NaOH-Catalyzed Fractionation of Rice Husk Followed by Concomitant Production of Bioethanol and Furfural for Improving Profitability in Biorefinery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Preparation
2.2. Structural and Compositional Analysis of RH
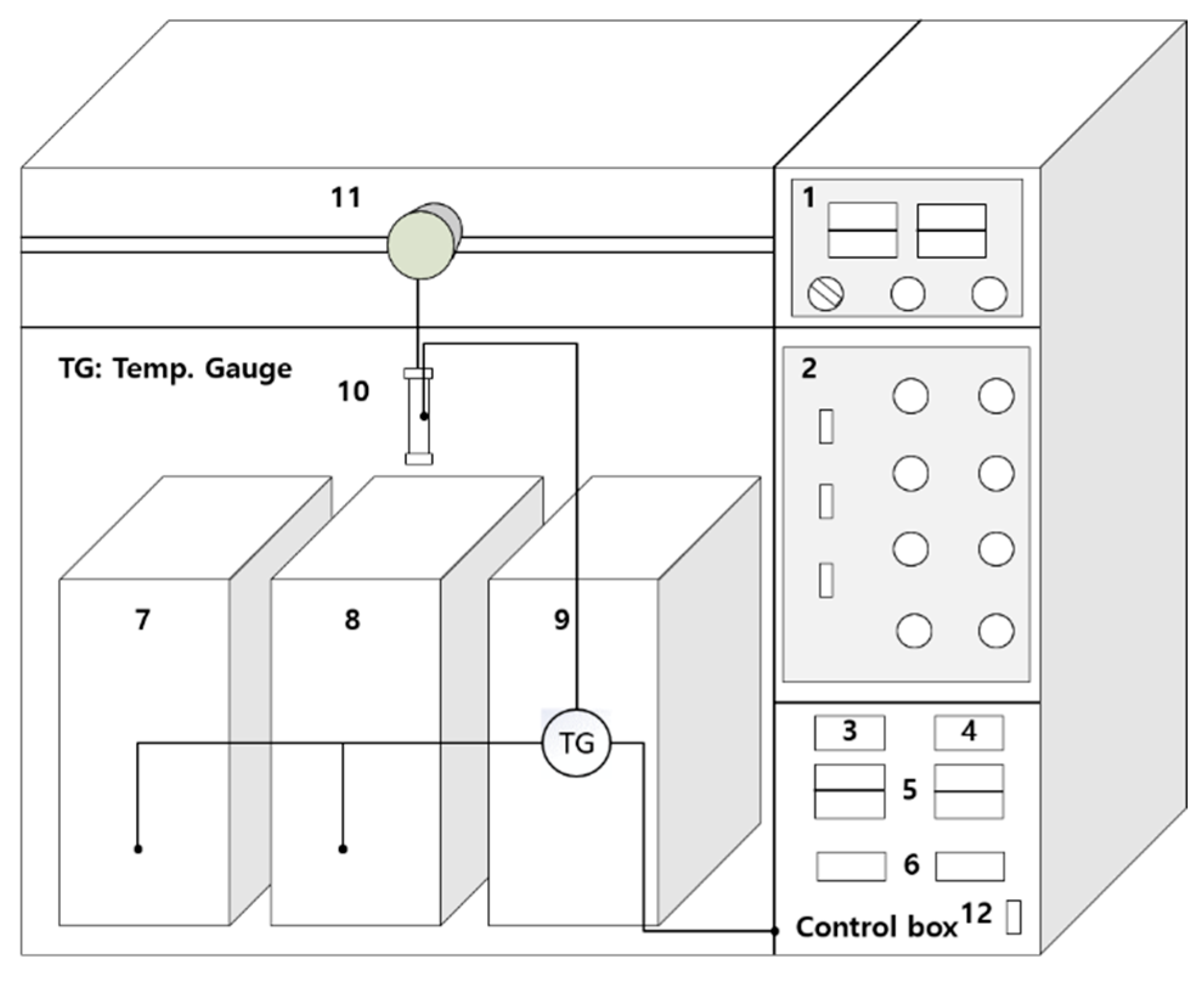
2.3. Experimental Setup and Operation of Bench-Scale Fractionation
2.4. Production of Furfural from Hemicellulose-Rich Hydrolysate
2.5. Evaluation of Alkaline Fractionation with Enzymatic Digestibility
2.6. Two-Stage Fed-Batch SSF with Glucan-Rich Solid Residues
3. Results and Discussions
3.1. Chemical Compositions of RH Based on Oven-Dry Biomass
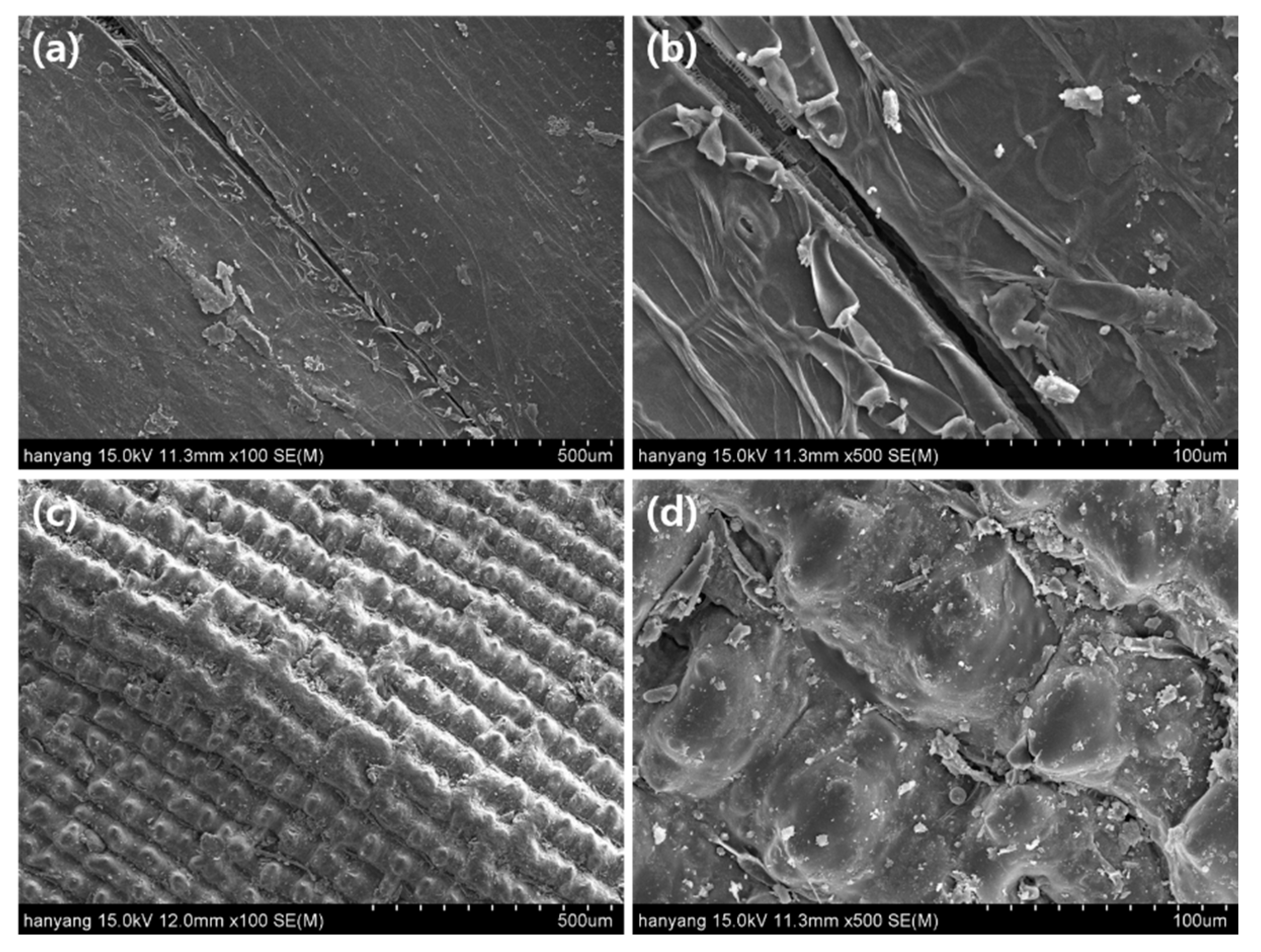
3.2. Structural Characteristics and Elemental Analysis of Raw RH
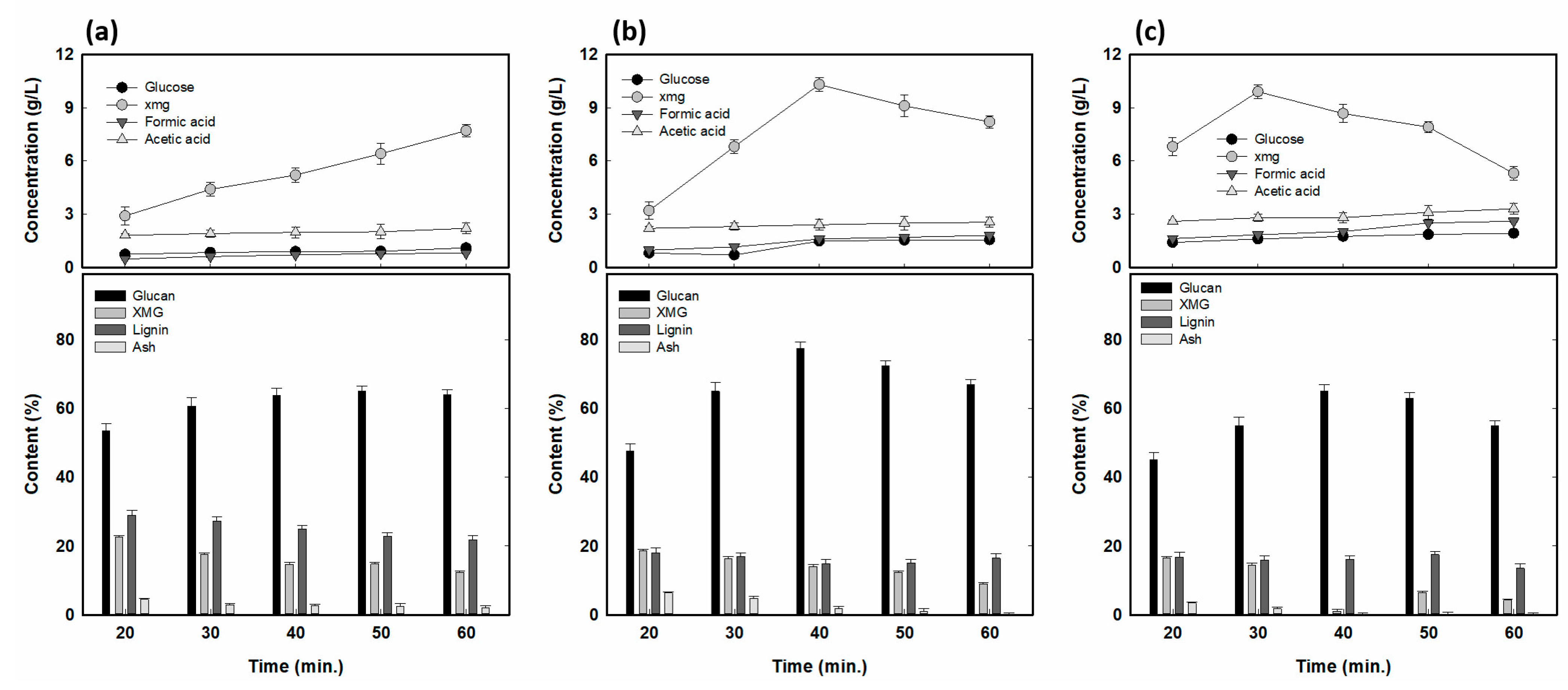
3.3. Alkaline Fractionation for High Glucan Content and XMG Yield
3.4. Bench-Scaled Alkaline Fractionation of RH at Optimized Conditions
3.5. Furfural Production from Acidified RH Hydrolysate
3.6. Enzymatic Digestibility of Fr. RH
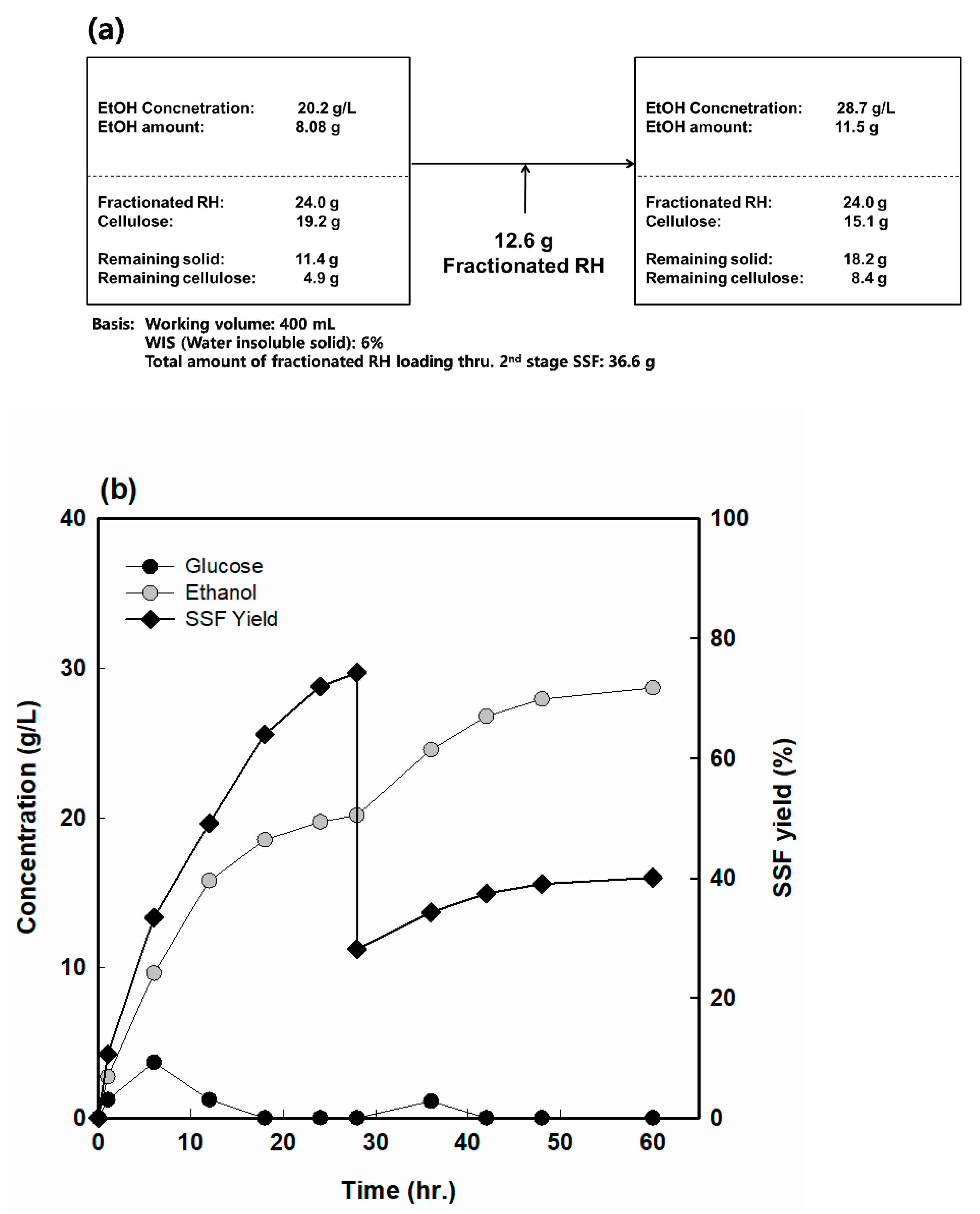
3.7. Fed-Batch Simultaneous Saccharification and Fermentation with Fr. RH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 524–534. [Google Scholar] [CrossRef]
- Salanti, A.; Zoia, L.; Orlandi, M.; Zanini, F.; Elegir, G. Structural characterization and antioxidant activity evaluation of lignins from rice husk. J. Agric. Food Chem. 2010, 58, 10049–10055. [Google Scholar] [CrossRef]
- Dizaji, H.B.; Zeng, T.; Hartmann, I.; Enke, D.; Schliermann, T.; Lenz, V.; Bidabadi, M. Generation of high quality biogenic silica by combustion of rice husk and rice straw combined with pre- and post-treatment strategies—A review. Appl. Sci. 2019, 9, 1083. [Google Scholar] [CrossRef] [Green Version]
- Werther, J.; Saenger, M.; Hartge, E.U.; Ogada, T.; Siagi, Z. Combustion of agricultural residues. Prog. Energy Combust. Sci. 2000, 26, 1–27. [Google Scholar] [CrossRef]
- Lee, T.; Othman, R.; Yeoh, F.Y. Development of photoluminescent glass derived from rice husk. Biomass Bioenergy 2013, 1–13. [Google Scholar] [CrossRef]
- Lau, B.B.; Yeung, T.; Patterson, R.J.; Aldous, L. A cation study on rice husk biomass pretreatment with aqueous hydroxides: Cellulose solubility does not correlate with improved enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2017, 5, 5320–5329. [Google Scholar] [CrossRef] [Green Version]
- Ang, T.N.; Ngoh, G.C.; Chua, A.S.M. Comparative study of various pretreatment reagents on rice husk and structural changes assessment of the optimized pretreated rice husk. Bioresour. Technol. 2013, 135, 116–119. [Google Scholar] [CrossRef]
- Kim, S.B.; Lee, S.J.; Lee, J.H.; Jung, Y.R.; Thapa, L.P.; Kim, J.S.; Um, Y.; Park, C.; Kim, S.W. Pretreatment of rice straw with combined process using dilute sulfuric acid and aqueous ammonia. Biotechnol. Biofuels 2013, 6, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Dagnino, E.P.; Chamorro, E.R.; Romano, S.D.; Felissia, F.E.; Area, M.C. Optimization of the acid pretreatment of rice hulls to obtain fermentable sugars for bioethanol production. Ind. Crop. Prod. 2013, 42, 363–368. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Banerjee, G.; Car, S.; Scott-Craig, J.S.; Hodge, D.B.; Walton, J.D. Alkaline peroxide pretreatment of corn stover: Effects of biomass, peroxide, and enzyme loading and composition on yields of glucose and xylose. Biotechnol. Biofuels 2011, 4, 16–30. [Google Scholar] [CrossRef] [Green Version]
- Ang, T.N.; Ngoh, G.C.; Chua, A.S.M.; Lee, M.G. Elucidation of the effect of ionic liquid pretreatment on rice husk via structural analyses. Biotechnol. Biofuels 2012, 5, 67–76. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Pang, Y.; Liu, Y.; Li, X.; Wang, K. Physicochemical characterization of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy Fuels 2008, 22, 2775–2781. [Google Scholar] [CrossRef]
- McIntosh, S.; Vancov, T. Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw. Biomass Bioenergy 2011, 35, 3094–3103. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ryu, H.J.; Oh, K.K. Acid-catalyzed hydrothermal severity on the fractionation of agricultural residues for xylose-rich hydrolyzates. Bioresour. Technol. 2013, 132, 84–90. [Google Scholar] [CrossRef]
- Sindhu, R.; Kuttiraja, M.; Binod, P.; Sukumaran, R.K.; Pandey, A. Physicochemical characterization of alkali pretreated sugarcane tops and optimization of enzymatic saccharification using response surface methodology. Renew. Energy 2014, 62, 362–368. [Google Scholar] [CrossRef]
- Spiridon, I.; Popa, V.I. Hemicelluloses: Major sources, properties and applications. In Monomers, Polymers and Composites from Renewable Resources, 1st ed.; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Boston, MA, USA, 2008; pp. 289–304. [Google Scholar]
- Mandalika, A.; Runge, T. Enabling integrated biorefineries through high-yield conversion of fractionated pentosans into furfural. Green Chem. 2012, 14, 3175–3184. [Google Scholar] [CrossRef]
- Avci, A.; Saha, B.; Kennedy, G.; Cotta, M. High temperature dilute phosphoric acid pretreatment of corn stover for furfural and ethanol production. Ind. Crop. Prod. 2013, 50, 478–484. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Ren, J.; Sun, R.; Zheng, J.; Sun, G.; Liu, S. An efficient process for dehydration of xylose to furfural catalyzed by inorganic salts in water/dimethyl sulfoxide system. Chin. J. Catal. 2014, 35, 741–747. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Wang, P.; Li, Y. Production of furfural from xylose, xylan and corncob in gamma-valerolactone using FeCl3•6H2O as catalyst. Bioresour. Technol. 2014, 151, 355–360. [Google Scholar] [CrossRef]
- Ambalkar, V.U.; Talib, M.I. Synthesis of Furfural from Lignocellulosic Biomass as Agricultural Residues: A Review. Int. J. Eng. Sci. 2012, 1, 30–36. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2011; NREL/TP-510-42618. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Tmpleton, D. Determination of Sugars, Byproducts and Degradation Products in Liquid Fraction Process Samples; National Renewable Energy Laboratory: Golden, CO, USA, 2008; NREL/TP-510-42623. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008; NREL/TP-510-42619. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008; NREL/TP-510-42622. [Google Scholar]
- Kim, S.J.; Um, B.H.; Im, D.J.; Lee, J.H.; Oh, K.K. Combined ball milling and ethanol organosolv pretreatment to improve the enzymatic digestibility of three types of herbaceous biomass. Energies 2018, 11, 2457. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Kwak, H.; Kim, T.H.; Oh, K.K. Extraction behaviors of lignin and hemicellulose-derived sugars during organosolv fractionation of agricultural residues using a bench-scale ball milling reactor. Energies 2020, 13, 352. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Jeon, Y.J.; Oh, K.K.; Kim, T.H. Production of furfural and cellulose from barley straw using acidified zinc chloride. Korean J. Chem. Eng. 2013, 30, 1339–1346. [Google Scholar] [CrossRef]
- Kim, T.H.; Oh, K.K.; Ryu, H.J.; Lee, K.H.; Kim, T.H. Hydrolysis of hemicellulose from barley straw and enhanced enzymatic saccharification of cellulose using acidified zinc chloride. Renew. Energy 2014, 65, 56–63. [Google Scholar] [CrossRef]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008; NREL/TP-510-42629. [Google Scholar]
- Boonsuk, P.; Sukolrat, A.; Bourkaew, S.; Kaewtatip, K.; Chantarak, S.; Kelarakis, A.; Chaibundit, C. Structure-properties relationships in alkaline treated rice husk reinforced thermoplastic cassava starch biocomposites. Int. J. Biol. Macromol. 2021, 167, 130–140. [Google Scholar] [CrossRef]
- Park, B.D.; Wi, S.G.; Lee, K.H.; Singh, A.P.; Yoon, T.H.; Kim, Y.S. Characterization of anatomical features and silica distribution in rice husk using microscopic and micro-analytical techniques. Biomass Bioenergy 2003, 25, 319–327. [Google Scholar] [CrossRef]
- Krishnarao, R.; Godkhindi, V. Distribution of silica in rice husks and its effect on the formation of silicon carbide. Ceram. Int. 1992, 18, 243–249. [Google Scholar] [CrossRef]
- Ruseckaite, R.A.; Ciannamea, E.M.; Leiva, P.; Stefan, P.M. Particleboards based on rice husk. In Polymer and Biopolymer Analysis and Characterization; Zaikov, G.E., Jimenez, A., Eds.; Nova Science Publishing Inc.: New York, NY, USA, 2007; pp. 1–12. [Google Scholar]
- Jung, H.J.; Kwak, H.; Chun, J.; Oh, K.K. Alkaline Fractionation and Subsequent Production of Nano-Structured Silica and Cellulose Nano-Fibrils for the Comprehensive Utilization of Rice Husk. Sustainability 2021, 13, 1951. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kim, Y.S.; Oh, K.K. Fractionation and delignification of empty fruit bunches with low reaction severity for high sugar recovery. Bioresour. Technol. 2013, 146, 176–183. [Google Scholar] [CrossRef]
- Xiang, Z.; Runge, T. Co-production of feed and furfural from dried distillers’ grains to improve corn ethanol profitability. Ind. Crops Prod. 2014, 55, 207–216. [Google Scholar] [CrossRef]
- Köchermann, J.; Mühlenberg, J.; Klemm, M. Kinetics of hydrothermal furfural production from organosolv hemicellulose and D-Xylose. Ind. Eng. Chem. Res. 2018, 57, 14417–14427. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Felby, C.; Jørgensen, H. Yield-determining factors in high-solids enzymatic hydrolysis of lignocellulose. Biotechnol. Biofuels 2009, 2, 11–20. [Google Scholar] [CrossRef] [Green Version]


| Component | Dry Solids (%, w/w) | |
|---|---|---|
| Carbohydrate | Glucan | 35.6 ± 0.8 |
| XMG (a) | 13.6 ± 0.4 | |
| Arabinan | 1.7 ± 0.1 | |
| Lignin | Acid insoluble lignin | 22.7 ± 0.3 |
| Acid soluble lignin | 0.7 ± 0.2 | |
| Extractives | Water extractives | 3.5 ± 0.1 |
| Ethanol extractives | 0.7 ± 0.1 | |
| Ash | 15.7 ± 0.2 | |
| Crude protein (b) | 3.2 | |
| Crude lipid (b) | 0.5 |
| Elements | Inner Surface | Outer Surface | ||
|---|---|---|---|---|
| Weight % | Atomic % | Weight % | Atomic % | |
| C | 45.99 | 53.89 | 23.91 | 33.64 |
| N | 4.53 | 4.55 | - | - |
| O | 44.61 | 39.24 | 45.58 | 48.14 |
| Si | 2.99 | 1.50 | 29.30 | 17.63 |
| S | 1.88 | 0.83 | 0.72 | 0.38 |
| Ca | - | - | 0.50 | 0.21 |
| Total | 100 | 100 | ||
| Components | Solid Remaining (%) | Fractionated Solid (%) | Fractionated Liquid (%) | EMB (a) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucan | XMG (b) | Lignin | Ash | Glucan | XMG | Ash | Glucan | XMG | Ash | ||||||
| Raw RH | 100 | 35.6 | 13.6 | 22.7 | 15.7 | ||||||||||
| Fractionated RH | 34.1 | 80.1 | 12.3 | 12.0 | - | 0.5 | 1.1 | 14.4 | 78.1 | 38.9 | 91.7 | ||||
| Fractionated RH (c) | 27.3 | 4.2 | 4.1 | - | |||||||||||
| Component retention | 76.7 | 30.9 | 18.1 | - | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.J.; Oh, K.K. NaOH-Catalyzed Fractionation of Rice Husk Followed by Concomitant Production of Bioethanol and Furfural for Improving Profitability in Biorefinery. Appl. Sci. 2021, 11, 7508. https://doi.org/10.3390/app11167508
Jung HJ, Oh KK. NaOH-Catalyzed Fractionation of Rice Husk Followed by Concomitant Production of Bioethanol and Furfural for Improving Profitability in Biorefinery. Applied Sciences. 2021; 11(16):7508. https://doi.org/10.3390/app11167508
Chicago/Turabian StyleJung, Hyun Jin, and Kyeong Keun Oh. 2021. "NaOH-Catalyzed Fractionation of Rice Husk Followed by Concomitant Production of Bioethanol and Furfural for Improving Profitability in Biorefinery" Applied Sciences 11, no. 16: 7508. https://doi.org/10.3390/app11167508
APA StyleJung, H. J., & Oh, K. K. (2021). NaOH-Catalyzed Fractionation of Rice Husk Followed by Concomitant Production of Bioethanol and Furfural for Improving Profitability in Biorefinery. Applied Sciences, 11(16), 7508. https://doi.org/10.3390/app11167508






