Direct Measurement and Modeling of Intraglottal, Subglottal, and Vocal Fold Collision Pressures during Phonation in an Individual with a Hemilaryngectomy
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
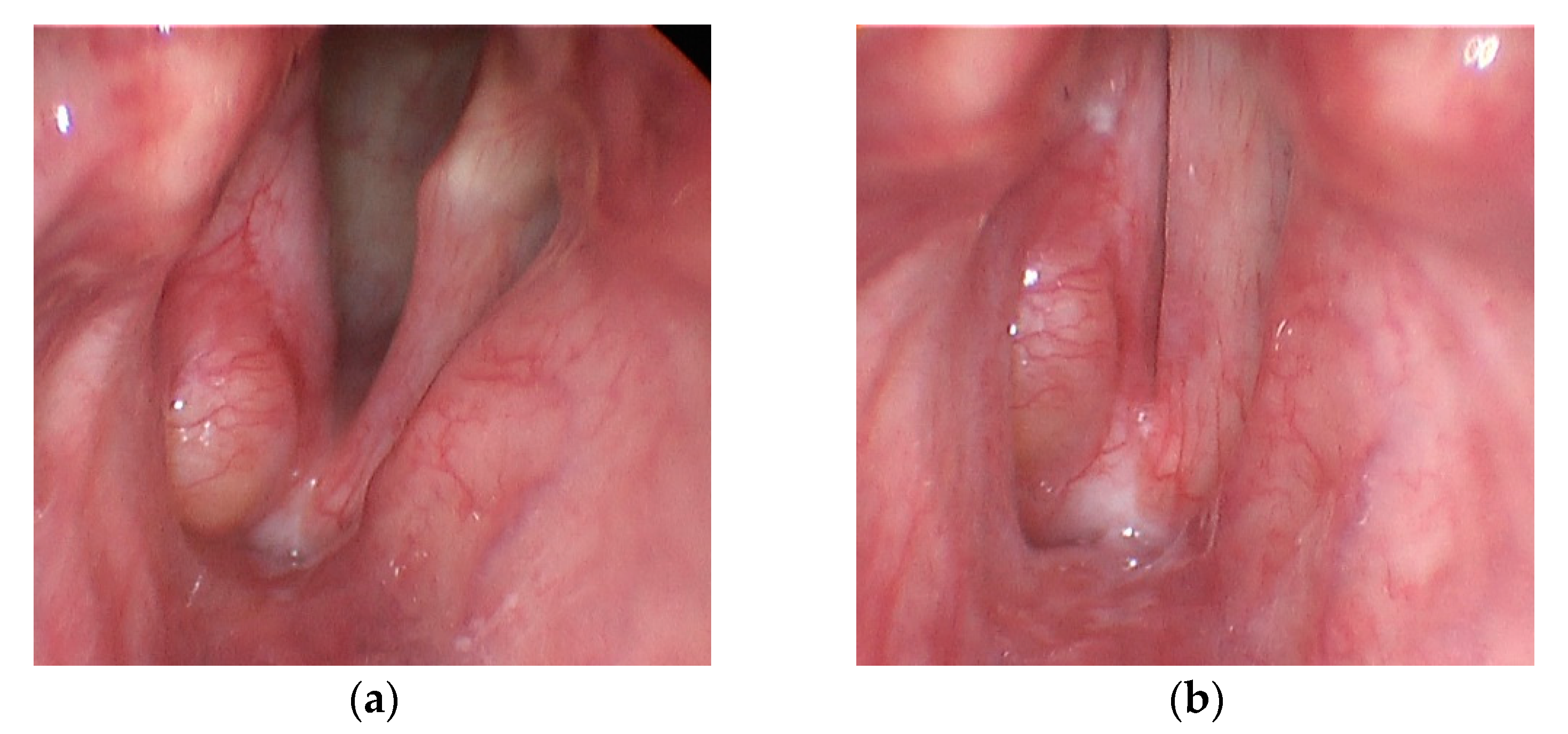
2.1. Participant Characteristics
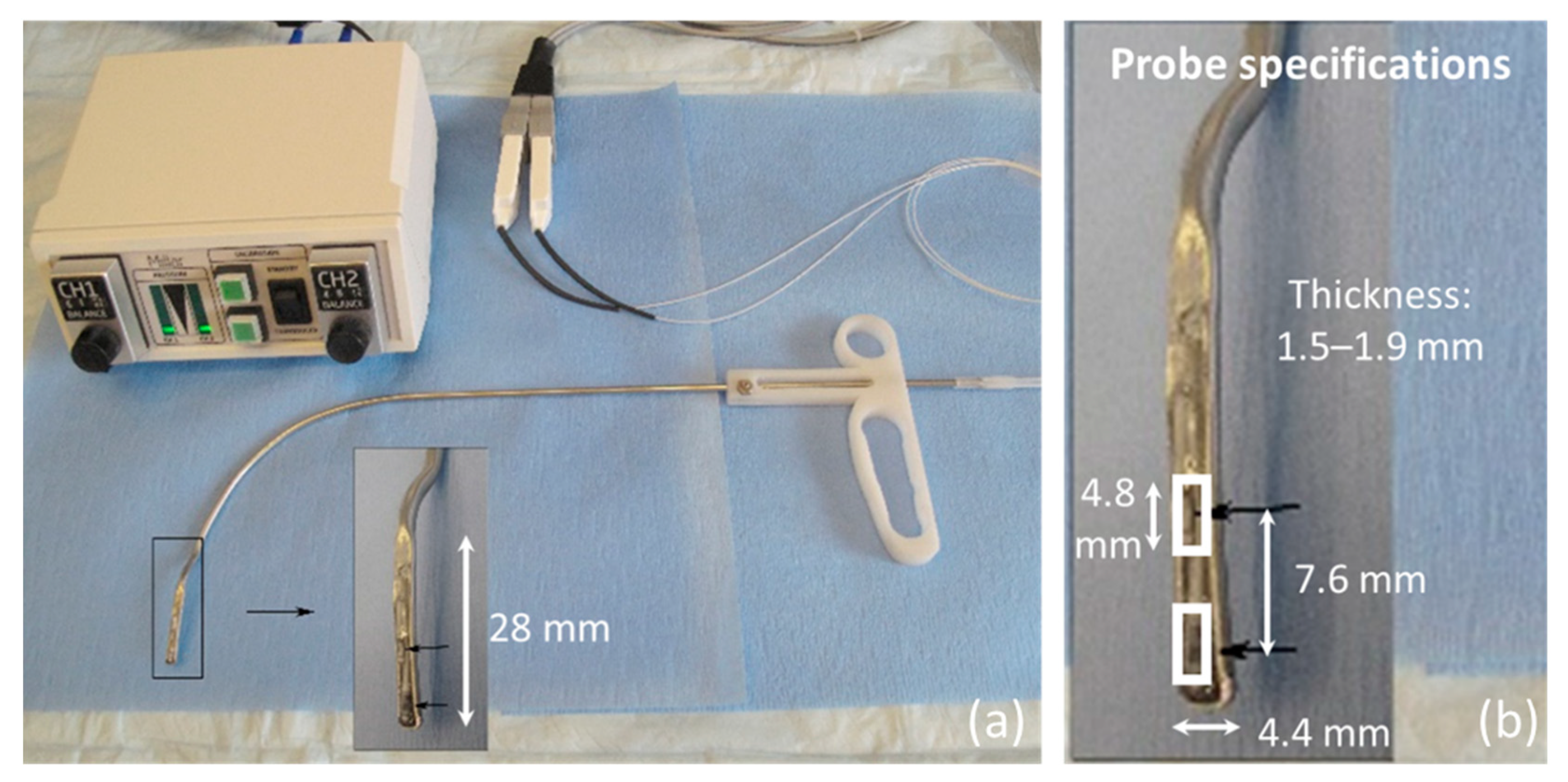
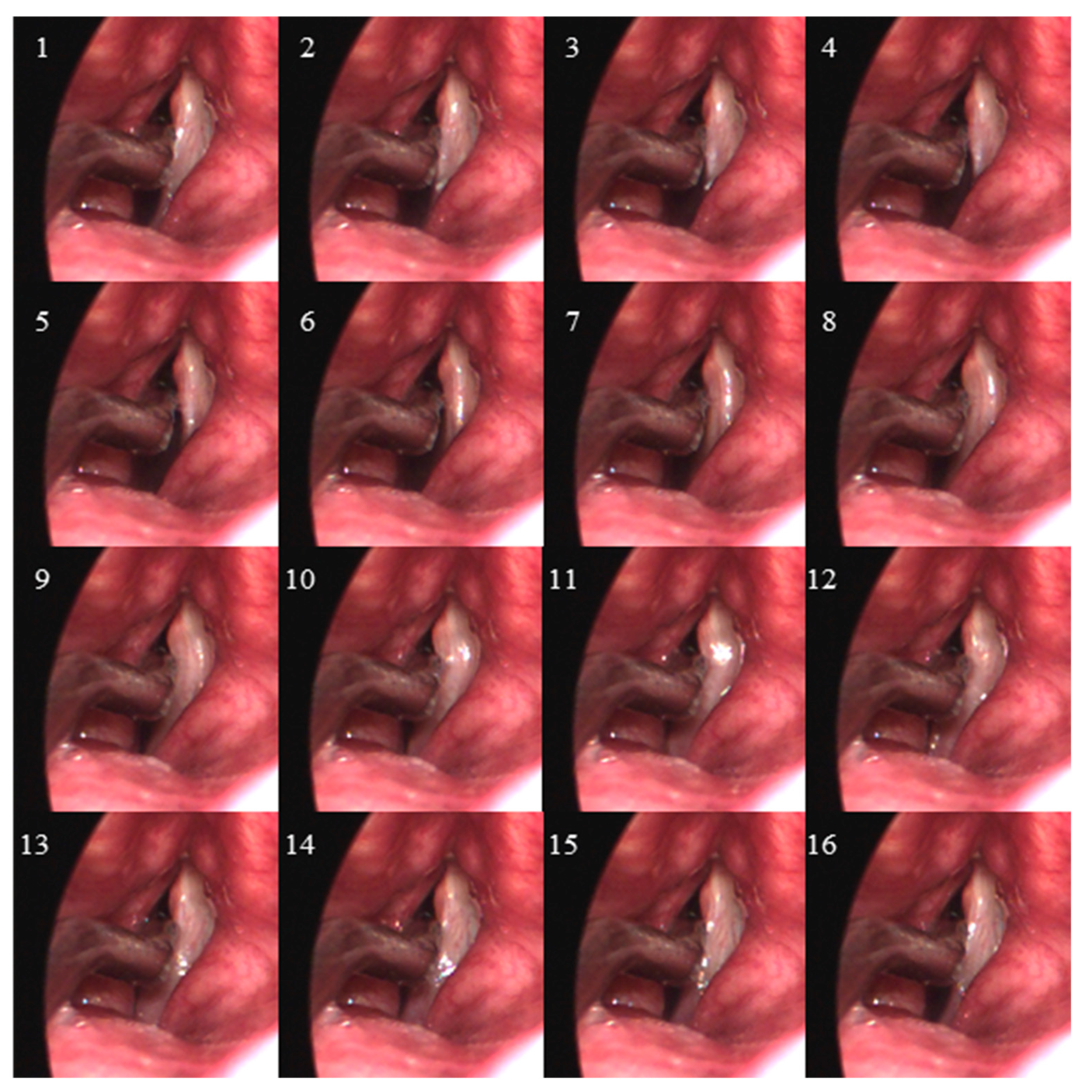
2.2. Data Collection
2.3. Data Analysis
3. Results
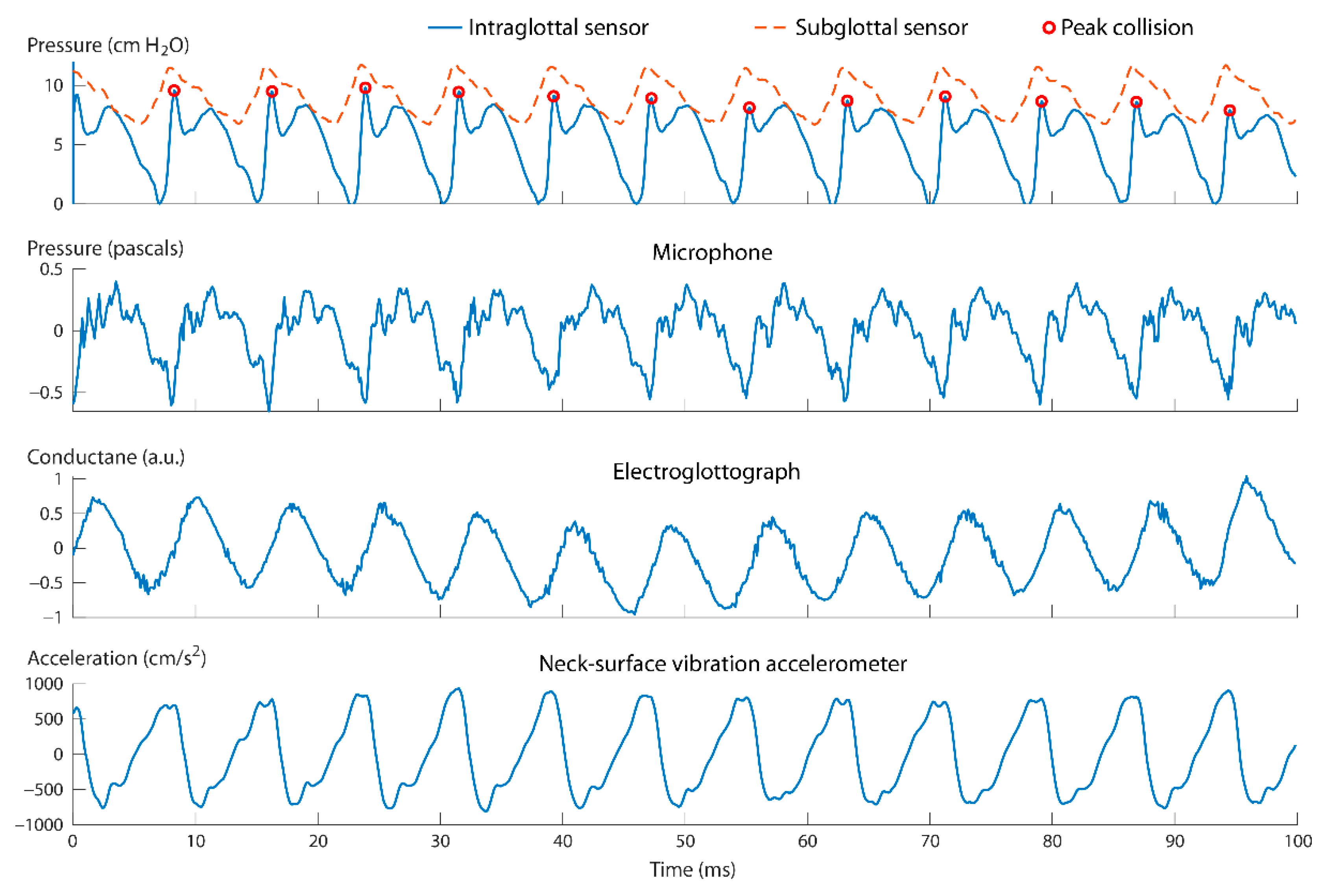
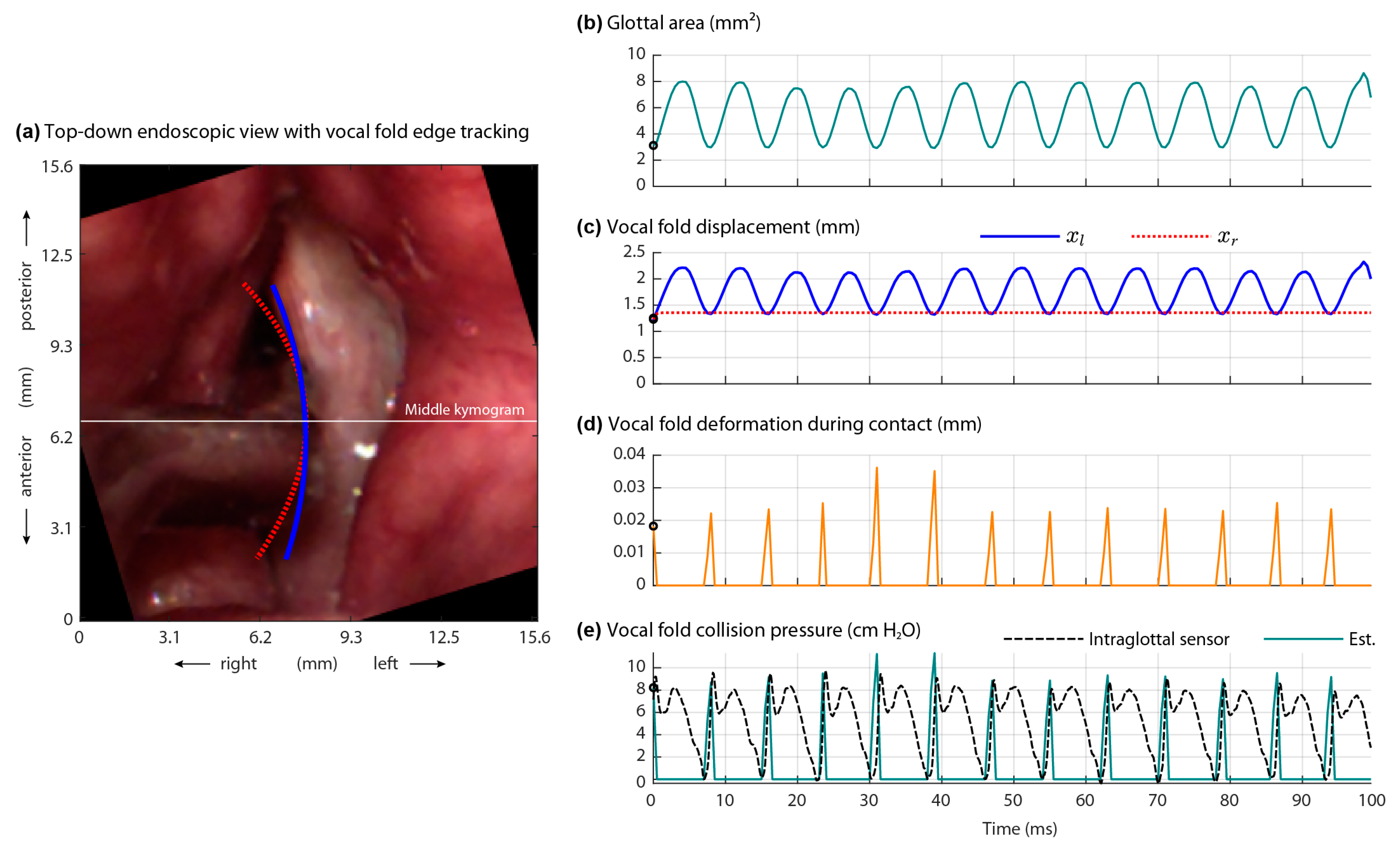
3.1. Direct Measurement of Aerodynamic and Acoustic Signals
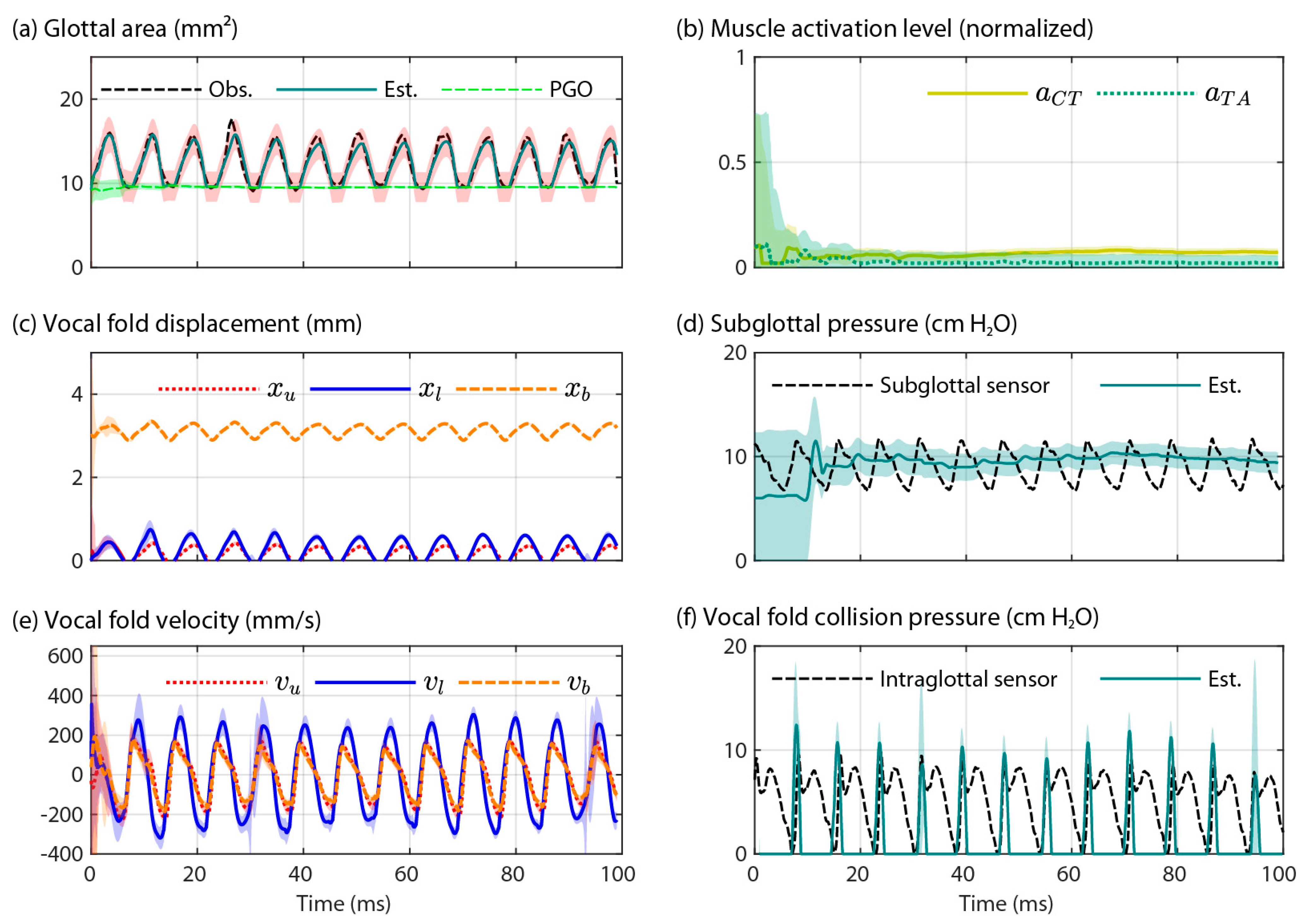
3.2. Validation of the Two Model-Based Estimates of Vocal Fold Collision Pressure
4. Discussion
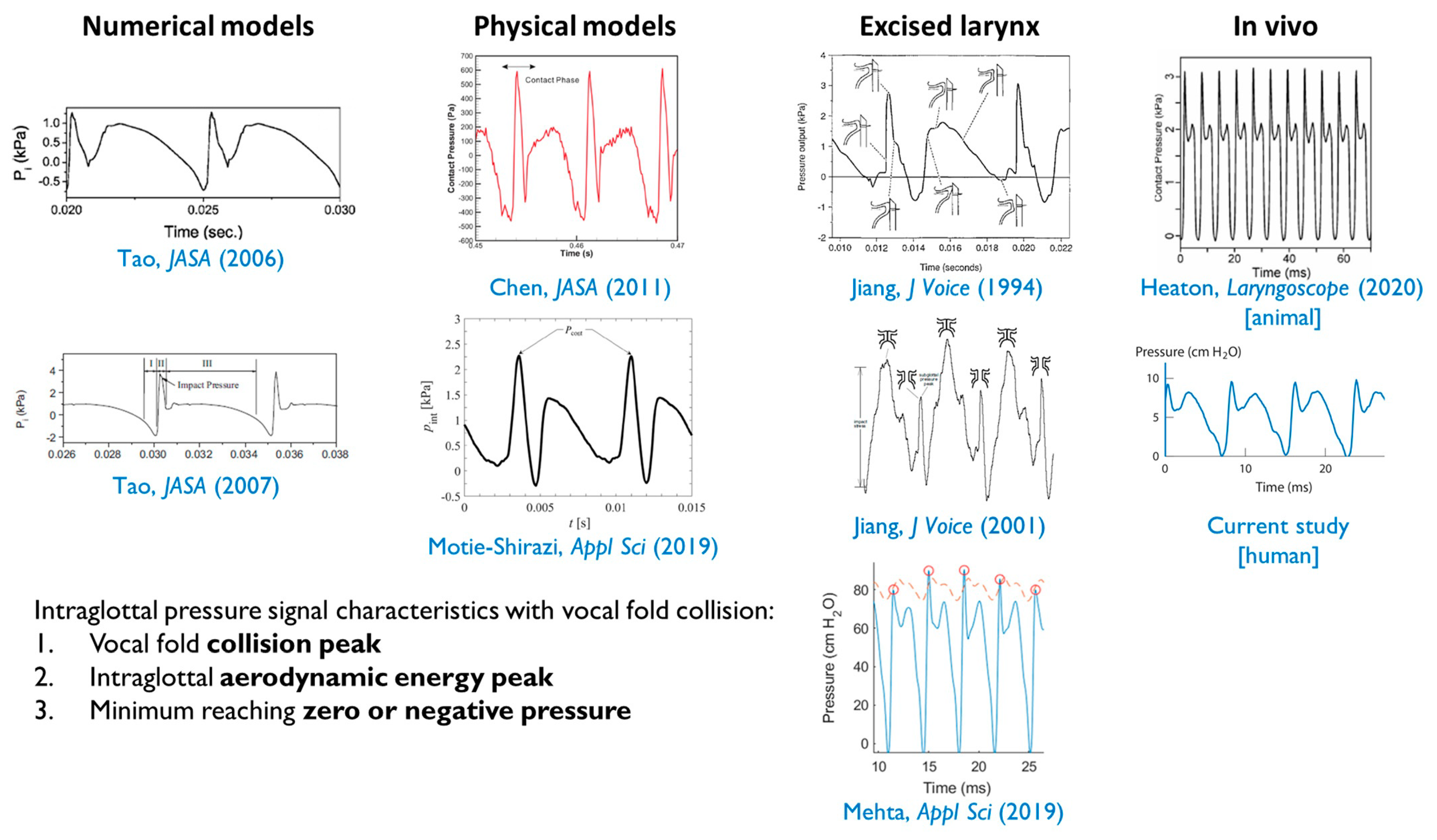
4.1. Intraglottal and Vocal Fold Collision Pressure Waveform Characteristics
- Endoscopic visualization is necessary to guide placement of the ISP probe such that the distal pressure sensor at the probe tip is positioned subglottally and the proximal sensor is positioned in the glottis in the phonatory strike zone to sense vocal fold impact collision pressure during phonation.
- In individuals with a hemilaryngectomy, the ISP probe should rest on the medialized scar band that replaces the excised vocal fold tissue, such that the pressure-sensing element comes into direct contact with the functioning vocal fold.
- The positioning of the intraglottal pressure sensor is in the phonatory strike zone if the following waveform characteristics are exhibited:
- An impulsive peak in the direction of increasing pressure at the instant of vocal fold contact;
- A more rounded peak following the impulsive peak that senses aerodynamic pressure build-up during the open phase; and
- A minimum value approaching zero or negative pressure immediately preceding the impulsive peak of the subsequent phonatory cycle, reflecting rapidly decreasing intraglottal pressure as airflow accelerates.
4.2. Validation of the Two Model-Based Estimates of Vocal Fold Collision Pressure
4.3. Clinical Implications
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, N.; Merrill, R.M.; Gray, S.D.; Smith, E.M. Voice disorders in the general population: Prevalence, risk factors, and occupational impact. Laryngoscope 2005, 115, 1988–1995. [Google Scholar] [CrossRef]
- Bhattacharyya, N. The prevalence of voice problems among adults in the United States. Laryngoscope 2014, 124, 2359–2362. [Google Scholar] [CrossRef]
- Hillman, R.E.; Stepp, C.E.; Van Stan, J.H.; Zañartu, M.; Mehta, D.D. An updated theoretical framework for vocal hyperfunction. Am. J. Speech Lang. Pathol. 2020, 29, 2254–2260. [Google Scholar] [CrossRef]
- Titze, I.R. Mechanical stress in phonation. J. Voice 1994, 8, 99–105. [Google Scholar] [CrossRef]
- Czerwonka, L.; Jiang, J.J.; Tao, C. Vocal nodules and edema may be due to vibration-induced rises in capillary pressure. Laryngoscope 2008, 118, 748–752. [Google Scholar] [CrossRef]
- Tao, C.; Jiang, J.J.; Czerwonka, L. Liquid accumulation in vibrating vocal fold tissue: A simplified model based on a fluid-saturated porous solid theory. J. Voice 2010, 24, 260–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvit, A.A.; Devine, E.E.; Jiang, J.J.; Vamos, A.C.; Tao, C. Characterizing liquid redistribution in a biphasic vibrating vocal fold using finite element analysis. J. Voice 2015, 29, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Gunter, H.E.; Howe, R.D.; Zeitels, S.M.; Kobler, J.B.; Hillman, R.E. Measurement of vocal fold collision forces during phonation: Methods and preliminary data. J. Speech Lang. Hear. Res. 2005, 48, 567–576. [Google Scholar] [CrossRef]
- Verdolini, K.; Hess, M.M.; Titze, I.R.; Bierhals, W.; Gross, M. Investigation of vocal fold impact stress in human subjects. J. Voice 1999, 13, 184–202. [Google Scholar] [CrossRef]
- Hess, M.M.; Verdolini, K.; Bierhals, W.; Mansmann, U.; Gross, M. Endolaryngeal contact pressures. J. Voice 1998, 12, 50–67. [Google Scholar] [CrossRef]
- Rothenberg, M. A multichannel electroglottograph. J. Voice 1992, 6, 36–43. [Google Scholar] [CrossRef]
- Motie-Shirazi, M.; Zañartu, M.; Peterson, S.D.; Mehta, D.D.; Kobler, J.B.; Hillman, R.E.; Erath, B.D. Toward development of a vocal fold contact pressure probe: Sensor characterization and validation using synthetic vocal fold models. Appl. Sci. 2019, 9, 3002. [Google Scholar] [CrossRef] [Green Version]
- Mehta, D.D.; Kobler, J.B.; Zeitels, S.M.; Zañartu, M.; Erath, B.D.; Motie-Shirazi, M.; Peterson, S.D.; Petrillo, R.H.; Hillman, R.E. Toward development of a vocal fold contact pressure probe: Bench-top validation of a dual-sensor probe using excised human larynx models. Appl. Sci. 2019, 9, 4360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-J.; Mongeau, L. Verification of two minimally invasive methods for the estimation of the contact pressure in human vocal folds during phonation. J. Acoust. Soc. Am. 2011, 130, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Gunter, H.E. A mechanical model of vocal-fold collision with high spatial and temporal resolution. J. Acoust. Soc. Am. 2003, 113, 994–1000. [Google Scholar] [CrossRef] [Green Version]
- Gunter, H.E. Modeling mechanical stresses as a factor in the etiology of benign vocal fold lesions. J. Biomech. 2004, 37, 1119–1124. [Google Scholar] [CrossRef]
- Horáček, J.; Šidlof, P.; Švec, J.G. Numerical simulation of self-oscillations of human vocal folds with Hertz model of impact forces. J. Fluids Struct. 2005, 20, 853–869. [Google Scholar] [CrossRef]
- Tao, C.; Jiang, J.J.; Zhang, Y. Simulation of vocal fold impact pressures with a self-oscillating finite-element model. J. Acoust. Soc. Am. 2006, 119, 3987–3994. [Google Scholar] [CrossRef]
- Tao, C.; Jiang, J.J. Mechanical stress during phonation in a self-oscillating finite-element vocal fold model. J. Biomech. 2007, 40, 2191–2198. [Google Scholar] [CrossRef]
- Horáček, J.; Laukkanen, A.M.; Šidlof, P.; Murphy, P.; Švec, J.G. Comparison of acceleration and impact stress as possible loading factors in phonation: A computer modeling study. Folia Phoniatr. Logop. 2009, 61, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Siegmund, T.; Mongeau, L. Determination of superior surface strains and stresses, and vocal fold contact pressure in a synthetic larynx model using digital image correlation. J. Acoust. Soc. Am. 2008, 123, 1089–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.J.; Titze, I.R. Measurement of vocal fold intraglottal pressure and impact stress. J. Voice 1994, 8, 132–144. [Google Scholar] [CrossRef]
- Verdolini, K.; Chan, R.; Titze, I.R.; Hess, M.; Bierhals, W. Correspondence of electroglottographic closed quotient to vocal fold impact stress in excised canine larynges. J. Voice 1998, 12, 415–423. [Google Scholar] [CrossRef]
- Berry, D.A.; Verdolini, K.; Montequin, D.W.; Hess, M.M.; Chan, R.W.; Titze, I.R. A quantitative output-cost ratio in voice production. J. Speech Lang. Hear. Res. 2001, 44, 29–37. [Google Scholar] [CrossRef]
- Jiang, J.J.; Shah, A.G.; Hess, M.M.; Verdolini, K.; Banzali, F.M., Jr.; Hanson, D.G. Vocal fold impact stress analysis. J. Voice 2001, 15, 4–14. [Google Scholar] [CrossRef]
- Heaton, J.T.; Kobler, J.B.; Hillman, R.E.; Zeitels, S.M. A new instrument for intraoperative assessment of individual vocal folds. Laryngoscope 2005, 115, 1223–1229. [Google Scholar] [CrossRef]
- Backshaei, H.; Yang, J.; Miri, A.K.; Mongeau, L. Determination of the stresses and strain on the superior surface of excised porcine larynges during phonation using digital image correlation. Proc. Meet. Acoust. 2013, 19, 060238. [Google Scholar]
- Weiss, S.; Sutor, A.; Rupitsch, S.J.; Kniesburges, S.; Doellinger, M.; Lerch, R. Development of a small film sensor for the estimation of the contact pressure of artificial vocal folds. Proc. Meet. Acoust. 2013, 19, 060307. [Google Scholar]
- Heaton, J.T.; Kobler, J.B.; Ottensmeyer, M.P.; Petrillo, R.H.; Tynan, M.A.; Mehta, D.D.; Hillman, R.E.; Zeitels, S.M. Aerodynamically driven phonation of individual vocal folds under general anesthesia in canines. Laryngoscope 2020, 130, 1980–1988. [Google Scholar] [CrossRef]
- Zeitels, S.M.; Jarboe, J.; Franco, R.A. Phonosurgical reconstruction of early glottic cancer. Laryngoscope 2001, 111, 1862–1865. [Google Scholar] [CrossRef]
- Zeitels, S.M.; Hillman, R.E.; Franco, R.A.; Bunting, G.W. Voice and treatment outcome from phonosurgical management of early glottic cancer. Ann. Otol. Rhinol. Laryngol. 2002, 111, 1–20. [Google Scholar] [CrossRef]
- Zeitels, S.M. Optimizing voice after endoscopic partial laryngectomy. Otolaryngol. Clin. N. Am. 2004, 37, 627–636. [Google Scholar] [CrossRef]
- Alipour, F.; Montequin, D.; Tayama, N. Aerodynamic profiles of a hemilarynx with a vocal tract. Ann. Otol. Rhinol. Laryngol. 2001, 110, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Doellinger, M.; Berry, D.A. Visualization and quantification of the medial surface dynamics of an excised human vocal fold during phonation. J. Voice 2006, 20, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.J.; Titze, I.R. A methodological study of hemilaryngeal phonation. Laryngoscope 1993, 103, 872–882. [Google Scholar] [CrossRef]
- Saraniti, C.; Speciale, R.; Santangelo, M.; Massaro, N.; Maniaci, A.; Gallina, S.; Serra, A.; Cocuzza, S. Functional outcomes after supracricoid modified partial laryngectomy. J. Biol. Regul. Homeost. Agents 2019, 33, 1903–1907. [Google Scholar] [PubMed]
- Zañartu, M.; Ho, J.C.; Mehta, D.D.; Hillman, R.E.; Wodicka, G.R. Subglottal impedance-based inverse filtering of voiced sounds using neck surface acceleration. IEEE Trans. Audio Speech Lang. Process. 2013, 21, 1929–1939. [Google Scholar] [CrossRef] [Green Version]
- Cheyne, H.A. Estimating glottal voicing source characteristics by measuring and modeling the acceleration of the skin on the neck. In Proceedings of the 3rd IEEE-EMBS International Summer School and Symposium on Medical Devices and Biosensors, Cambridge, MA, USA, 4–6 September 2006; pp. 118–121. [Google Scholar]
- Cortés, J.P.; Espinoza, V.M.; Ghassemi, M.; Mehta, D.D.; Van Stan, J.H.; Hillman, R.E.; Guttag, J.V.; Zañartu, M. Ambulatory assessment of phonotraumatic vocal hyperfunction using glottal airflow measures estimated from neck-surface acceleration. PLoS ONE 2018, 13, e0209017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, D.D.; Van Stan, J.H.; Zañartu, M.; Ghassemi, M.; Guttag, J.V.; Espinoza, V.M.; Cortés, J.P.; Cheyne, H.A., II; Hillman, R.E. Using ambulatory voice monitoring to investigate common voice disorders: Research update. Front. Bioeng. Biotechnol. 2015, 3, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popolo, P.S.; Švec, J.G.; Titze, I.R. Adaptation of a Pocket PC for use as a wearable voice dosimeter. J. Speech Lang. Hear. Res. 2005, 48, 780–791. [Google Scholar] [CrossRef]
- Cheyne, H.A.; Hanson, H.M.; Genereux, R.P.; Stevens, K.N.; Hillman, R.E. Development and testing of a portable vocal accumulator. J. Speech Lang. Hear. Res. 2003, 46, 1457–1467. [Google Scholar] [CrossRef]
- Winholtz, W.S.; Titze, I.R. Conversion of a head-mounted microphone signal into calibrated SPL units. J. Voice 1997, 11, 417–421. [Google Scholar] [CrossRef]
- Boersma, P.; Weenink, D. Praat: Doing Phonetics by Computer; University of Amsterdam: Amsterdam, The Netherlands, 2003; Available online: http://www.praat.org (accessed on 21 July 2003).
- Díaz-Cádiz, M.E.; Peterson, S.D.; Galindo, G.E.; Espinoza, V.M.; Motie-Shirazi, M.; Erath, B.D.; Zañartu, M. Estimating vocal fold contact pressure from raw laryngeal high-speed videoendoscopy using a Hertz contact model. Appl. Sci. 2019, 9, 2384. [Google Scholar] [CrossRef] [Green Version]
- Alzamendi, G.A.; Manríquez, R.; Hadwin, P.J.; Deng, J.J.; Peterson, S.D.; Erath, B.D.; Mehta, D.D.; Hillman, R.E.; Zañartu, M. Bayesian estimation of vocal function measures using laryngeal high-speed videoendoscopy and glottal airflow estimates: An in vivo case study. J. Acoust. Soc. Am. 2020, 147, EL434–EL439. [Google Scholar] [CrossRef] [PubMed]
- Zañartu, M.; Galindo, G.E.; Erath, B.D.; Peterson, S.D.; Wodicka, G.R.; Hillman, R.E. Modeling the effects of a posterior glottal opening on vocal fold dynamics with implications for vocal hyperfunction. J. Acoust. Soc. Am. 2014, 136, 3262–3271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkholz, P. GlottalImageExplorer—An open source tool for glottis segmentation in endoscopic high-speed videos of the vocal folds. In Studientexte zur Sprachkommunikation: Elektronische Sprachsignalverarbeitung; Jokisch, O., Ed.; TUDPress: Dresden, Germany, 2016. [Google Scholar]
- Titze, I.R. Theoretical analysis of maximum flow declination rate versus maximum area declination rate in phonation. J. Speech Lang. Hear. Res. 2006, 49, 439–447. [Google Scholar] [CrossRef]
- Titze, I.R.; Hunter, E.J. Comparison of vocal vibration-dose measures for potential-damage risk criteria. J. Speech Lang. Hear. Res. 2015, 58, 1425–1439. [Google Scholar] [CrossRef] [Green Version]
- Motie-Shirazi, M.; Zañartu, M.; Peterson, S.D.; Erath, B.D. Vocal fold dynamics in a synthetic self-oscillating model: Contact pressure and dissipated energy dose. J. Acoust. Soc. Am. 2021, 150, 478–489. [Google Scholar] [CrossRef]
- Hillman, R.E.; Heaton, J.T.; Masaki, A.; Zeitels, S.M.; Cheyne, H.A. Ambulatory monitoring of disordered voices. Ann. Otol. Rhinol. Laryngol. 2006, 115, 795–801. [Google Scholar] [CrossRef]
- Szabo Portela, A.; Granqvist, S.; Ternström, S.; Södersten, M. Vocal behavior in environmental noise: Comparisons between work and leisure conditions in women with work-related voice disorders and matched controls. J. Voice 2018, 32, 126.e23–126.e38. [Google Scholar] [CrossRef] [Green Version]
- Van Stan, J.H.; Mehta, D.D.; Ortiz, A.J.; Burns, J.A.; Toles, L.E.; Marks, K.L.; Vangel, M.; Hron, T.; Zeitels, S.; Hillman, R.E. Differences in weeklong ambulatory vocal behavior between female patients with phonotraumatic lesions and matched controls. J. Speech Lang. Hear. Res. 2020, 63, 372–384. [Google Scholar] [CrossRef]
- Van Stan, J.H.; Mehta, D.D.; Ortiz, A.J.; Burns, J.A.; Marks, K.L.; Toles, L.E.; Stadelman-Cohen, T.; Krusemark, C.; Muise, J.; Hron, T.; et al. Changes in a Daily Phonotrauma Index after laryngeal surgery and voice therapy: Implications for the role of daily voice use in the etiology and pathophysiology of phonotraumatic vocal hyperfunction. J. Speech Lang. Hear. Res. 2020, 63, 3934–3944. [Google Scholar] [CrossRef] [PubMed]
- Van Stan, J.H.; Ortiz, A.J.; Cortés, J.P.; Marks, K.L.; Toles, L.E.; Mehta, D.D.; Burns, J.A.; Hron, T.; Stadelman-Cohen, T.; Krusemark, C.; et al. Differences in daily voice use measures between female patients with nonphonotraumatic vocal hyperfunction and matched controls. J. Speech Lang. Hear. Res. 2021, 64, 1457–1470. [Google Scholar] [CrossRef] [PubMed]
- Van Stan, J.; Ortiz, A.; Marks, K.; Toles, L.; Mehta, D.; Burns, J.; Hron, T.; Stadelman-Cohen, T.; Krusemark, C.; Muise, J.; et al. Changes in the Daily Phonotrauma Index (DPI) following the use of voice therapy as the sole treatment for phonotraumatic vocal hyperfunction in females. J. Speech Lang. Hear. Res. 2021, in press. [Google Scholar]
- Toles, L.E.; Ortiz, A.J.; Marks, K.L.; Burns, J.A.; Hron, T.; Van Stan, J.H.; Mehta, D.D.; Hillman, R.E. Differences between female singers with phonotrauma and vocally healthy matched controls in singing and speaking voice use during 1 week of ambulatory monitoring. Am. J. Speech Lang. Pathol. 2021, 30, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Švec, J.G.; Titze, I.R.; Popolo, P.S. Estimation of sound pressure levels of voiced speech from skin vibration of the neck. J. Acoust. Soc. Am. 2005, 117, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Titze, I.R.; Švec, J.G.; Popolo, P.S. Vocal dose measures: Quantifying accumulated vibration exposure in vocal fold tissues. J. Speech Lang. Hear. Res. 2003, 46, 919–932. [Google Scholar] [CrossRef]
- Fryd, A.S.; Van Stan, J.H.; Hillman, R.E.; Mehta, D.D. Estimating subglottal pressure from neck-surface acceleration during normal voice production. J. Speech Lang. Hear. Res. 2016, 59, 1335–1345. [Google Scholar] [CrossRef] [Green Version]
- Marks, K.L.; Lin, J.Z.; Fox, A.B.; Toles, L.E.; Mehta, D.D. Impact of nonmodal phonation on estimates of subglottal pressure from neck-surface acceleration in healthy speakers. J. Speech Lang. Hear. Res. 2019, 62, 3339–3358. [Google Scholar] [CrossRef]
- Lin, J.Z.; Espinoza, V.M.; Marks, K.L.; Zañartu, M.; Mehta, D.D. Improved subglottal pressure estimation from neck-surface vibration in healthy speakers producing non-modal phonation. IEEE J. Sel. Top. Signal Process. 2020, 14, 449–460. [Google Scholar] [CrossRef]
- Marks, K.; Lin, J.Z.; Burns, J.A.; Hron, T.A.; Hillman, R.E.; Mehta, D.D. Estimation of subglottal pressure from neck surface vibration in patients with voice disorders. J. Speech Lang. Hear. Res. 2020, 63, 2202–2218. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehta, D.D.; Kobler, J.B.; Zeitels, S.M.; Zañartu, M.; Ibarra, E.J.; Alzamendi, G.A.; Manriquez, R.; Erath, B.D.; Peterson, S.D.; Petrillo, R.H.; et al. Direct Measurement and Modeling of Intraglottal, Subglottal, and Vocal Fold Collision Pressures during Phonation in an Individual with a Hemilaryngectomy. Appl. Sci. 2021, 11, 7256. https://doi.org/10.3390/app11167256
Mehta DD, Kobler JB, Zeitels SM, Zañartu M, Ibarra EJ, Alzamendi GA, Manriquez R, Erath BD, Peterson SD, Petrillo RH, et al. Direct Measurement and Modeling of Intraglottal, Subglottal, and Vocal Fold Collision Pressures during Phonation in an Individual with a Hemilaryngectomy. Applied Sciences. 2021; 11(16):7256. https://doi.org/10.3390/app11167256
Chicago/Turabian StyleMehta, Daryush D., James B. Kobler, Steven M. Zeitels, Matías Zañartu, Emiro J. Ibarra, Gabriel A. Alzamendi, Rodrigo Manriquez, Byron D. Erath, Sean D. Peterson, Robert H. Petrillo, and et al. 2021. "Direct Measurement and Modeling of Intraglottal, Subglottal, and Vocal Fold Collision Pressures during Phonation in an Individual with a Hemilaryngectomy" Applied Sciences 11, no. 16: 7256. https://doi.org/10.3390/app11167256





