Effects of Organic Additives on Chemical, Microbiological and Plant Pathogen Suppressive Properties of Aerated Municipal Waste Compost Teas
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction Procedure: Technical and Operative Details
2.2. CTs in Comparison
- CT-Wa = 7 L of compost + 35 L of dechlorinated tap water
- CT-Wh1 = 7 L of compost + 3 L of whey + 32 L of dechlorinated tap water
- CT-Wh2 = 7 L of compost + 5 L of whey + 30 L of dechlorinated tap water
- CT-M1 = 7 L of compost + 105 g of molasses + 35 L of dechlorinated tap water
- CT-M2 = 7 L of compost + 175 g of molasses + 35 L of dechlorinated tap water
- CT-M3 = 7 L of compost + 350 g of molasses + 35 L of dechlorinated tap water
2.3. Chemical Analyses
2.3.1. Electrical Conductivity and pH
2.3.2. Total Organic Carbon, Humic and Fulvic Acids
2.3.3. Total and Ammonia Nitrogen
2.3.4. Heavy Metals, Alkali Metals and Alkaline Earth Metals
2.4. Microbiological Analyses
2.4.1. Counting of Microbial Populations in CTs
2.4.2. Biolog Analyses and Bacterial Community Levels of Physiological Profiles
2.4.3. In Vitro Suppression Assay of CTs
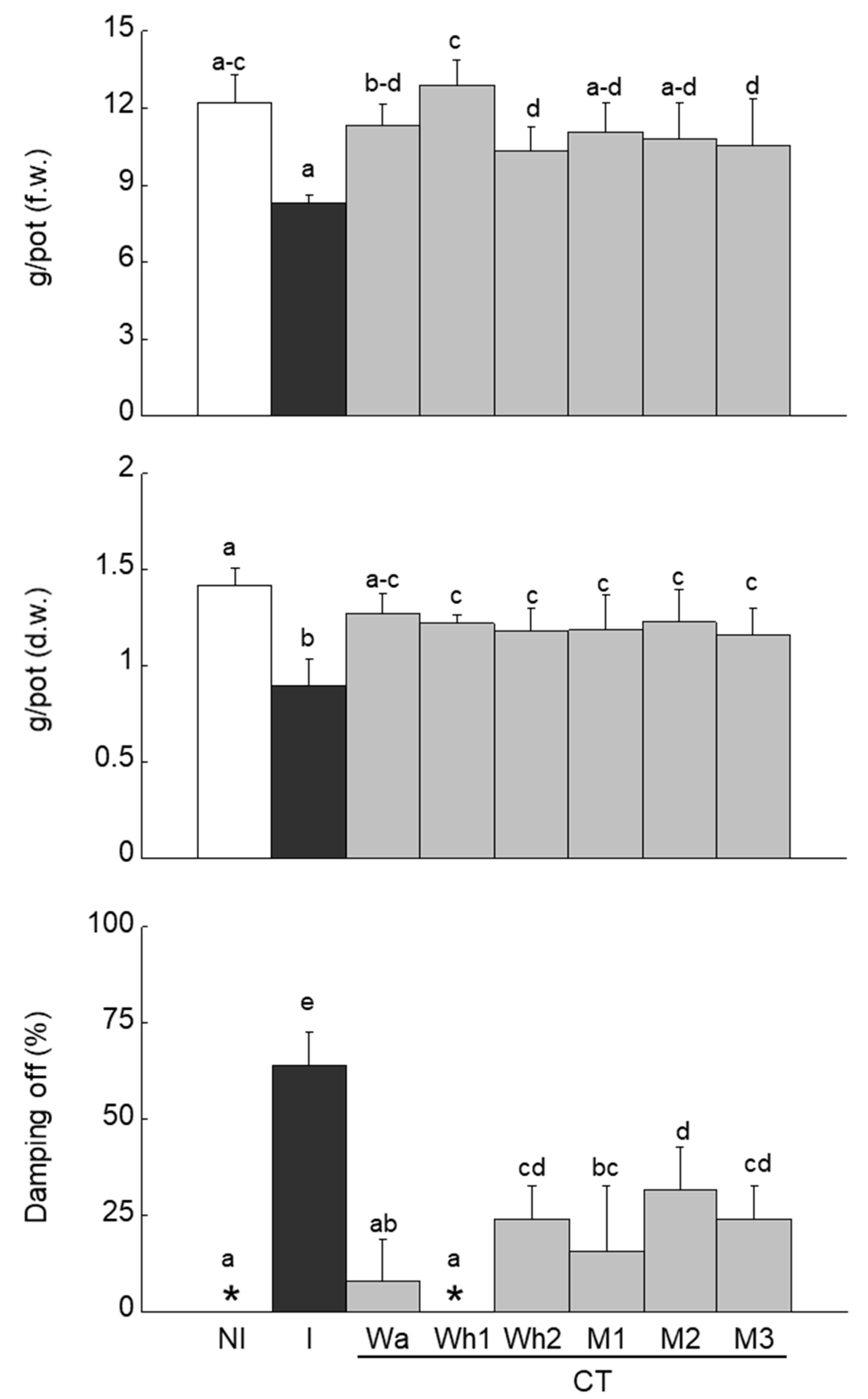
2.4.4. Rhizoctonia Disease Suppressiveness Assay by CTs
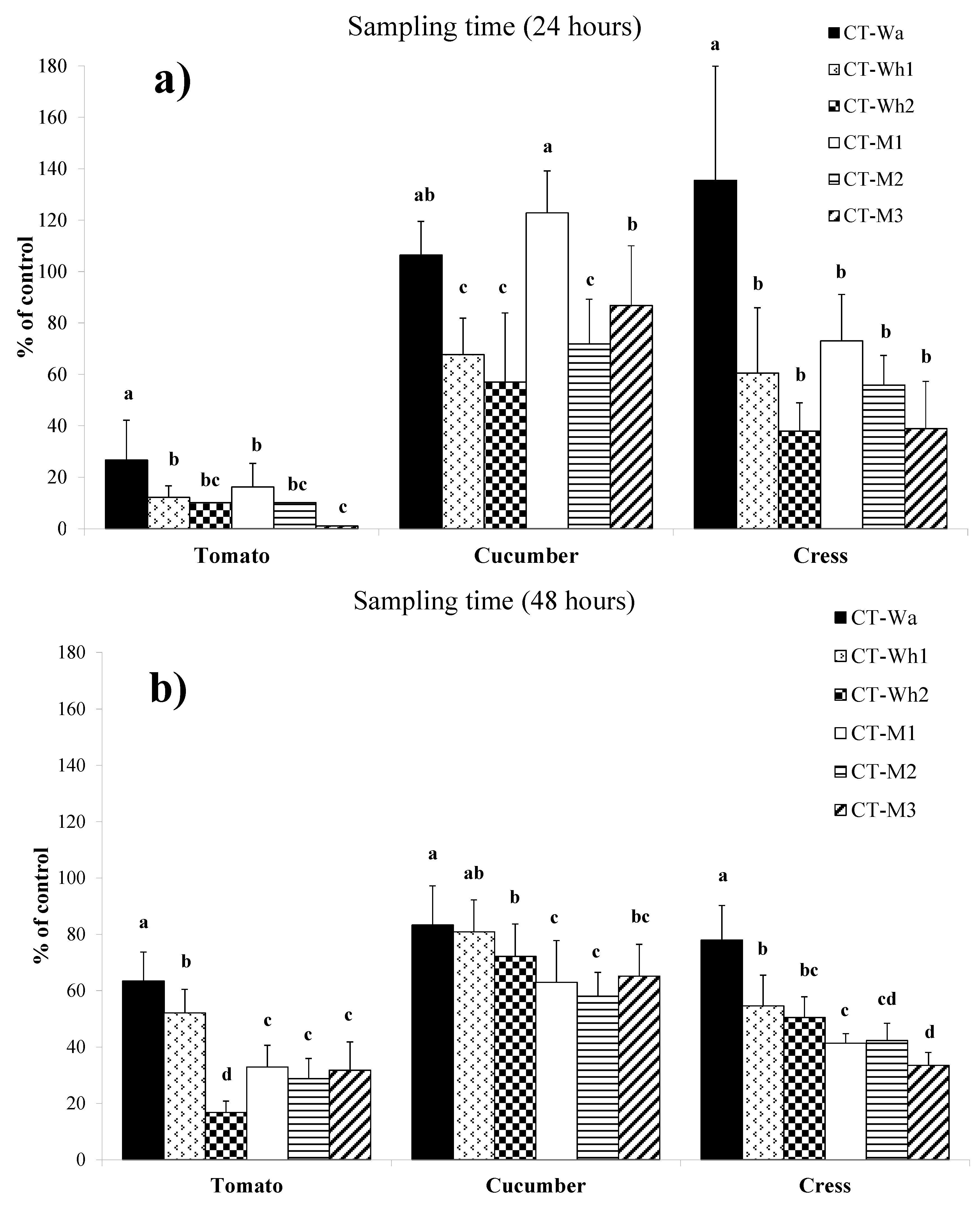
2.5. Phytotoxicity Assays
2.6. Statistical Analyses
3. Results
3.1. Substrates and Additives Characteristics
3.2. Evolution of the Measured Parameters during the Production Process of CTs
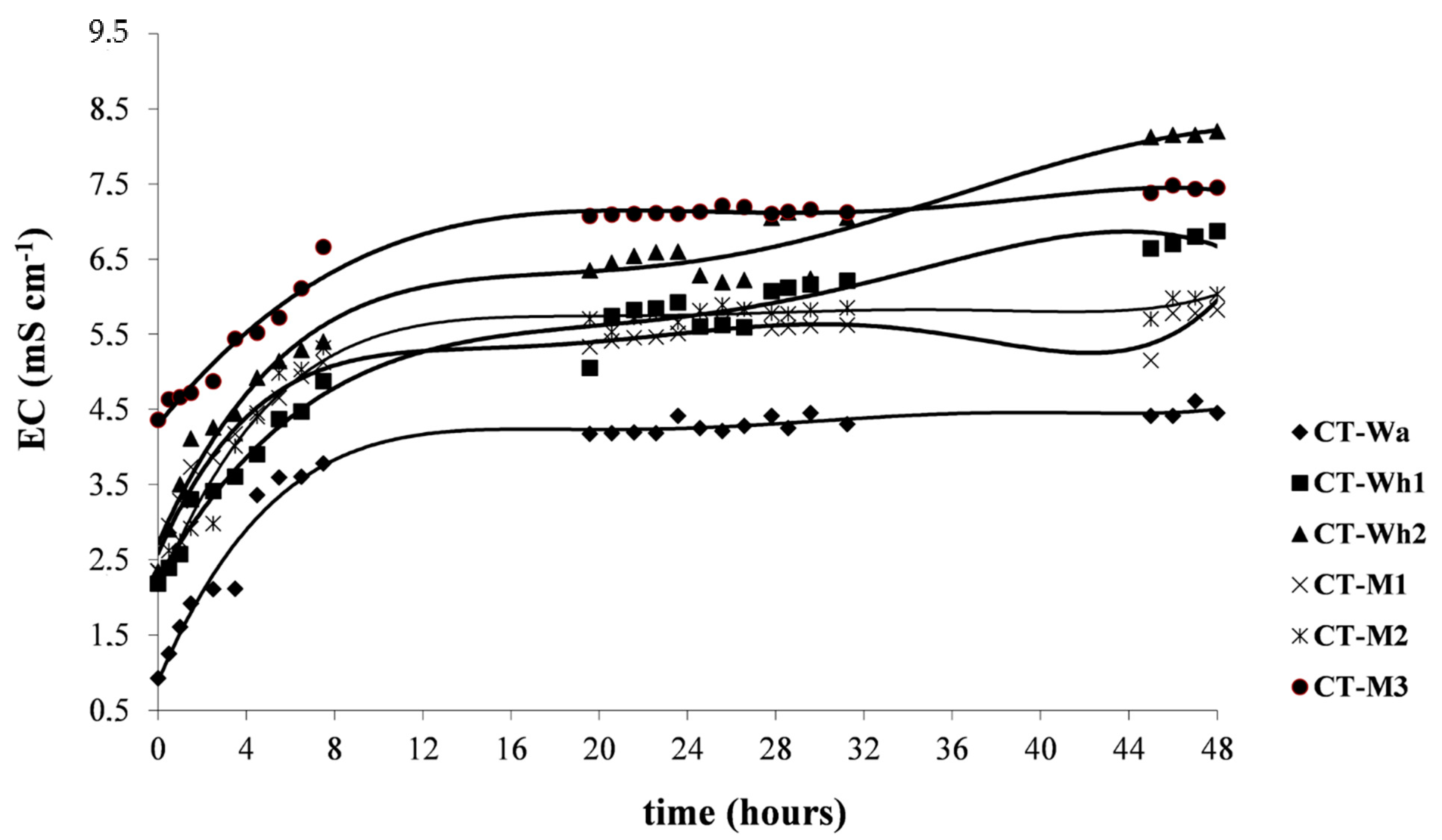
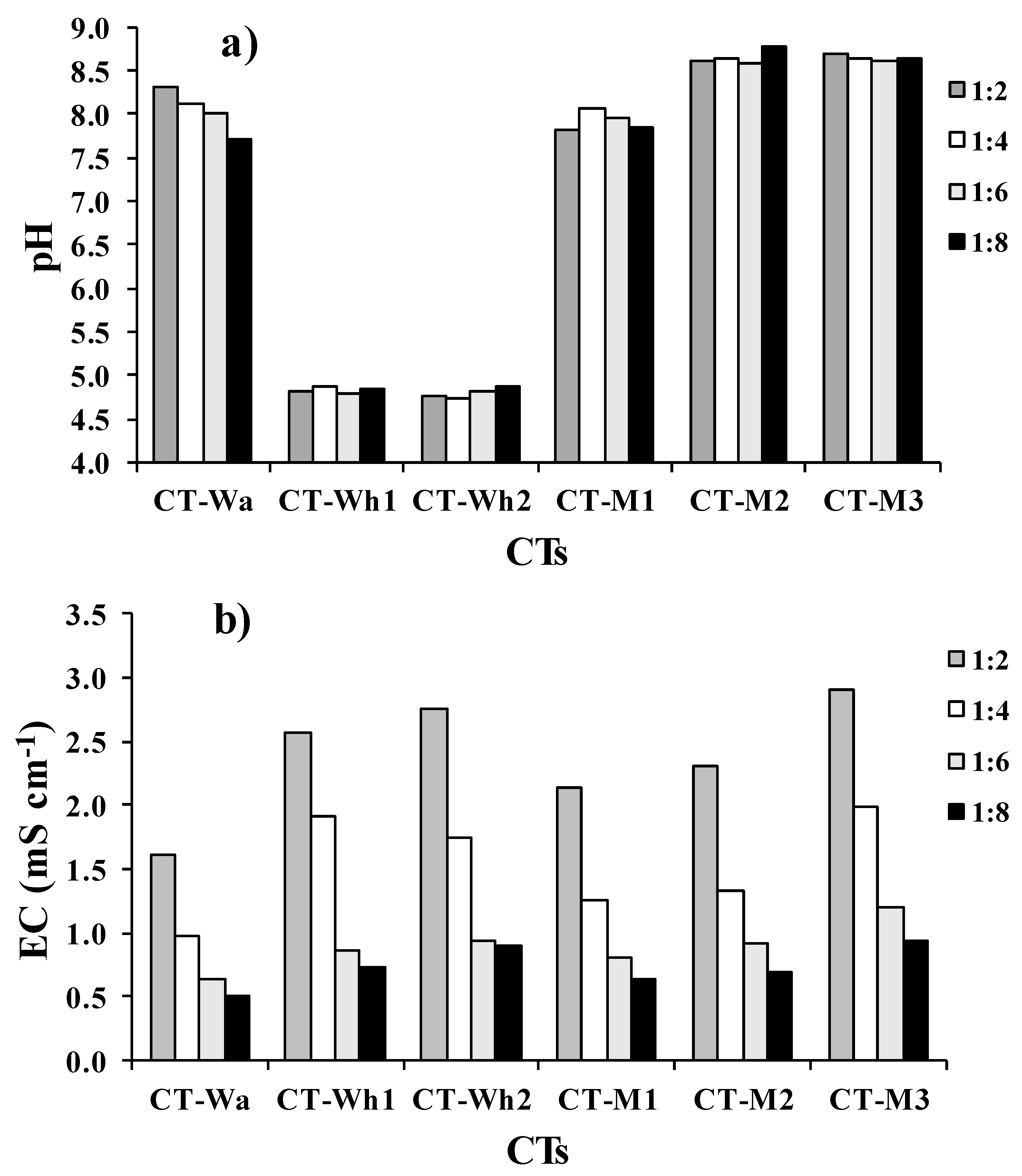
3.2.1. Electrical Conductivity and pH
3.2.2. Total Organic Carbon, Humic and Fulvic Acids
3.3. Final Characteristics of the Obtained CTs
3.3.1. Chemical Features of the Final CTs and Their Dilutions
3.3.2. Microbiological Features
3.4. CTs Fungal Pathogen Suppressiveness
3.5. Phytotoxicity Assays on Seeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Diver, S. Compost Tea for Plant Disease Control. ATTRA, Publ. 2002. Available online: http://www.attra.org/attra-pub/comptea.htm (accessed on 30 October 2020).
- Scheuerell, S.J.; Mahaffee, W.F. Variability Associated with Suppression of Gray Mold (Botrytis cinerea) on Geranium by Foliar Applications of Non aerated and Aerated Compost Teas. Plant Dis. 2006, 90, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Weltzien, H.C. Some effects of composted organic materials on plant health. Agric. Ecosyst. Environ. 1989, 27, 439–446. [Google Scholar] [CrossRef]
- Weltzien, H.C. The use of composted materials for leaf disease suppression in field crops. Monogr. Br. Crop. Prot. Counc. 1990, 45, 115–120. [Google Scholar]
- Scheuerell, S.J.; Mahaffee, W.F. Compost tea: Principles and prospects for plant disease control. Compos. Sci. Util. 2002, 10, 313–338. [Google Scholar] [CrossRef]
- Hargreaves, J.C.; Adl, M.S.; Warman, P.R. The effects of municipal solid waste compost and compost tea on mineral element uptake and fruit quality of strawberries. Compos. Sci. Util. 2009, 17, 85–94. [Google Scholar] [CrossRef]
- Naidu, Y.; Meon, S.; Siddiqui, Y. Foliar application of microbial-enriched compost tea enhances growth: Yield and quality of muskmelon (Cucumis melo L.) cultivated under fertigation system. Sci. Hortic. 2013, 159, 33–40. [Google Scholar] [CrossRef]
- Pane, C.; Palese, A.M.; Spaccini, R.; Piccolo, A.; Celano, G.; Zaccardelli, M. Enhancing sustainability of a processing tomato cultivation system by using bioactive compost teas. Sci. Hortic. 2016, 202, 117–124. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Pane, C.; Villecco, D.; Palese, A.M.; Celano, G. Compost tea spraying increases yield performance of pepper (Capsicum annuum L.) grown in greenhouse under organic farming system. Ital. J. Agron. 2018, 13, 229–234. [Google Scholar] [CrossRef]
- Koepf, H.H. Research in Biodynamic Agriculture: Methods and Results; Bio-Dynamic Farming and Gardening Association: Kimberton, PA, USA, 1993; p. 78. [Google Scholar]
- Diver, S.; Greer, L. Sustainable Small-Scale Nursery Production. ATTRA, Publ. 2001. Available online: http://www.microfarms.com/technical/greenhousecd/greenhouse/nursery/nursery.pdf (accessed on 30 October 2020).
- Morard, P.; Eyheraguibel, B.; Morard, M.; Silvestre, J. Direct effects of humic-like substance on growth, water, and mineral nutrition of various species. J. Plant Nutr. 2011, 34, 46–59. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Muscolo, A.; Vianello, A. Physiological effects of humic substances on higer plants. Soil Biol. Biochem. 2002, 34, 1527–1536. [Google Scholar] [CrossRef]
- Mengesha, W.K.; Powel, S.M.; Evans, K.J.; Barry, K.M. Diverse microbial communities in non-aerated compost teas suppress bacterial wilt. World J. Microb. Biot. 2017, 33, 49. [Google Scholar] [CrossRef]
- Scheuerell, S.J.; Mahaffee, W.F. Compost tea as a container medium drench for suppressing seedling damping—Off by Pythium ultimum. Phytopathology 2004, 94, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Weltzien, H.C. Biocontrol of foliar fungal disease with compost extracts. In Microbial Ecology of Leaves; Andrews, J.H., Hirano, S.S., Eds.; Springer: New York, NY, USA, 1991; pp. 430–450. [Google Scholar]
- Ingham, E.R. The Compost Tea Brewing Manual, 5th ed.; Soil Foodweb: Corvallis, OR, USA, 2005. [Google Scholar]
- Wang, H.; Ding, J.; Liu, S.; Bai, X.; Xue, L. Different carbonic supplements induced changes of microflora in two types of compost teas and biocontrol efficiency against Pythium aphanidermatum. Biocontrol Sci. Technol. 2019, 29, 924–939. [Google Scholar] [CrossRef]
- Brinton, W.; Storms, P.; Evans, E.; Hill, J. Compost teas: Microbial hygiene and quality in relation to method of preparation. Biodynamics 2004, 2, 36–45. [Google Scholar]
- Duffy, B.; Sarreal, C.; Ravva, S.; Stanker, L. Effect of molasses on regrowth of E. coli O157:H7 and Salmonella in compost teas. Compos. Sci. Util. 2004, 12, 93–96. [Google Scholar] [CrossRef]
- Ingram, D.T.; Millner, P.D. Factors affecting compost tea as a potential source of Escherichia coli and Salmonella on fresh produce. J. Food Prot. 2007, 70, 828–834. [Google Scholar] [CrossRef][Green Version]
- Legislative Decree, No. 75, 29 April 2010, Gazzetta Ufficiale No.121, Rome, Italy, 26 May 2010. Riordino e Revisione Della Disciplina in Materia di Fertilizzanti, a Norma Dell’articolo 13 Della Legge 7 Luglio 2009, n. 88. Available online: https://www.gazzettaufficiale.it/eli/id/2010/05/26/010G0096/sg (accessed on 2 March 2021).
- ANPA. Metodi di Analisi del compost. In Manuali e Linee Guida; ANPA: Roma, Italy, 2001; ISBN 88-448-0258-9. [Google Scholar]
- Pane, C.; Spaccini, R.; Piccolo, A.; Celano, G.; Zaccardelli, M. Disease suppressiveness of agricultural greenwaste composts as related to chemical and bio-based properties shaped by different on-farm composting methods. Biol. Control 2019, 137, 104026. [Google Scholar] [CrossRef]
- Scher, F.M.; Baker, R. Effect of Pseudomonas putida and a synthetic iron chelator on induction of soil suppressiveness to Fusarium Wilt pathogens. Phytopathology 1982, 72, 1567–1573. [Google Scholar] [CrossRef]
- Sadfi, N.; Cherif, M.; Fliss, I.; Boudabbous, A.; Antoun, H. Evaluation of bacterial isolates from salty soils and Bacillus thurigiensis strains for the biocontrol of Fusarium dry rot of potato tubers. J. Plant Pathol. 2001, 83, 101–118. [Google Scholar]
- Pane, C.; Piccolo, A.; Spaccini, R.; Celano, G.; Villecco, D.; Zaccardelli, M. Agricultural waste-based composts exhibiting suppressivity to diseases caused by the phytopathogenic soil-borne fungi Rhizoctonia solani and Sclerotinia minor. Appl. Soil Ecol. 2013, 65, 43–51. [Google Scholar] [CrossRef]
- Pane, C.; Spaccini, R.; Piccolo, A.; Scala, F.; Bonanomi, G. Compost amendments enhance peat suppressiveness to Pythium ultimum, Rhizoctonia solani and Sclerotinia minor. Biol. Control 2011, 56, 115–124. [Google Scholar] [CrossRef]
- APAT & CTN TES. Proposta di guida tecnica su metodi di analisi per il suolo e i siti contaminati; utilizzo di indicatori biologici ed ecotossicologici. RTI CTN_TES 1/2004. p. 160. Available online: http://www.isprambiente.gov.it/files/biodiversita/APAT_Guida_tecnica_indicatori_2004.pdf (accessed on 7 January 2020).
- Kim, M.J.; Shim, C.K.; Kim, Y.K.; Hong, S.J.; Park, J.H.; Han, E.J.; Kim, J.H.; Kim, S.C. Effect of aerated compost tea on the growth promotion of lettuce, soybean, and sweet corn in organic cultivation. Plant Pathol. J. 2015, 31, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, H.G. General Microbiology, 7th ed.; Cambridge University Press: New York, NY, USA, 1993. [Google Scholar]
- Caballero, P.; Rodríguez-Morgado, B.; Macías, S.; Tejada, M.; Parrado, J. Obtaining plant and soil biostimulants by waste whey fermentation. Waste Biomass Valor. 2020, 11, 3281–3292. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture. FAO Irrigation and Drainage Paper 29 Rev. 1; FAO: Rome, Italy, 1985. [Google Scholar]
- Brinton, W.; Droffner, M. The control of plant pathogenic fungi by use of compost teas. Biodynamics 1995, 197, 12–15. [Google Scholar]
- Brodeur, C. Actively Aerated Compost Tea and Trees. Ecological Landscape Alliance. 2011. Available online: https://www.ecolandscaping.org/05/developing-healthy-landscapes/composting-compost-tea/actively-aerated-compost-tea-and-trees/ (accessed on 1 August 2021).
- Scheuerell, S.J. Understanding how compost tea can control disease. BioCycle 2003, 44, 20–25. [Google Scholar]
- Dearborn, Y. Compost Tea-Literature Review on Production, Application and Plant Disease Management; San Francisco Department of Environment Toxic Reduction Program: IPM Task Order, 3–18; College of Agriculture, University of Arizona: Tucson, AZ, USA, 2011. [Google Scholar]
- Eudoxie, G.; Martin, M. Compost Tea Quality and Fertility, Organic Fertilizers—History, Production and Applications, Marcelo Larramendy and Sonia Soloneski; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/68380 (accessed on 23 June 2021). [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 96, 15–27. [Google Scholar] [CrossRef]
- Decree of Ministry for Environment, No. 185, 12 June 2003, Gazzetta Ufficiale No.169, Rome, Italy, 23 July 2003. Italian Technical Guidelines for Wastewater Reuse. Available online: https://www.gazzettaufficiale.it/eli/id/2003/07/23/003G0210/sg (accessed on 23 January 2021).
- Legislative Decree, No. 99, 27 January 1992, Gazzetta Ufficiale No. 38, Rome, Italy, 15 February 1992, Implementation of the Directive 86/278/EC Concerning the Protection of the Environment and in Particular of Soil, for the Use of Sewage Sludge in Agriculture. Available online: https://www.gazzettaufficiale.it/eli/id/1992/02/15/092G0139/sg (accessed on 23 January 2021).
- Pane, C.; Celano, G.; Villecco, D.; Zaccardelli, M. Control of Botrytis cinerea, Alternaria alternata and Pyrenochaeta lycopersici on tomato with whey compost-tea application. Crop. Prot. 2012, 38, 80–86. [Google Scholar] [CrossRef]
- Naidu, Y.; Meon, S.; Kadir, J.; Siddiqui, Y. Microbial starter for the enhancement of biological activity of compost tea. Int. J. Agric. Biol. 2010, 12, 51–56. [Google Scholar]
- Zaccardelli, M.; Sorrentino, R.; Caputo, M.; Scotti, R.; De Falco, E.; Pane, C. Stepwise-Selected Bacillus amyloliquefaciens and B. subtilis Strains from Composted Aromatic Plant Waste Able to Control Soil-Borne Diseases. Agriculture 2020, 10, 30. [Google Scholar] [CrossRef]
- Mohd Din, A.R.J.; Hanapi, S.Z.; Futamata, H.; Sarmidi, M.R. Utilization of compost tea for biochemical response assessment associated with resistance to phytopathogen causing leaf spot in Melicope ptelefolia. Org. Agric. 2019, 9, 445–456. [Google Scholar] [CrossRef]
- Pane, C.; Zaccardelli, M. Principles of Compost-based Plant Diseases Control and Innovative New Developments. In Composting for Sustainable Agriculture. Sustainable Development and Biodiversity; Maheshwari, D., Ed.; Springer: Cham, Switzerland, 2014; Volume 3. [Google Scholar]
- Scotti, R.; Mitchell, A.L.; Pane, C.; Finn, R.D.; Zaccardelli, M. Microbiota Characterization of Agricultural Green Waste-Based Suppressive Composts Using Omics and Classic Approaches. Agriculture 2020, 10, 61. [Google Scholar] [CrossRef]
- Pane, C.; Celano, G.; Zaccardelli, M. Metabolic patterns of bacterial communities in aerobic compost teas associated with potential biocontrol of soilborne plant diseases. Phytopathol. Mediterr. 2014, 53, 277–286. [Google Scholar]

| Parameter | C | Wa | Wh | M | |
|---|---|---|---|---|---|
| pH | 8.0 | 7.8 | 6.0 | 8.5 | |
| EC | mS cm−1 | 2.96 | 0.75 | - | - |
| Total-N | % d.w. or L−1 Z | 1.4 | - | 0.61 | 3.0 |
| N-NH4+ | Ppm | 959 | 0 | 19 | . |
| N-NH4+/Total-N | 0.07 | 0.00 | 0.03 | . | |
| TOC | g kg−1 | 313 | 0 | 33 | 10 |
| HA + FA | % d.m. | 11 | - | - | - |
| Ca | mg kg−1 or mg L−1 Y | 65,800 | 21 | 137 | 835 |
| Mg | ’’ | 4000 | 4 | 39 | 304 |
| K | ’’ | 11,800 | 2 | 592 | 1150 |
| Na | ’’ | 2700 | 3 | 942 | 3400 |
| Fe | ’’ | 11,400 | 0.09 | 0.26 | 0.09 |
| Cu | ’’ | 45.98 | 0.02 | 0.04 | 0.61 |
| Zn | ’’ | 247.43 | 0.46 | 0.27 | 5.00 |
| Mn | ’’ | 427.30 | 0.01 | 0.01 | 7.39 |
| Cr | ’’ | 16.050 | 0.015 | 0.012 | 0.069 |
| Cd | ’’ | 0.300 | 0.000 | 0.000 | 0.005 |
| Ni | ’’ | 13.3 | 0.013 | 0.011 | 1.731 |
| Pb | ’’ | 28.150 | 0.024 | 0.027 | 0.250 |
| Metal | CT-Wh1 | CT-Wh2 | CT-M1 | CT-M2 | CT-M3 |
|---|---|---|---|---|---|
| g Supplied | |||||
| Ca | 411 | 685 | 88 | 146 | 292 |
| Mg | 117 | 195 | 32 | 53 | 106 |
| K | 1776 | 2960 | 121 | 201 | 403 |
| Na | 2826 | 4710 | 357 | 595 | 1190 |
| Fe | 0.780 | 1.300 | 0.009 | 0.016 | 0.032 |
| Cu | 0.120 | 0.200 | 0.064 | 0.107 | 0.214 |
| Zn | 0.810 | 1.350 | 0.525 | 0.875 | 1.750 |
| Mn | 0.030 | 0.050 | 0.776 | 1.293 | 2.587 |
| Cr | 0.036 | 0.060 | 0.007 | 0.012 | 0.024 |
| Cd | 0.000 | 0.000 | 0.001 | 0.001 | 0.002 |
| Ni | 0.033 | 0.055 | 0.182 | 0.303 | 0.606 |
| Pb | 0.081 | 0.135 | 0.026 | 0.044 | 0.088 |
| CT | pH | EC | TOC | (HA + FA) | [(HA + FA)/TOC] × 100 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mS cm−1 | g L−1 | g L−1 | ||||||||||
| 0 h | 24 h | 48 h | 0 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| CT-Wa | 8.00 | 8.47 | 8.60 | 0.92 | 4.41 | 4.45 | 1.67 | 1.53 | 0.19 | 0.03 | 11.41 | 1.66 |
| CT-Wh1 | 6.46 | 4.90 | 5.00 | 2.18 | 5.92 | 6.87 | 2.14 | 2.18 | 0.30 | 0.52 | 13.99 | 23.91 |
| CT-Wh2 | 6.05 | 4.81 | 6.02 | 2.34 | 6.60 | 8.20 | 2.73 | 2.89 | 0.61 | 0.66 | 22.33 | 22.64 |
| CT-M1 | 8.04 | 8.54 | 8.72 | 2.35 | 5.51 | 5.82 | 2.49 | 1.97 | 0.43 | 0.25 | 17.35 | 12.90 |
| CT-M2 | 8.24 | 8.60 | 8.77 | 2.34 | 5.66 | 6.03 | 2.40 | 1.61 | 0.18 | 0.41 | 7.43 | 25.30 |
| CT-M3 | 8.32 | 8.62 | 8.99 | 4.36 | 7.10 | 7.45 | 2.14 | 1.76 | 0.31 | 0.53 | 14.54 | 30.32 |
| Parameter | CT-Wa | CT-Wh1 | CT-Wh2 | CT-M1 | CT-M2 | CT-M3 | Limits Imposed by the Italian Law | |
|---|---|---|---|---|---|---|---|---|
| pH | U pH | 8.60 | 5.00 | 6.02 | 8.72 | 8.77 | 8.99 | - |
| EC | mS cm−1 | 4.45 | 6.87 | 8.20 | 5.82 | 6.03 | 7.45 | - |
| SAR | 3.6 | 3.4 | 2.4 | 5.6 | 7.1 | 8.1 | - | |
| Total-N | mg L−1 | 97 | 138 | 197 | 151 | 194 | 288 | - |
| N-NH4+/ | ’’ | 24 | 21 | 50 | 39 | 47 | 50 | - |
| N-NH4+/Total-N | 0.25 | 0.15 | 0.25 | 0.26 | 0.24 | 0.17 | - | |
| TOC | g L−1 | 1.53 | 2.18 | 2.89 | 1.97 | 1.61 | 1.76 | - |
| HA + FA | g L−1 | 0.03 | 0.52 | 0.66 | 0.25 | 0.41 | 0.53 | - |
| Ca | mg L−1 | 51 | 226 | 416 | 59 | 59 | 74 | - |
| Mg | ’’ | 12 | 33 | 60 | 14 | 16 | 17 | - |
| K | ’’ | 373 | 545 | 731 | 524 | 567 | 615 | - |
| Na | ’’ | 103 | 199 | 187 | 170 | 218 | 275 | - |
| Fe | ’’ | 1.86 | 1.6 | 3.37 | 2.62 | 2.19 | 2.43 | 2.0 |
| Cu | ’’ | 0.20 | 0.15 | 1.16 | 0.23 | 0.25 | 0.20 | 1.0 |
| Zn | ’’ | 0.32 | 0.37 | 0.43 | 0.53 | 0.44 | 0.45 | 0.5 |
| Mn | ’’ | 0.18 | 1.07 | 1.91 | 0.30 | 0.28 | 0.28 | 0.2 |
| Cr | ’’ | 0.244 | 0.029 | 0.034 | 0.027 | 0.021 | 0.045 | 0.1 |
| Cd | ’’ | 0.000 | 0.004 | 0.001 | 0.002 | 0.000 | 0.000 | 0.005 |
| Ni | ’’ | 0.037 | 0.029 | 0.054 | 0.064 | 0.071 | 0.105 | 0.2 |
| Pb | ’’ | 0.04 | 0.05 | 0.07 | 0.05 | 0.06 | 0.05 | 0.1 |
| Metal | A1 | A2 | A3 | A4 | A5 | A6 | B1 | B2 | B2 | B4 | B5 | B6 | Bi/Ai | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wa | +Wh1 | +Wh2 | +M1 | +M2 | +M3 | CT-Wa | CT-Wh1 | CT-Wh2 | CT-M1 | CT-M2 | CT-M3 | I = 1 | 2 | 3 | 4 | 5 | 6 | |
| Ca | 21 | 31 | 38 | 24 | 25 | 29 | 51 | 226 | 416 | 59 | 59 | 74 | 2.4 | 7.3 | 11.1 | 2.5 | 2.3 | 2.5 |
| Mg | 4 | 7 | 9 | 5 | 6 | 7 | 12 | 33 | 60 | 14 | 16 | 17 | 3.0 | 4.7 | 6.7 | 2.9 | 2.9 | 2.4 |
| K | 2 | 53 | 86 | 5 | 8 | 14 | 373 | 545 | 731 | 524 | 567 | 615 | 186.5 | 10.4 | 8.5 | 96.1 | 73.2 | 45.6 |
| Na | 3 | 83 | 137 | 13 | 20 | 37 | 103 | 199 | 187 | 170 | 218 | 275 | 34.3 | 2.4 | 1.4 | 12.9 | 10.9 | 7.4 |
| Fe | 0.09 | 0.10 | 0.11 | 0.090 | 0.090 | 0.091 | 1.86 | 1.60 | 3.37 | 2.62 | 2.19 | 2.43 | 20.7 | 15.3 | 29.5 | 29.0 | 24.2 | 26.7 |
| Cu | 0.02 | 0.02 | 0.02 | 0.022 | 0.023 | 0.026 | 0.2 | 0.15 | 1.16 | 0.23 | 0.25 | 0.2 | 10.0 | 6.9 | 50.8 | 10.5 | 10.8 | 7.7 |
| Zn | 0.46 | 0.44 | 0.43 | 0.475 | 0.485 | 0.510 | 0.32 | 0.37 | 0.43 | 0.53 | 0.44 | 0.45 | 0.7 | 0.8 | 1.0 | 1.1 | 0.9 | 0.9 |
| Mn | 0.01 | 0.01 | 0.01 | 0.032 | 0.047 | 0.084 | 0.18 | 1.07 | 1.91 | 0.3 | 0.28 | 0.28 | 18.0 | 107.0 | 191.0 | 9.3 | 6.0 | 3.3 |
| Cr | 0.015 | 0.01 | 0.01 | 0.015 | 0.015 | 0.016 | 0.244 | 0.03 | 0.03 | 0.027 | 0.021 | 0.045 | 16.3 | 2.0 | 2.3 | 1.8 | 1.4 | 2.9 |
| Cd | 0 | 0.00 | 0.00 | 0.000 | 0.000 | 0.000 | 0 | 0.004 | 0.001 | 0.002 | 0 | 0 | - | - | - | 133.3 | 0.0 | 0.0 |
| Ni | 0.013 | 0.01 | 0.01 | 0.018 | 0.022 | 0.030 | 0.037 | 0.03 | 0.05 | 0.064 | 0.071 | 0.105 | 2.8 | 2.3 | 4.2 | 3.5 | 3.3 | 3.5 |
| Pb | 0.024 | 0.02 | 0.02 | 0.025 | 0.025 | 0.027 | 0.04 | 0.05 | 0.07 | 0.05 | 0.06 | 0.05 | 1.7 | 2.1 | 2.9 | 2.0 | 2.4 | 1.9 |
| COMPOST TEAS | Fungi (LogCFU ml−1) | Yeast (LogCFU ml−1) | Bacteria (LogCFU ml−1) | Fungi/Bacteria Ratio | Fungi/Yeast Ratio | Yeast/Bacteria Ratio | Pseudomonas spp. (LogCFU ml−1) | Bacillus spp. (LogCFU ml−1) |
|---|---|---|---|---|---|---|---|---|
| CT-Wa | 3.1c | 4.4 c | 5.6 c | 0.562 | 0.711 | 0.789 | 3.8 d | 4.1 a |
| CT-Wh1 | 6.3 a | 5.0 c | 7.2 bc | 0.868 | 1.264 | 0.687 | 5.1 d | 4.0 a |
| CT-Wh2 | 6.1 b | 5.9 b | 8.3 a | 0.741 | 1.044 | 0.710 | 6.8 b | 4.3 a |
| CT-M1 | 4.4 c | 6.3 a | 7.4 bc | 0.601 | 0.699 | 0.859 | 7.2 a | 4.4 a |
| CT-M2 | 3.6 c | 5.3 c | 7.8 b | 0.462 | 0.683 | 0.676 | 6.3 cd | 3.7 a |
| CT-M3 | 3.4 c | 5.5 c | 7.6 bc | 0.453 | 0.623 | 0.727 | 6.8 bc | 3.6 a |
| CTs | Biolog CLPPs | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fungi | Yeast | Bacteria | Fungi/Bacteria | Fungi/Yeast | Yeast/Bacteria | Pseudomonas spp. | Bacillus spp. | Boltzmann Function of AWCD | Metabolic Biodiversity | |||||
| LogCFU ml−1 | LogCFU ml−1 | A2 | x0 | dx | R2 | VC | H′ | |||||||
| CT-Wa | 3.1c | 4.4 c | 5.6 c | 0.562 | 0.711 | 0.789 | 3.8 d | 4.1 a | 1.47 | 45.21 | 11.01 | 0.996 | 2.013 d | 3.32 d |
| CT-Wh1 | 6.3 a | 5.0 c | 7.2 bc | 0.868 | 1.264 | 0.687 | 5.1 d | 4.0 a | 1.11 | 43.26 | 13.40 | 0.996 | 1.362 d | 3.32 d |
| CT-Wh2 | 6.1 b | 5.9 b | 8.3 a | 0.741 | 1.044 | 0.710 | 6.8 b | 4.3 a | 1.22 | 36.71 | 14.26 | 0.991 | 1.869 d | 3.37 c |
| CT-M1 | 4.4 c | 6.3 a | 7.4 bc | 0.601 | 0.699 | 0.859 | 7.2 a | 4.4 a | 1.71 | 41.36 | 13.74 | 0.996 | 3.128 c | 3.40 b |
| CT-M2 | 3.6 c | 5.3 c | 7.8 b | 0.462 | 0.683 | 0.676 | 6.3 cd | 3.7 a | 1.80 | 37.84 | 14.19 | 0.993 | 4.500 b | 3.41 a |
| CT-M3 | 3.4 c | 5.5 c | 7.6 bc | 0.453 | 0.623 | 0.727 | 6.8 bc | 3.6 a | 1.70 | 37.96 | 15.12 | 0.990 | 5.180 a | 3.41 a |
| Compost Teas | Mycelial Inhibition Zone (%) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fusarium solani | Fusarium oxysporum | Fusarium sambucinum | Fusarium semitectum | Alternaria alternata | Botrytis cinerea | Verticillium dahliae | Colletotrichum lindemutianum | Pyrenochaeta lycopersici | Rhizoctonia solani | |||||||||||
| 1:5 | 1:10 | 1:5 | 1:10 | 1:5 | 1:10 | 1:5 | 1:10 | 1:5 | 1:10 | 1:5 | 1:10 | 1:5 | 1:10 | 1:5 | 1:10 | 1:5 | 1:10 | 1:5 | 1:10 | |
| CT-Wa | 42 bc | 41 ab | 42 b | 41 ab | 54 a | 61 a | 45 a | 45 ab | 41 ab | 39 b | 46 ab | 47 a | 58 a | 40 b | 35 a | 33 a | 44 bc | 67 a | 25 b | 24 bc |
| CT-S1 | 35 c | 43 ab | 38 b | 33 b | 31 b | 35 b | 37 b | 40 c | 50 a | 38 b | 45 ab | 44 ab | 47 c | 34 c | 29 a | 28 ab | 21 d | 41 b | 27 b | 26 b |
| CT-S2 | 35 c | 30 c | 37 b | 49 a | 41 ab | 38 b | 43 ab | 49 a | 55 a | 39 b | 44 ab | 44 ab | 47 c | 42 b | 23 b | 22 b | 33 cd | 28 c | 33 a | 32 a |
| CT-M1 | 49 ab | 46 a | 52 a | 22 c | 42 ab | 43 b | 45 a | 47 ab | 36 bc | 53 a | 56 a | 45 ab | 54 ab | 61 a | 27 ab | 35 a | 23 d | 27 c | 24 b | 22 bc |
| CT-M2 | 54 a | 41 ab | 44 b | 38 b | 41 ab | 39 b | 44 ab | 42 bc | 28 c | 32 b | 28 c | 41 ab | 51 bc | 44 b | 28 ab | 27 ba | 64 a | 25 c | 26 b | 21 c |
| CT-M3 | 54 a | 33 bc | 40 b | 49 a | 38 ab | 36 b | 39 ab | 40 c | 43 ab | 43 b | 38 b | 34 b | 49 c | 42 b | 30 ab | 35 a | 55 ab | 20 d | 29 ab | 26 b |
| Element | CT-Wa | CT-Wh2 | CT-M3 | Maximum Annual Amounts Allowed by the ltalian Law |
|---|---|---|---|---|
| g ha−1 | ||||
| Fe | 5.6 | 10.1 | 7.3 | - |
| Cu | 0.6 | 3.5 | 0.6 | 5000 |
| Zn | 1.0 | 1.3 | 1.4 | 12,500 |
| Mn | 0.5 | 5.7 | 0.8 | - |
| Cr | 0.7 | 0.1 | 0.1 | - |
| Cd | 0.0 | 0.0 | 0.0 | 100 |
| Ni | 0.1 | 0.2 | 0.3 | 1500 |
| Pb | 0.1 | 0.2 | 0.2 | 3750 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palese, A.M.; Pane, C.; Villecco, D.; Zaccardelli, M.; Altieri, G.; Celano, G. Effects of Organic Additives on Chemical, Microbiological and Plant Pathogen Suppressive Properties of Aerated Municipal Waste Compost Teas. Appl. Sci. 2021, 11, 7402. https://doi.org/10.3390/app11167402
Palese AM, Pane C, Villecco D, Zaccardelli M, Altieri G, Celano G. Effects of Organic Additives on Chemical, Microbiological and Plant Pathogen Suppressive Properties of Aerated Municipal Waste Compost Teas. Applied Sciences. 2021; 11(16):7402. https://doi.org/10.3390/app11167402
Chicago/Turabian StylePalese, Assunta Maria, Catello Pane, Domenica Villecco, Massimo Zaccardelli, Gessica Altieri, and Giuseppe Celano. 2021. "Effects of Organic Additives on Chemical, Microbiological and Plant Pathogen Suppressive Properties of Aerated Municipal Waste Compost Teas" Applied Sciences 11, no. 16: 7402. https://doi.org/10.3390/app11167402
APA StylePalese, A. M., Pane, C., Villecco, D., Zaccardelli, M., Altieri, G., & Celano, G. (2021). Effects of Organic Additives on Chemical, Microbiological and Plant Pathogen Suppressive Properties of Aerated Municipal Waste Compost Teas. Applied Sciences, 11(16), 7402. https://doi.org/10.3390/app11167402









