The Association of Salivary Conductivity with Cardiomegaly in Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
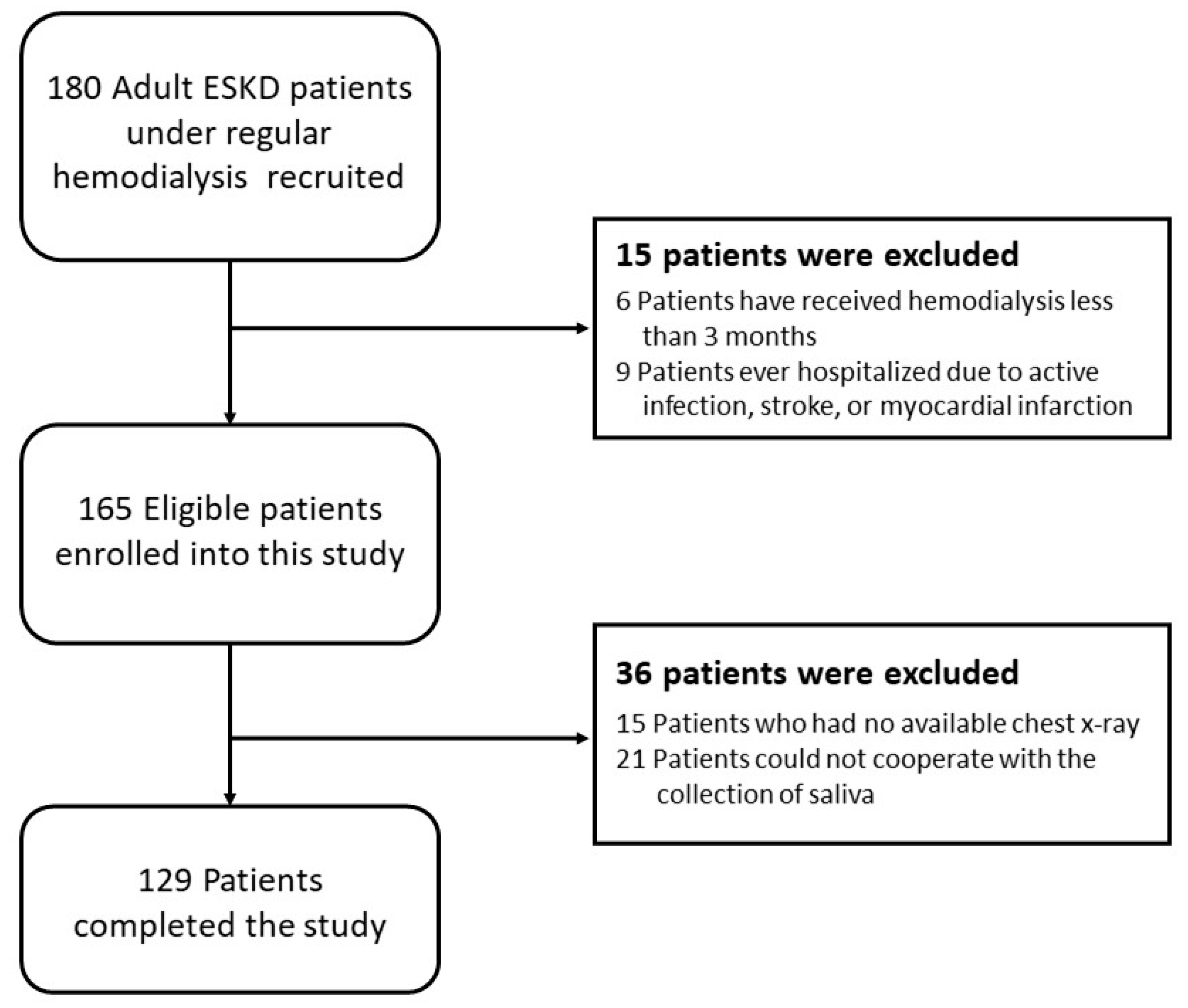
2.1. Study Subjects
2.2. Experimental Substance
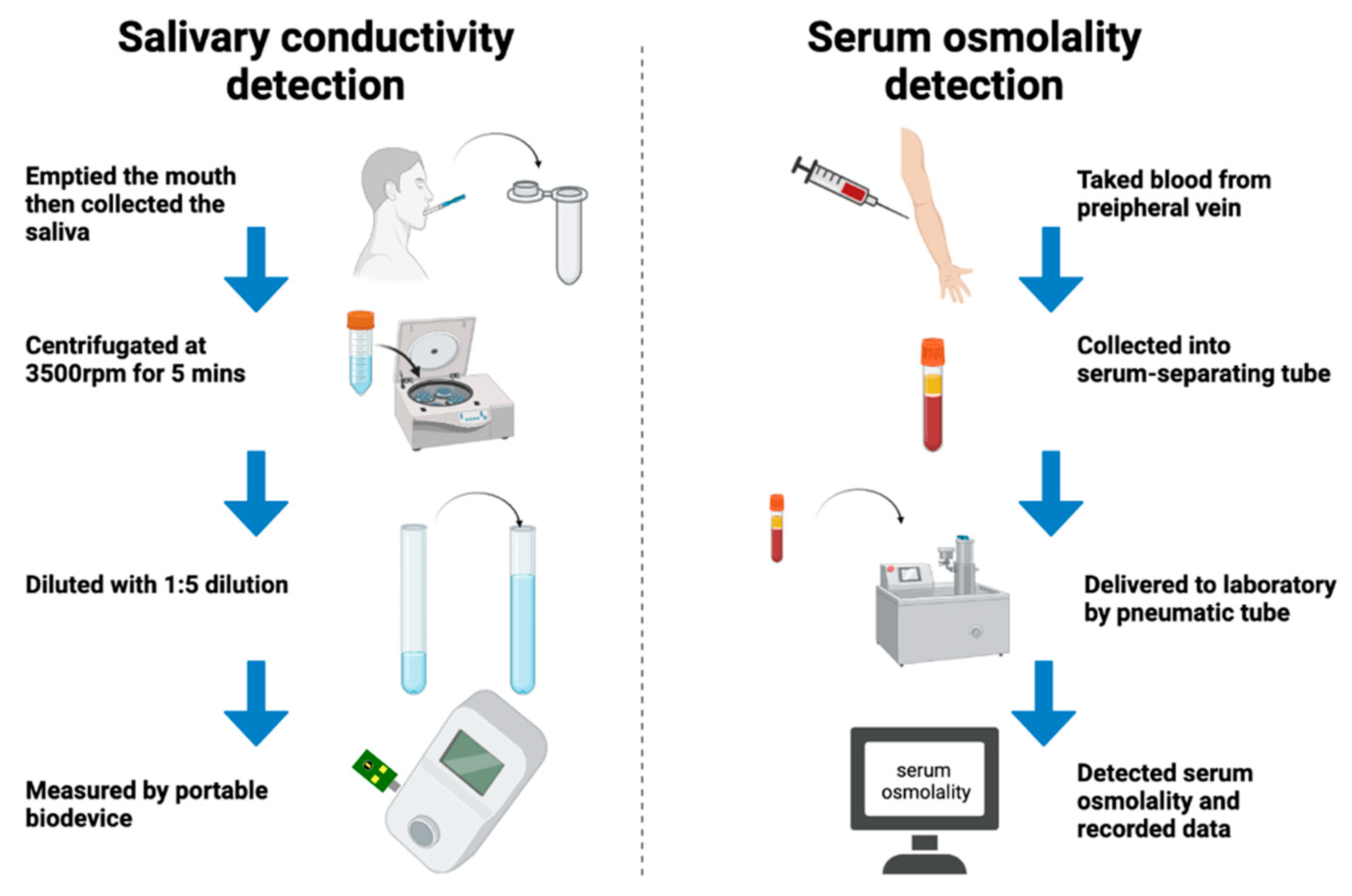
2.3. Collection and Analysis of Saliva
- Participant swallowed and emptied their mouth.
- We collected saliva with a 1 mL dropper and placed the collected salivary sample into a 1.5 mL Eppendorf tube.
- Saliva was centrifugated at 3500 rpm for 5 min and was collected from the bottom of the 1.5 mL Eppendorf tube for the storage of the samples.
- Upon measurement, we took 100 μL of a saliva sample which was diluted with 400 μL ultrapure water. The conductivity is 0.055 µSiemens/cm of the water, while the resistivity is 18.2 MΩ × cm at 25 °C.
- The salivary conductivity was analyzed through the developed portable monitor with a disposable printed-circuit board electrode.
- The above steps were repeated 3 times, then we measured the averaged values, and recorded the data of the salivary conductivity (Table S1 in Supplemental Materials).
2.4. Detection of Serum Osmolality
2.5. Assessment of Thirst Intensity Scales
2.6. Evaluation of CTR and the Definition of Cardiomegaly
2.7. Statistical Analysis
3. Results
3.1. Demographics of the 129 Hemodialysis Patients
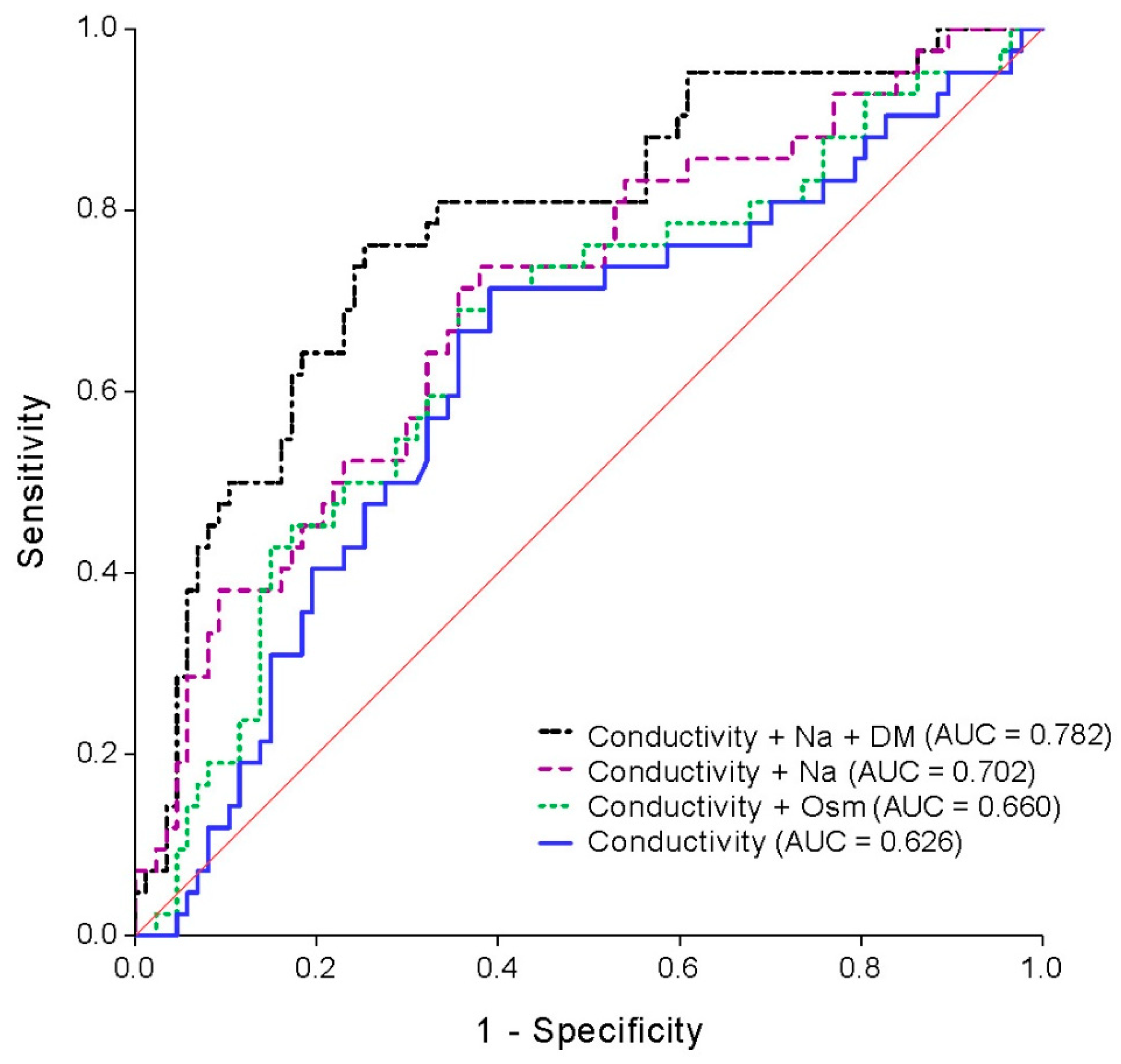
3.2. The Prediction of Cardiothoracic Ratio
3.3. Association between Salivary Conductivity and Cardiomegaly
3.4. Determinant of Salivary Conductivity from Clinical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klinger, M.; Madziarska, K. Mortality predictor pattern in hemodialysis and peritoneal dialysis in diabetic patients. Adv. Clin. Exp. Med. 2019, 28, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Ahmadmehrabi, S.; Tang, W.H.W. Hemodialysis-induced cardiovascular disease. Semin. Dial. 2018, 31, 258–267. [Google Scholar] [CrossRef]
- Bansal, N. Evolution of Cardiovascular Disease During the Transition to End-Stage Renal Disease. Semin. Nephrol. 2017, 37, 120–131. [Google Scholar] [CrossRef]
- Chen, S.C.; Teh, M.; Huang, J.C.; Wu, P.Y.; Chen, C.Y.; Tsai, Y.C.; Chiu, Y.W.; Chang, J.M.; Chen, H.C. Increased Aortic Arch Calcification and Cardiomegaly is Associated with Rapid Renal Progression and Increased Cardiovascular Mortality in Chronic Kidney Disease. Sci. Rep. 2019, 9, 5354. [Google Scholar] [CrossRef] [PubMed]
- Yotsueda, R.; Taniguchi, M.; Tanaka, S.; Eriguchi, M.; Fujisaki, K.; Torisu, K.; Masutani, K.; Hirakata, H.; Kitazono, T.; Tsuruya, K. Cardiothoracic Ratio and All-Cause Mortality and Cardiovascular Disease Events in Hemodialysis Patients: The Q-Cohort Study. Am. J. Kidney Dis. 2017, 70, 84–92. [Google Scholar] [CrossRef]
- Amin, H.; Siddiqui, W.J. Cardiomegaly; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Gunal, A.I. How to determine ‘dry weight’? Kidney Int. Suppl. 2013, 3, 377–379. [Google Scholar] [CrossRef][Green Version]
- Dasgupta, S.; Kelleman, M.; Slesnick, T.; Oster, M.E. Cardiomegaly on chest radiographs as a predictor of heart disease in the pediatric population. Am. J. Emerg. Med. 2020, 38, 855–859. [Google Scholar] [CrossRef]
- Ogata, H.; Kumasawa, J.; Fukuma, S.; Mizobuchi, M.; Kinugasa, E.; Fukagawa, M.; Fukuhara, S.; Akizawa, T. The cardiothoracic ratio and all-cause and cardiovascular disease mortality in patients undergoing maintenance hemodialysis: Results of the MBD-5D study. Clin. Exp. Nephrol. 2017, 21, 797–806. [Google Scholar] [CrossRef]
- Okute, Y.; Shoji, T.; Hayashi, T.; Kuwamura, Y.; Sonoda, M.; Mori, K.; Shioi, A.; Tsujimoto, Y.; Tabata, T.; Emoto, M.; et al. Cardiothoracic Ratio as a Predictor of Cardiovascular Events in a Cohort of Hemodialysis Patients. J. Atheroscler. Thromb. 2017, 24, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Jotterand, M.; Faouzi, M.; Dedouit, F.; Michaud, K. New formula for cardiothoracic ratio for the diagnosis of cardiomegaly on post-mortem CT. Int. J. Legal. Med. 2020, 134, 663–667. [Google Scholar] [CrossRef]
- Wittbrodt, M.T.; Espinoza, S.; Millard-Stafford, M.L. Biological variation of plasma osmolality obtained with capillary versus venous blood. Clin. Chem. Lab. Med. 2015, 53, 1613–1619. [Google Scholar] [CrossRef]
- Tanaka, S.; Nakano, T.; Tokumoto, M.; Masutani, K.; Tsuchimoto, A.; Ooboshi, H.; Kitazono, T. Estimated plasma osmolarity and risk of end-stage kidney disease in patients with IgA nephropathy. Clin. Exp. Nephrol. 2020, 24, 910–918. [Google Scholar] [CrossRef]
- Gennari, F.J. Serum osmolality. N. Engl. J. Med. 1984, 310, 1608–1610. [Google Scholar] [CrossRef]
- Morley, J.E. Dehydration, Hypernatremia, and Hyponatremia. Clin. Geriatr. Med. 2015, 31, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.R.; Cote, T.R.; Lawhorne, L.; Levenson, S.A.; Rubenstein, L.Z.; Smith, D.A.; Stefanacci, R.G.; Tangalos, E.G.; Morley, J.E.; Dehydration, C. Understanding clinical dehydration and its treatment. J. Am. Med. Dir. Assoc. 2008, 9, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Bunn, D.; Jimoh, F.O.; Fairweather-Tait, S.J. Water-loss dehydration and aging. Mech. Ageing Dev. 2014, 136, 50–58. [Google Scholar] [CrossRef]
- Andreoli, T.E. Water: Normal balance, hyponatremia, and hypernatremia. Ren. Fail. 2000, 22, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Yilmaz, S.; Dincer, N.; Ortiz, A.; Sag, A.A.; Covic, A.; Sanchez-Lozada, L.G.; Lanaspa, M.A.; Cherney, D.Z.I.; Johnson, R.J.; et al. Antidiuretic Hormone and Serum Osmolarity Physiology and Related Outcomes: What Is Old, What Is New, and What Is Unknown? J. Clin. Endocrinol. Metab. 2019, 104, 5406–5420. [Google Scholar] [CrossRef]
- Fasanella d’Amore, T.; Wauters, J.P.; Waeber, B.; Nussberger, J.; Brunner, H.R. Response of plasma vasopressin to changes in extracellular volume and/or plasma osmolality in patients on maintenance hemodialysis. Clin. Nephrol. 1985, 23, 299–302. [Google Scholar]
- Wallace, H.A.; Perera, T.B. Necrotizing Fasciitis; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Aykanat Girgin, B.; Gol, I. Reducing Pain and Fear in Children During Venipuncture: A Randomized Controlled Study. Pain Manag. Nurs. 2020, 21, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Tummalapalli, S.L.; Chu, C.D.; Tuot, D.S. Avoiding Overuse of Venipuncture and Laboratory Testing in Hospitalized Patients on Hemodialysis. J. Patient Saf. 2020. [Google Scholar] [CrossRef]
- Chojnowska, S.; Baran, T.; Wilinska, I.; Sienicka, P.; Cabaj-Wiater, I.; Knas, M. Human saliva as a diagnostic material. Adv. Med. Sci. 2018, 63, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Matczuk, J.; Zalewska, A.; Lukaszuk, B.; Knas, M.; Maciejczyk, M.; Garbowska, M.; Ziembicka, D.M.; Waszkiel, D.; Chabowski, A.; Zendzian-Piotrowska, M.; et al. Insulin Resistance and Obesity Affect Lipid Profile in the Salivary Glands. J. Diabetes Res. 2016, 2016, 8163474. [Google Scholar] [CrossRef]
- Kulak-Bejda, A.; Waszkiewicz, N.; Bejda, G.; Zalewska, A.; Maciejczyk, M. Diagnostic Value of Salivary Markers in Neuropsychiatric Disorders. Dis. Markers 2019, 2019, 4360612. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Bielas, M.; Zalewska, A.; Gerreth, K. Salivary Biomarkers of Oxidative Stress and Inflammation in Stroke Patients: From Basic Research to Clinical Practice. Oxid Med. Cell. Longev. 2021, 2021, 5545330. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Szulimowska, J.; Skutnik, A.; Taranta-Janusz, K.; Wasilewska, A.; Wisniewska, N.; Zalewska, A. Salivary Biomarkers of Oxidative Stress in Children with Chronic Kidney Disease. J. Clin. Med. 2018, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Flint, P.W.; Haughey, B.H.; Robbins, K.T.; Thomas, J.R.; Niparko, J.K.; Lund, V.J.; Lesperance, M.M. Cummings Otolaryngology: Head and Neck Surgery; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; pp. 1139–1148. [Google Scholar]
- Lu, Y.P.; Huang, J.W.; Lee, I.N.; Weng, R.C.; Lin, M.Y.; Yang, J.T.; Lin, C.T. A Portable System to Monitor Saliva Conductivity for Dehydration Diagnosis and Kidney Healthcare. Sci. Rep. 2019, 9, 14771. [Google Scholar] [CrossRef]
- Munoz, C.X.; Johnson, E.C.; Demartini, J.K.; Huggins, R.A.; McKenzie, A.L.; Casa, D.J.; Maresh, C.M.; Armstrong, L.E. Assessment of hydration biomarkers including salivary osmolality during passive and active dehydration. Eur. J. Clin. Nutr. 2013, 67, 1257–1263. [Google Scholar] [CrossRef]
- Smith, D.L.; Shalmiyeva, I.; Deblois, J.; Winke, M. Use of salivary osmolality to assess dehydration. Prehosp. Emerg. Care 2012, 16, 128–135. [Google Scholar] [CrossRef]
- Villiger, M.; Stoop, R.; Vetsch, T.; Hohenauer, E.; Pini, M.; Clarys, P.; Pereira, F.; Clijsen, R. Evaluation and review of body fluids saliva, sweat and tear compared to biochemical hydration assessment markers within blood and urine. Eur. J. Clin. Nutr. 2018, 72, 69–76. [Google Scholar] [CrossRef]
- Prager, D.J.; Bowman, R.L. Freezing-Point Depression: New Method for Measuring Ultramicro Quantities of Fluids. Science 1963, 142, 237–239. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Z.; Li, H.; Sun, Y.; Xie, G.; Cheng, B.; Ji, F.; Fang, X. Effects of preoperative oral carbohydrates on patients undergoing ESD surgery under general anesthesia: A randomized control study. Medicine 2019, 98, e15669. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J.; Wood, R.J.; Rolls, E.T.; Lind, H.; Lind, W.; Ledingham, J.G. Thirst following water deprivation in humans. Am. J. Physiol. 1980, 239, R476–R482. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.A.; Campbell, R.G. Hunger in humans induced by 2-deoxy-D-glucose: Glucoprivic control of taste preference and food intake. Science 1977, 198, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.C.; Winters, M.E. Congestive Heart Failure. Emerg. Med. Clin. N. Am. 2015, 33, 553–562. [Google Scholar] [CrossRef]
- Shotan, A.; Dacca, S.; Shochat, M.; Kazatsker, M.; Blondheim, D.S.; Meisel, S. Fluid overload contributing to heart failure. Nephrol. Dial. Transpl. 2005, 20 (Suppl. 7), vii24–vii27. [Google Scholar] [CrossRef]
- Ueki, R.; Okutani, R.; Saga, T.; Wada, T.; Tashiro, C. Fluid management after off-pump CABG in a patient receiving preoperative continuous ambulatory peritoneal dialysis. Masui Jpn. J. Anesthesiol. 2000, 49, 292–294. [Google Scholar]
- Buffington, M.A.; Abreo, K. Hyponatremia: A Review. J. Intensive Care Med. 2016, 31, 223–236. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef] [PubMed]
- Konigsbrugge, O.; Lorenz, M.; Auinger, M.; Schmaldienst, S.; Klauser-Braun, R.; Kletzmayr, J.; Grilz, E.; Posch, F.; Antlanger, M.; Pabinger, I.; et al. Venous thromboembolism and vascular access thrombosis in patients with end-stage renal disease on maintenance hemodialysis: Cross-sectional results of the Vienna InVestigation of AtriaL fibrillation and thromboembolism in patients on hemoDIalysis (VIVALDI). Thromb. Res. 2017, 158, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Kwan, B.C.; Chow, K.M.; Chung, K.Y.; Leung, C.B.; Li, P.K.; Szeto, C.C. Measurements on the routine chest radiograph as prognostic markers in Chinese peritoneal dialysis patients. Clin. Nephrol. 2011, 76, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.M.; Ayus, J.C.; Kalantar-Zadeh, K. Hyponatremia in the Dialysis Population. Kidney Int. Rep. 2019, 4, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Kara, B. Determinants of thirst distress in patients on hemodialysis. Int. Urol. Nephrol. 2016, 48, 1525–1532. [Google Scholar] [CrossRef]
- Bossola, M.; Calvani, R.; Marzetti, E.; Picca, A.; Antocicco, E. Thirst in patients on chronic hemodialysis: What do we know so far? Int. Urol. Nephrol. 2020, 52, 697–711. [Google Scholar] [CrossRef]
- Sprick, J.D.; Nocera, J.R.; Hajjar, I.; O’Neill, W.C.; Bailey, J.; Park, J. Cerebral blood flow regulation in end-stage kidney disease. Am. J. Physiol. Renal. Physiol. 2020, 319, F782–F791. [Google Scholar] [CrossRef]
- Voroneanu, L.; Gavrilovici, C.; Covic, A. Overhydration, underhydration, and total body sodium: A tricky “menage a trois” in dialysis patients. Semin. Dial. 2018, 31, 21–25. [Google Scholar] [CrossRef]
- Singh, A.T.; Mc Causland, F.R. Osmolality and blood pressure stability during hemodialysis. Semin. Dial. 2017, 30, 509–517. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Marti, C.N.; Mentz, R.J.; Greene, S.J.; Ambrosy, A.P.; Subacius, H.P.; Fonarow, G.C.; Chioncel, O.; Bazari, H.; Maggioni, A.P.; et al. Serum Osmolality and Postdischarge Outcomes After Hospitalization for Heart Failure. Am. J. Cardiol. 2016, 117, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Du, K. Hyposmolarity may be also associated with worse outcomes in patients with heart failure. Int. J. Cardiol. 2017, 229, 53. [Google Scholar] [CrossRef]
- Bergomi, P.; Scudeller, L.; Pintaldi, S.; Dal Molin, A. Efficacy of Non-pharmacological Methods of Pain Management in Children Undergoing Venipuncture in a Pediatric Outpatient Clinic: A Randomized Controlled Trial of Audiovisual Distraction and External Cold and Vibration. J. Pediatric Nurs. 2018, 42, e66–e72. [Google Scholar] [CrossRef]
- Mima, A. Hemodialysis vascular access dysfunction: Molecular mechanisms and treatment. Ther. Apher. Dial. 2012, 16, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Bindroo, S.; Quintanilla Rodriguez, B.S.; Challa, H.J. Renal Failure; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]


| Variable | All Patient (n = 129) | Non-Cardiomegaly (n = 87) | Cardiomegaly (n = 42) | p Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 61.36 ± 11.30 | 60.99 ± 11.74 | 62.12 ± 10.43 | 0.60 |
| Female sex, n (%) | 49 (38.0) | 30 (34.5) | 19 (45.2) | 0.24 |
| Height, cm | 161.33 ± 8.30 | 162.25 ± 7.91 | 159.41 ± 8.83 | 0.07 |
| Weight, kg | 66.49 ± 13.41 | 67.38 ± 13.53 | 64.65 ± 13.13 | 0.28 |
| Underlying disease | ||||
| Hypertension, n (%) | 120 (93.0) | 81 (93.1) | 39 (92.9) | 0.96 |
| Diabetes mellitus, n (%) | 81 (62.8) | 54 (62.1) | 27 (64.3) | 0.81 |
| Hyperlipidemia, n (%) | 63 (48.8) | 44 (50.6) | 19 (45.2) | 0.57 |
| Gouty arthritis, n (%) | 37 (28.7) | 22 (25.3) | 15 (35.7) | 0.22 |
| Coronary arterial disease, n (%) | 23 (17.8) | 16 (18.4) | 7 (16.7) | 0.81 |
| Atrial fibrillation, n (%) | 10 (7.8) | 4 (4.6) | 6 (14.3) | 0.05 * |
| Liver disease, n (%) | 44 (34.1) | 29 (33.3) | 15 (35.7) | 0.79 |
| Old stroke, n (%) | 12 (9.3) | 6 (6.9) | 6(14.3) | 0.18 |
| Laboratory parameters | ||||
| BUN, mg/dL | 68.25 ± 17.67 | 68.94 ± 18.53 | 66.83 ± 15.85 | 0.77 |
| Creatinine, mg/dL | 10.44 ± 2.16 | 10.60 ± 2.24 | 10.10 ± 1.96 | 0.22 |
| BUN/Cr ratio | 6.69 ± 1.74 | 6.63 ± 1.66 | 6.82 ± 1.90 | 0.66 |
| eGFR, mL/min/1.73 m2 | 4.80 ± 1.26 | 4.80 ± 1.36 | 4.80 ± 1.05 | 0.43 |
| Serum osmolality, mOsm/kgH2O | 310.35 ± 10.36 | 311.56 ± 9.72 | 307.83 ± 11.28 | 0.05 * |
| Glucose, mg/dL | 136.33 ± 73.49 | 137.77 ± 78.24 | 133.33 ± 63.28 | 0.85 |
| Sodium, mEq/L | 136.80 ± 3.58 | 137.25 ± 3.27 | 135.88 ± 4.01 | 0.04 * |
| Potassium, mEq/L | 4.43 ± 0.78 | 4.39 ± 0.81 | 4.51 ± 0.74 | 0.41 |
| Cardiothoracic ratio | 0.48 ± 0.06 | 0.45 ± 0.03 | 0.55 ± 0.04 | <0.01 * |
| Salivary conductivity, μs/cm | 7562.05 ± 2348.91 | 7833.52 ± 2372.98 | 6999.71 ± 2221.09 | 0.02 * |
| Thirst intensity scale scores | ||||
| Thirst scale (VAS) | 3.26 ± 2.44 | 3.29 ± 2.35 | 3.19 ± 2.65 | 0.70 |
| Thirst scale (CS) | 3.02 ± 1.46 | 3.07 ± 1.44 | 2.90 ± 1.53 | 0.50 |
| Model 1 | Cardiothoracic Ratio | ||
| Standardized Beta Coefficient (Standard Error) | t | Significance | |
| Age, per year | 0.200 (0.000) | 2.121 | 0.036 * |
| Salivary conductivity, μs/cm | −0.203 (0.000) | −2.230 | 0.028 * |
| Serum sodium, mEq/L | −0.130 (0.001) | −1.486 | 0.140 |
| Serum potassium, mEq/L | 0.122 (0.007) | 1.368 | 0.174 |
| R2 = 0.30 | |||
| Model 2 | Cardiothoracic Ratio | ||
| Standardized Beta Coefficient (Standard Error) | t | Significance | |
| Serum sodium, mEq/L | −0.178 (0.001) | −1.486 | 0.044 * |
| R2 = 0.18 | |||
| Salivary Conductivity | |||
|---|---|---|---|
| Standardized Beta Coefficient (Standard Error) | t | Significance | |
| Age, per year | 0.309 (0.000) | 3.694 | <0.001 * |
| Serum osmolality, mOsm/kgH2O | 0.192 (0.000) | 2.300 | 0.023 * |
| R2 = 0.35 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.-T.; Lu, Y.-P.; Chen, C.-H.; Chang, C.-H.; Tsai, Y.-H.; Tung, C.-W.; Yang, J.-T. The Association of Salivary Conductivity with Cardiomegaly in Hemodialysis Patients. Appl. Sci. 2021, 11, 7405. https://doi.org/10.3390/app11167405
Lee A-T, Lu Y-P, Chen C-H, Chang C-H, Tsai Y-H, Tung C-W, Yang J-T. The Association of Salivary Conductivity with Cardiomegaly in Hemodialysis Patients. Applied Sciences. 2021; 11(16):7405. https://doi.org/10.3390/app11167405
Chicago/Turabian StyleLee, An-Ting, Yen-Pei Lu, Chun-Hao Chen, Chia-Hao Chang, Yuan-Hsiung Tsai, Chun-Wu Tung, and Jen-Tsung Yang. 2021. "The Association of Salivary Conductivity with Cardiomegaly in Hemodialysis Patients" Applied Sciences 11, no. 16: 7405. https://doi.org/10.3390/app11167405
APA StyleLee, A.-T., Lu, Y.-P., Chen, C.-H., Chang, C.-H., Tsai, Y.-H., Tung, C.-W., & Yang, J.-T. (2021). The Association of Salivary Conductivity with Cardiomegaly in Hemodialysis Patients. Applied Sciences, 11(16), 7405. https://doi.org/10.3390/app11167405






