of two wood-rot fungi. J. Environ. Manag. 2017, 203, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Crognale, S.; D’Annibale, A.; Federici, F.; Fenice, M.; Quaratino, D.; Petruccioli, M. Olive oil mill wastewater valorisation by fungi. J. Chem. Technol. Biotechnol. 2006, 81, 1547–1555. [Google Scholar] [CrossRef]
- Cordova, J.; Nemmaoui, M.; Ismaіli-Alaoui, M.; Morin, A.; Roussos, S.; Raimbault, M.; Benjilali, B. Lipase production by solid state fermentation of olive cake and sugar cane bagasse. J. Mol. Catal. B Enzym. 1998, 5, 75–78. [Google Scholar] [CrossRef]
- Federici, F. Production, purification and partial characterization of an endopolygalacturonase from Cryptococcus albidus var. albidus. Antonie van Leeuwenhoek 1985, 51, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Federici, F.; Montedoro, G.; Servili, M.; Petruccioli, M. Pectic enzyme production by Cryptococcus albidus var. albidus on olive vegetation waters enriched with sunflower calathide meal. Biol. Wastes 1988, 25, 291–301. [Google Scholar] [CrossRef]
- Petruccioli, M.; Servili, M.; Montedoro, G.F.; Federici, F. Development of a recycle procedure for the utilisation of vegetation waters in the olive-oil extraction process. Biotechnol. Lett. 1988, 10, 55–60. [Google Scholar] [CrossRef]
- Nadour, M.; Laroche, C.; Pierre, G.; Delattre, C.; Moulti-Mati, F.; Michaud, P. Structural characterization and biological activities of polysaccharides from olive mill wastewater. Appl. Biochem. Biotechnol. 2015, 177, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Petri, D. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Lopez, M.; Ramos-Cormenzana, A. Xanthan production from olive-mill wastewaters. Int. Biodeterior. Biodegrad. 1996, 38, 263–270. [Google Scholar] [CrossRef]
- Morillo, J.A.; Del Águila, V.G.; Aguilera, M.; Ramos-Cormenzana, A.; Monteoliva-Sánchez, M. Production and characterization of the exopolysaccharide produced by Paenibacillus jamilae grown on olive mill-waste waters. World J. Microbiol. Biotechnol. 2007, 23, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Bravo, A.; Jimenez-Valera, M.; Moreno, E.; Guerra, V.; Ramos-Cormenzana, A. Biological response modifier activity of an exopolysaccharide from Paenibacillus jamilae CP-7. Clin. Diagn. Lab. Immunol. 2001, 8, 706–710. [Google Scholar] [CrossRef]
- Hill, J. Environmental costs and benefits of transportation biofuel production from food-and lignocellulose-based energy crops: A Review. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 125–139. [Google Scholar]
- Moraes, B.; Zaiat, M.; Bonomi, A. Anaerobic digestion of vinasse from sugarcane ethanol production in Brazil: Challenges and perspectives. Renew. Sustain. Energy Rev. 2015, 44, 888–903. [Google Scholar] [CrossRef]
- Lercker, G. Reflui oleari, i metodi per renderli “buoni”. Olivo E Olio 2014, 9, 37–41. [Google Scholar]
- Borja, R.; Alba, J.; Garrido, S.E.; Martinez, L.; Garcia, M.P.; Incerti, C.; Ramos-Cormenzana, A. Comparative study of anaerobic digestion of olive mill wastewater (OMW) and OMW previously fermented with Aspergillus terreus. Bioprocess. Eng. 1995, 13, 317–322. [Google Scholar] [CrossRef]
- Massadeh, M.I.; Modallal, N. Ethanol production from olive mill wastewater (OMW) pretreated with Pleurotus sajor-caju. Energy Fuels 2007, 22, 150–154. [Google Scholar] [CrossRef]
- Sarris, D.; Matsakas, L.; Aggelis, G.; Koutinas, A.A.; Papanikolaou, S. Aerated vs non-aerated conversions of molasses and olive mill wastewaters blends into bioethanol by Saccharomyces cerevisiae under non-aseptic conditions. Ind. Crop. Prod. 2014, 56, 83–93. [Google Scholar] [CrossRef]
- Nikolaou, A.; Kourkoutas, Y. Exploitation of olive oil mill wastewaters and molasses for ethanol production using immobilized cells of Saccharomyces cerevisiae. Environ. Sci. Pollut. Res. 2017, 25, 7401–7408. [Google Scholar] [CrossRef]
- Heinrich, A.; Zanichelli, D.; Carloni, F.; Hasanaji, E.; D’Andrea, N.; Filippini, A.; Setti, L. Production of ethanol by an integrated valorization of olive oil byproducts. The role of phenolic inhibition (2 pp). Environ. Sci. Pollut. Res. 2006, 14, 5–6. [Google Scholar] [CrossRef]
- Taccari, M.; Ciani, M. Use of Pichia fermentans and Candida sp. strains for the biological treatment of stored olive mill wastewater. Biotechnol. Lett. 2011, 33, 2385–2390. [Google Scholar] [CrossRef]
- Sarris, D.; Stoforos, N.G.; Mallouchos, A.; Kookos, I.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Production of added-value metabolites by Yarrowia lipolytica growing in olive mill wastewater-based media under aseptic and non-aseptic conditions. Eng. Life Sci. 2017, 17, 695–709. [Google Scholar] [CrossRef]
- Berbel, J.; Posadillo, A. Opportunities for the bioeconomy of olive oil byproducts. Biomed. J. Sci. Tech. Res. 2018, 2, 2094–2096. [Google Scholar]
- Makri, S.; Kafantaris, I.; Savva, S.; Ntanou, P.; Stagos, D.; Argyroulis, I.; Kotsampasi, B.; Christodoulou, V.; Gerasopoulos, K.; Petrotos, K.; et al. Novel feed including olive oil mill wastewater bioactive compounds enhanced the redox status of lambs. In Vivo 2018, 32, 291–302. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Petrotos, K.; Kokkas, S.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with polyphenolic byproduct from olive mill wastewater processing improves the redox status in blood and tissues of piglets. Food Chem. Toxicol. 2015, 86, 319–327. [Google Scholar] [CrossRef]
- Varricchio, E.; Coccia, E.; Orso, G.; Lombardi, V.; Imperatore, R.; Vito, P.; Paolucci, M. Influence of polyphenols from olive mill wastewater on the gastrointestinal tract, alveolar macrophages and blood leukocytes of pigs. Ital. J. Anim. Sci. 2019, 18, 574–586. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Kokkas, S.; Petrotos, K.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015, 82, 42–49. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Fidalgo, A.; Ilharco, L.M.; Pagliaro, M. Extraction, benefits and valorization of olive polyphenols. Eur. J. Lipid Sci. Technol. 2015, 118, 503–511. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). 2014. Available online: http://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices&id=459 (accessed on 1 January 2021).
- Galanakis, C.M. A Study for the Implementation of Polyphenols from Olive Mill Wastewater in Foodstuff and Cosmetics; General Secretariat for Research and Technology (GSRT): Athens, Greece, 2015. [Google Scholar]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and removal of phenolic compounds from olive mill wastewater. J. Am. Oil Chem. Soc. 2013, 91, 1–18. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Schieber, A. Editorial. Special issue on recovery and utilization of valuable compounds from food processing by-products. Food Res. Int. 2014, 65, 299–300. [Google Scholar] [CrossRef]
- Veneziani, G.; Novelli, E.; Esposto, S.; Taticchi, A.; Servili, M. Applications of recovered bioactive compounds in food products. In Olive Mill Waste; Academic Press: Cambridge, MA, USA, 2017; pp. 231–253. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Meydani, M. Nutrition, immune cells, and atherosclerosis. Nutr. Rev. 1998, 56, S177–S182. [Google Scholar] [CrossRef]
- Visioli, F.; Davalos, A.; Hazas, M.D.C.L.D.L.; Crespo, M.D.C.; Tomé-Carneiro, J. An overview of the pharmacology of olive oil and its active ingredients. Br. J. Pharmacol. 2019, 177, 1316–1330. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar]
- Aissa, I.; Kharrat, N.; Aloui, F.; Sellami, M.; Bouaziz, M.; Gargouri, Y. Valorization of antioxidants extracted from olive mill wastewater. Biotechnol. Appl. Biochem. 2017, 64, 579–589. [Google Scholar] [CrossRef]
- Girotti, A.W. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 1998, 39, 1529–1542. [Google Scholar] [CrossRef]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Furneri, P.M.; Piperno, A.; Sajia, A.; Bisignano, G. Antimycoplasmal activity of hydroxytyrosol. Antimicrob. Agents Chemother. 2004, 48, 4892–4894. [Google Scholar] [CrossRef]
- Boudet, A.-M. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef] [PubMed]
- Tafesh, A.; Najami, N.; Jadoun, J.; Halahlih, F.; Riepl, H.; Azaizeh, H. Synergistic antibacterial effects of polyphenolic compounds from olive mill wastewater. Evid. Based Complement. Altern. Med. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, G.; Tomaino, A.; Cascio, R.L.; Crisafi, G.; Uccella, N.; Saija, A. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999, 51, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Farag, R.S.; Mahmoud, E.A.; Basuny, A. Use crude olive leaf juice as a natural antioxidant for the stability of sunflower oil during heating. Int. J. Food Sci. Technol. 2007, 42, 107–115. [Google Scholar] [CrossRef]
- Jaber, H.; Ayadi, M.; Makni, J.; Rigane, G.; Sayadi, S.; Bouaziz, M. Stabilization of refined olive oil by enrichment with chlorophyll pigments extracted from Chemlali olive leaves. Eur. J. Lipid Sci. Technol. 2012, 114, 1274–1283. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jafari, S.M.; Assadpour, E.; Esfanjani, A.F. Nano-encapsulation of olive leaf phenolic compounds through WPC–pectin complexes and evaluating their release rate. Int. J. Biol. Macromol. 2016, 82, 816–822. [Google Scholar] [CrossRef]
- Obied, H.K.; Karuso, P.; Prenzler, P.D.; Robards, K. Novel secoiridoids with antioxidant activity from Australian olive mill waste. J. Agric. Food Chem. 2007, 55, 2848–2853. [Google Scholar] [CrossRef]
- Serra, A.T.; Matias, A.; Nunes, A.V.M.; Leitão, M.; Brito, D.; Bronze, M.; Silva, S.; Pires, A.; Crespo, M.T.B.; Romão, M.S.; et al. In vitro evaluation of olive- and grape-based natural extracts as potential preservatives for food. Innov. Food Sci. Emerg. Technol. 2008, 9, 311–319. [Google Scholar] [CrossRef]
- Hazas, M.D.C.L.D.L.; Piñol, C.; Macià, A.; Romero-Fabregat, M.-P.; Pedret, A.; Solà, R.; Rubió, L.; Motilva, M.J. Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef]
- Medina, E.; De Castro, A.; Romero, C.; Brenes, M. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: Correlation with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef]
- Fasolato, L.; Cardazzo, B.; Balzan, S.; Carraro, L.; Taticchi, A.; Montemurro, F.; Novelli, E. Minimum bactericidal concentration of phenols extracted from oil vegetation water on spoilers, starters and food-borne bacteria. Ital. J. Food Saf. 2015, 4. [Google Scholar] [CrossRef]
- Galanakis, C.M. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci. Technol. 2018, 79, 98–105. [Google Scholar] [CrossRef]
- Barbier, C. The Behaviour of Some Antioxidants in an In Vitro Meat Model. Master’s Thesis, Department of Food Technology, Engineering and Nutrition, Lund University, Lund, Sweden, 2009. [Google Scholar]
- Lopez, C.C.; Serio, A.; Mazzarrino, G.; Martuscelli, M.; Scarpone, E.; Paparella, A. Control of household mycoflora in fermented sausages using phenolic fractions from olive mill wastewaters. Int. J. Food Microbiol. 2015, 207, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Rizzello, C.G.; Taticchi, A.; Esposto, S.; Urbani, S.; Mazzacane, F.; Di Maio, I.; Selvaggini, R.; Gobbetti, M.; Di Cagno, R. Functional milk beverage fortified with phenolic compounds extracted from olive vegetation water, and fermented with c-aminobutyric acid (GABA)-producing and potential probiotic lactic acid bacteria. Int. J. Food Microbiol. 2011, 147, 45–52. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Lembo, G.; Aretini, A.; Nag, A. Studies on oxidative stabilisation of lard by natural antioxidants recovered from olive-oil mill wastewater. Food Chem. 2007, 100, 998–1004. [Google Scholar] [CrossRef]
- Troise, A.D.; Fiore, A.; Colantuono, A.; Kokkinidou, S.; Peterson, D.G.; Fogliano, V. Effect of olive mill wastewater phenol compounds on reactive carbonyl species and Maillard reaction end-products in ultrahigh-temperature-treated milk. J. Agric. Food Chem. 2014, 62, 10092–10100. [Google Scholar] [CrossRef] [PubMed]
- Mikdame, H.; Kharmach, E.; Mtarfi, N.E.; Alaoui, K.; Ben Abbou, M.; Rokni, Y.; Majbar, Z.; Taleb, M.; Rais, Z. By-products of olive oil in the service of the deficiency of food antioxidants: The case of butter. J. Food Qual. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Roila, R.; Valiani, A.; Ranucci, D.; Ortenzi, R.; Servili, M.; Veneziani, G.; Branciari, R. Antimicrobial efficacy of a polyphenolic extract from olive oil by-product against “Fior di latte” cheese spoilage bacteria. Int. J. Food Microbiol. 2019, 295, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Tsatalas, P.; Charalambous, Z.; Galanakis, I.M. Control of microbial growth in bakery products fortified with polyphenols recovered from olive mill wastewater. Environ. Technol. Innov. 2018, 10, 1–15. [Google Scholar] [CrossRef]
- Cedola, A.; Cardinali, A.; D’Antuono, I.; Conte, A.; Del Nobile, M.A. Cereal foods fortified with by-products from the olive oil industry. Food Biosci. 2019, 33, 100490. [Google Scholar] [CrossRef]
- Zbakh, H.; El Abbassi, A. Potential use of olive mill wastewater in the preparation of functional beverages: A review. J. Funct. Foods 2012, 4, 53–65. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The Future of Food and Agriculture, Trends and Challenges; Food and Agriculture Organization: Rome, Italy, 2017; Volume 4, ISBN 9789251095515. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).