Abstract
Active dressings acting on multiple fronts are requested in the field of care for chronic skin ulcers in order to ameliorate patient compliance and tissue restoration. Currently, three-dimensional polymeric hydrogels are widely investigated; however, no prototypes aiming to control oxidative stress and bacterial proliferation in the wound bed have been developed up until now. The present work describes the formulation of a novel chitosan-based printable material containing α-tocopherol at stable dosages to obtain reproducible 3D scaffolds possessing antioxidant and antimicrobial activity without the use of organic solvents. Stability assays mimicking the manufacturing process and storage conditions reveal no significant drug loss. Chemico-physical characterizations including porosity and behavior after dehydration/hydration demonstrate that the dressings are highly porous, can be dehydrated up to 80%, and can recover more than 90% of water upon 1 h of rehydration. Elasticity determined by stress/strain tests was higher than human skin and was sufficiently resistant for potential clinical manipulation. Footage of fibroblasts in in vitro cultures demonstrated the biocompatibility of the constructs over 28 days. Finally, scaffolds loaded with α-tocopherol showed dose-dependent antioxidant activity (up to 80% in less than 1 h), while antimicrobial action versus multi-drug resistant strains of Pseudomonas aeruginosa and Staphilococcus aureus was assessed by inhibition rings obtained through the Kirby–Bauer technique. The proposed hydrogels can be useful as dressings for the treatment of chronically infected wounds.
1. Introduction
Skin is the most extended organ of the human body, and it mainly works as a physical and selective barrier, protecting the body from the external environment and from mechanical traumas and microbial/viral infections in particular. Numerous pathological conditions associated with aging can directly or indirectly affect the skin, such as diabetes, peripheral neuropathy, venous insufficiency, and prolonged decubitus, often leading to the development of lesions which can deeply alter the functions of the skin [1,2,3].
Specifically, skin is a highly regenerative organ, the majority of which is composed of stem cells that in healthy conditions, continuously proliferate and differentiate [4]. However, in the case of the above-mentioned clinical pictures, their capability to re-grow correctly and to maintain their metabolic activity is significantly lowered and altered [5]. Repair mechanisms are deeply impaired by the scarce peripherical blood flow, by the damages to the micro vessels irrorating the deepest skin layers of the extremities, and by the low diffusion rate of nutrients through the tissue [6].
In physiological conditions, the restoring process normally takes place in a complicated sequence of four partially overlapping steps, including blood clotting and inflammation, which is necessary for the concomitant release of growth factors and cytokines, the generation of new tissue, and final remodeling [7,8].
During the inflammation phase, Reactive Oxygen Species (ROS) are produced by macrophages invading the wound site in large amounts, playing a crucial role as signaling molecules for angiogenesis induction as well as for working in the same way as antimicrobial compounds, hindering microbial infection [9]. On the other hand, excessive and sustained oxidative stress can contribute to the insurgence and persistence of chronic wounds [10] since ROS can damage healthy tissues due to their high chemical reactivity and their capability to oxidize cellular macromolecules [11].
Extra Cellular Matrix (ECM) proteins, indeed, are subject to destruction by ROS and proteases activity, and this results in uncertain, delayed, or impeded regeneration [12].
Moreover, this micro-environment predisposes the wound bed to infection from multidrug resistant species of hazardous bacteria pathogens commonly inhabiting clinical settings, such as Staphylococcus aureus and Pseudomonas aeruginosa. These lead to the development of pathological situations that require a high degree of care rather than frequent hospitalization, both representing a consistent burden for healthcare-related expenditures [13].
Detoxification from excesses of ROS occurs in vivo through specific detoxifying enzymes and through the action of endogenous (glutathione, ubiquinones, uric acid, lipoic acid) and exogenous (vitamin E, ascorbic acid, carotenoids, phenolic compounds) antioxidants. Furthermore, studies conducted over the last two decades indicate a strong correlation between the impaired healing of chronic wounds and low levels of antioxidants with a low molecular weight [14], suggesting that a supplementation of these compounds could enhance and ameliorate the regeneration process [15].
This hypothesis was confirmed by several specific experiments conducted on animal wound models; for instance, Musalmah et al. found that the administration of α-tocopherol (VitE) led to the drastic lowering of lipid peroxydes in diabetic mice, resulting in a significant enhancement of the wound healing process [16].
Clinically, the standard procedures for the management and treatment of chronic ulcers are represented by the periodical debridement of necrotic tissue and the surgical drainage of abscesses, the management of infections (antibiotic therapies), negative pressure sessions, and the application of medicated dressings [17]. The latter are more and more investigated and employed as adjuvant treatments since novel materials are continuously being developed as are the manufacturing techniques used to manipulate them.
Nowadays, several alternatives are widely available on the market, ranging from highly absorbent patches to moisturizing ones, produced by exploiting both naturally occurring or synthetic materials. Many of the aforementioned products are loaded with silver or other antimicrobial substances for the control of ongoing infections [18]. Nevertheless, these active ingredients often result in high toxicity and might delay cell proliferation and differentiation from the wound bed [19].
Among medicated dressings, active ones are conceived to promote healing by providing a biocompatible microenvironment that is able to host cell proliferation. The fundamental element of this approach is the presence of an ECM biomimetic scaffold: for this reason, the choice of the material is of primary importance in terms of physical form, hydration, mechanical strength, and porosity as well as shaping/structuration. Taking naturally derived polymers into account [20], chitosan is considered to be unique because of its combination of biological properties, such as hemostatic action, biocompatibility [21], and wide spectrum antimicrobial activity [22]. Furthermore, this cationic polysaccharide, which is composed of repeated sequences of acetyl-glucosamine and glucosamine, has a great chemical similarity with the glycosaminoglycans present in the ECM, prompting its use as a scaffolding material for tissue regeneration [23]. In the literature, other works have described the useful association of chitosan and vitamin E in wound dressings [24,25,26], but preparation methods include the use of other polymers and organic crosslinkers or gamma radiation to induce chitosan gelation, which could leave potentially toxic residues. In this work, a freeze-deposition 3D printing method is proposed, offering the chance to avoid toxic solvents while providing a controlled porosity of the hydrogel.
The three-dimensionality of chitosan scaffolds was recently investigated by Intini et al. [27] in terms of wound healing enhancement, exploiting it for their production of a freeze-deposition additive manufacturing system developed to obtain reproducible, micro-structured, biocompatible chitosan hydrogels, as previously reported by Elviri et al. [28]. The use of three-dimensional printing provides the chance to design more complex devices in terms of geometry and control of drug release towards the target of tailored personalized medicine with respect to traditional manufacturing processes [29,30].
Certain 3D chitosan constructs have also favored human fibroblast/keratinocyte adhesion and three-dimensional growth in vitro, spurring the cell proliferation rate compared to bi-dimensional sheets of the same material. Additionally, these patch prototypes implanted in vivo on a diabetic model in Wistar rats led to faster and improved wound healing [27].
In this work, the primary challenge was to develop a novel biocompatible three-dimensionally-structured dressing prototype including defined dosages of VitE. Moreover, the fine tuning of its chemico-physical properties, manipulability, and capability to control bacterial burden while providing antioxidant action would represent desirable features in order to potentially ameliorate the wound healing environment, boosting tissue reparation.
2. Materials and Methods
2.1. Evaluation of VitE Stability during the Manufacturing Process
2.1.1. A-Tocopherol Stability during Scaffold Production Process-Acid Degradation
A methanol:water 1:1 solution of DL-α-tocopherol (Ph.Eur. grade, from A.C.E.F., Fiorenzuola, Italy) was prepared at a concentration of 20 mg/mL in 2 mL amber vials. Glacial acetic acid was then added to each vial at the final concentration of 2% v/v (pH 2.4). Samples were diluted 1:1000 and were immediately analyzed over time at determined time intervals (0, 0.25 h, 1 h, 3 h, 6 h, and 24 h). The analysis was performed through high performance liquid chromatography using an LC Agilent 1200 binary pump (Agilent Technologies, Santa Clara, CA, USA) equipped with a UV/VIS detector (HPLC-UV/VIS). The experiment was conducted in triplicate.
Chromatographic separation was achieved on a C18 column (Alltech 5 μm; 150 mm × 4.6 mm, Columbia, MD, USA) thermostated at 25 °C; the mobile phase consisted of ultrapure water:methanol at the ratio 1:99. The elution was isocratic at a flow rate of 1500 μL/min, the detector wavelength was set at 292 nm, and the injection volume was 20 μL. The calibration curve was built by injecting standard amounts of VitE dissolved in methanol:water 1:1 ranging from 1 to 40 μg/mL (y = 4.8335x; R² = 0.999).
2.1.2. A-Tocopherol Stability during Scaffold Gelation-Alkaline Degradation
The stability of the VitE exposed to an alkaline environment was tested by simulating the gelation step of the manufacturing process. A methanol:water 1:1 solution of VitE at the concentration of 20 mg/mL was prepared in an amber 2 mL vial. A total of 10 µL of a 6 mM bromothymol blue (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) solution were added as a pH indicator (toning from yellow to blue in the pH range 6–7.5). There were eight exposure time intervals that were chosen to measure the vapors generated by a 28% ammonia solution (VWR Internationals Srl, Milano, Italy), ranging from 1 to 60 min. Pictures were collected at each time point in order to better evaluate toning over time.
Once the time needed for the ammonia to completely diffuse into the vials was determined, the alkaline degradation of VitE was in intervals up to 24 h by placing fresh opened samples in a desiccator saturated with ammonia vapors. Vials were thus removed at the chosen time points from the gelling reactor, diluted 1:1000 with methanol, and were analyzed according to the chromatographic method described in Section 2.1.1.
2.2. Formulation
2.2.1. Ink Preparation for 3D Printing
Chitosan powder (ChitoClear TM4030: DD 75.5%; Primex, Siglufjordur, Iceland) was dispersed in ultrapure water (UP) (0.055 μS/cm, TOC 1ppb Purelab pulse + Flex ultra-pure water; Elga Veolia, Milan, Italy) at the concentration of 6% w/v in a beaker and was magnetically stirred until homogeneous dispersion was achieved. Glacial acetic acid (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) was added dropwise to obtain a final concentration of 2% v/v. The blend was stirred for 12 h to achieve complete dissolution of the polymer. Raffinose was then added as a rheological agent at the concentration 290 mM, and stirring was conducted for a further 12 h to allow the sugar to dissolve completely [31]. The removal of gases from the resulting solution took place through the immersion of the sealed beaker in an ultrasonic bath for 30 min at 24 °C (Branson 2510; OPTO-LAB, Modena, Italy). The method was adopted according to the description of chitosan solution preparation for 3D printing reported by Elviri et al. [28].
Dispersions were prepared by weighing α-tocopherol (A.C.E.F., Fiorenzuola, Italy) and adding it to the chitosan solution to obtain final VitE concentrations of 1 and 0.1% w/v (from now on referred as VitE 1 and VitE 0.1). Afterwards the formulation was further magnetically stirred for 1 h and was finally homogenized through a homogenizer (Ultraturrax TP 18/10–10N; IKA-Werke GmbH, Staufen, Germany) for 20 min at 26,000 rpm using an 8 Gauge dispersing tool.
2.2.2. Stability over 48 h
Dispersions were intended to be used for printing within about 24 h. For this reason, stability tests were conducted at room temperature over this time period.
Aliquots of 5 mL of the VitE 1 and VitE 0.1 formulations were stored at room temperature (24 °C) for 48 h. At pre-fixed time points (0, 5, 19, 24, and 48 h) 4 withdrawals of 150 µL were made from each formulation, were dissolved in 1 mL of 2% acetic acid, were shaken by means of Vortex®, and were opportunely diluted with methanol until the achievement of a concentration range between 1 and 25 µg/mL. Standard solutions of VitE prepared in methanol as well as the prepared samples were injected in the range from 1 to 25 µg/mL, according to the chromatographic method reported in Section 2.1.1; a new calibration curve was plotted, and the corresponding regression equation calculated (y = 4.8335x; R² = 0.9982).
2.3. Production Process
2.3.1. Scaffold Design
Scaffold 3D models were drawn by means of the Computer Aided Design (CAD) software, SolidworksTM (Dassault systems, USA), which allows the creation of an “.stl” file (STereo Lithography interface format). The latter is further processed by a slicing program, Slic3rTM (RepRap), which generates a machine code (g.Code) file providing the information needed from the 3D printer software to build up the designed object.
The three-dimensional model was constructed by overlapping two figures presenting parallel strands and rotating them by 90°. In total, five layers were superimposed, forming an orthogonal grid with fixed distances between filaments of 200 μm and creating a three-dimensional “gauze”. The total sizes of the objects corresponded to 15, 15, and 1.3 mm for the width, length, and thickness, respectively (named as regular scaffolds). The cross section diameter taken from the strands was considered to be 260 µm for the design.
2.3.2. 3D Printer and Scaffold Manufacturing
The machinery employed in the experiment was designed and developed in-house ad hoc for the production of three-dimensional scaffolds based on naturally derived polymers [24]. This machinery is composed by the structure of a 3-axes Fused Deposition Manufacturing system to which Peltier cells and liquid/air exchangers were added in order to provide a plate for the instantaneous freezing of the deposited ink. The ink formulation used for scaffold realization was loaded into a 5 mL syringe and was properly fixed to the printer chassis.
The material was then extruded through a 26 G transversely cut needle (inner diameter 0.260 mm) by the application of controlled pression on the syringe through a mechanically assisted piston; robotic arms moved along three axes (x, y, z) following the directives imposed by the g.code file. Deposition took place on a stainless-steel plate cooled at −15/−10 °C by Peltier cells that instantaneously froze the filament at the moment of deposition, allowing the construction and maintenance of the three-dimensional item. Once completed, the frozen scaffold underwent a gelation reaction that permitted the 3D structure formed in the stiff hydrogel to stiffen within a short period of time. In particular, the process occurred by placing the frozen scaffold at 24 °C in a desiccator with the bottom filled with an ammonia solution 28% v/v that saturated the internal ambient vapors (pH 13) according to the gelation method described by Bergonzi et al. [32]. After 1 h, the scaffolds were immersed in UP water to eliminate ammonia residues and raffinose according to the method described by Bettini et al. [31]. Litmus paper was used to check the achievement of pH neutrality in the washing medium. Samples were always stocked in UP water (pH 7) in the fridge at 4 °C until they were needed for further use.
2.3.3. Effect of Disinfection Procedure on VitE Title
The experiment was conducted by keeping triplicates of the scaffolds soaked in 25 mL of 70% vol ethanol (VWR Internationa, Milan, Italy) for 0, 6, 18, and 24 h. At each time point, the scaffolds were picked up, rinsed twice for 10 min with UP water, and were gently blotted on filter paper for a few seconds for the removal of excess water. Samples were then prepared by dissolving each scaffold in 1 mL of 2% v/v acetic acid. Dissolution was assisted by shaking with the Vortex® for 2 min and then by opportunely diluting with methanol for chromatographic analysis.
The theoretical value of VitE in the produced scaffolds was calculated before experiments took place by weighting 10 scaffolds and deriving the mass of VitE. An experimental titer of VitE was determined using the HPLC-UV method reported in Section 2.1.2. Standard amounts of VitE in methanol ranging from 50 to 220 µg/mL were injected. A calibration curve was plotted, obtaining the equation y = 5.368x + 105.54 (R2 = 0.9758).
2.4. Dosage Uniformity Studies in Scaffolds
VitE1 scaffolds were placed on graph paper and cut in 4 equal parts using a sharp scalpel. Each scaffold quarter was blotted on filter paper to remove excess water from the hydrogel and were weighted on an analytical scale. The scaffold quarters were then singularly dissolved in 1 mL of 2% v/v acetic acid, stirred with the Vortex®, and diluted with methanol for HPLC-UV analysis. The experiment was conducted in triplicate. Data were used to evaluate VitE concentration in the scaffolds, and pie charts were plotted to visualize concentration distribution within the four quarters of the scaffolds and the overall types of specimens present. Data normalization was calculated through the formulas:
and
where [αT] equals the experimental concentration in each scaffold quarter, [αT] theoretical equals the theoretical concentration of each scaffold, and the scaffold weight % was obtained by relating the weight of the quarters to the whole scaffold weight.
Data were used to statistically compare the scaffold quarters of each scaffold. Reproducibility was evaluated by the comparison of the concentration means for each scaffold.
2.5. Chemico-Physical Characterization
2.5.1. Scaffold Rehydration Capability
For the experiment, 20 scaffolds for each dispersion (VitE 0.1; VitE1) were prepared. Each scaffold was produced and gelled following the production method and the disinfection previously described, thus undergoing gelation for 1 h in ammonia vapors and soaking for 1 h in the 70% ethanol sterilizing solution. Scaffolds were then washed twice for 10 min and were blotted on filter paper for excess water removal. Each scaffold was consequently weighted; the value of 100% of hydration was attributed to the weight obtained for the following calculations.
Scaffolds were then placed in a thermostatic oven in triplicate at 40 °C for 8, 20, 33, 40, and 960 min in order to obtain the hydration statuses of around 80, 60, 45, 30 and 10%. At each time point, the scaffolds were removed from the oven and were weighted on an analytical scale. Dehydration times were established according to previously reported studies [32]. After each weighing, the triplicates of the scaffolds were soaked in 10 mL of UP water for 1 h at 24 °C with the purpose of rehydrating them as much as possible. Finally, the scaffolds were picked up, were blotted on filter paper, and were weighed one more time.
Maximum rehydration in one hour was calculated as
and Strain (ε) was calculated as
where Δl equals the net linear elongation, and l equals the distance between clips.
The slope of the elastic portion (linear) of each curve was calculated, corresponding to the elastic modulus (Young’s modulus), derived from the formula:
2.5.2. Scanning Electron Microscopy (SEM) Characterization
Scaffolds with a concentration of 0.1 or 1% of VitE were freeze-dried for 24 h by a Christ Alpha 2–4 LSC plus Freeze-Dryer. Dried samples were mounted on aluminum stubs and were metallized to cover the scaffolds with a 60 nm thick gold film. Samples were observed with a Philips SEM501. Collected photographs were processed by means of the software ImageJ (ImageJ JTM) to evaluate pore size distribution on the surface and in cross sections of the scaffolds. Moreover, the analysis of the increasing concentrations of VitE in the scaffolds was performed with the purpose of evaluating whether concentration had an effect on the porosity and overall morphology.
Each image was analyzed by randomly collecting 150 pore Feret’s diameter measurements. For each image that was analyzed, pore size categories were assigned. In particular, the smallest pore was considered to be the inferior limit of the first category; multiplying this value by √2 the superior limit was also defined. The same mathematical correlation was adopted for all of the following increasing categories up to the biggest pore value (superior limit of the last category). Each class included a certain number of pores that had a Feret’s diameter value included in it.
The number of pores included in each range was converted to a cumulative percentage value, and cumulative pore size distribution curves were plotted. At least 10 measurements of strand diameters and holes were collected of the top and the bottom of the scaffolds taken on micrographs at 40× of magnification by means of ImageJ software. Mean values and the standard deviation were reported and compared to theoretical CAD parameters (reported in Section 2.2).
2.6. Pharmacological Activity and Biocompatibility
2.6.1. Antioxidant Activity on 2,2-Diphenyl-1-Picrylhydrazyl (DPPH)
The antioxidant scavenging activity of the VitE loaded scaffolds was measured by a DPPH test [28]. VitE 0.1 and VitE 1 scaffolds were produced as described for the previous experiments. A test solution composed of 8.5 mL of acetonitrile, 1 mL of a stock solution of 1 mM DPPH in ethanol (final concentration 100 mM) and 0.5 mL of deionized water was prepared before the experiment. The test specimens (regular scaffolds) were placed in amber jars at the different concentrations (n = 3 per concentration) together with 10 mL of test solution and were stirred in the dark on an orbital shaker for 60 min at room temperature. A control sample only consisting of the test solution was measured as a control. At predetermined time intervals (each 20 min), 1 mL of each sample was collected and immediately analyzed. The absorbance of the collected samples was systematically read through a Perkin Elmer–Lambda 25 spectrophotometer measuring the absorbance at 517 nm. A solution of ethanol/UP water (95:5 v/v) was used as blank sample during the experiment. The scavenging effect at each time point was calculated to a percentage according to following equation:
where A0 is the absorbance of the control sample, and A is the absorbance in the presence of the sample at any time. De-coloration and the subsequent decrease of absorbance at 517 nm indicated a proportional antioxidant charge increase of the teste samples.
2.6.2. Cell Cultures on Scaffold with VitE
Briefly, human dermal fibroblasts (hDF), coded as C84, were isolated and expanded from an underarm explant from a healthy, normolipaemic 45-year-old female and were used at passage 26. hDF were cultured in a 75 cm2 cell culture flask in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), antibiotic solution (streptomycin 100 μg/mL and penicillin 100 U/mL, Sigma Chem. Co), and 2 mM L-glutamine. The hDF were incubated at 37 °C in a wet atmosphere with 5% CO2 and 95% air.
VitE 0.1 and VitE 1 scaffolds were stamped circularly (diameter 6 mm) before disinfection. Afterwards, scaffolds were washed twice with sterile UP water for 10 min and were then immersed in sterile Phosphate Buffer Saline (PBS) solution. After 1 h, the PBS was removed, and the scaffolds were placed in a 48 multi-well plate (Corning). The hDF suspension was inoculated on scaffolds at the concentration of 100 × 103 cells/well. Regular chitosan scaffolds free of VitE, as reported in a previous work by Elviri et al. [28], were used as controls. The number of scaffolds that were seeded was sufficient for cell viability evaluation in duplicate once a week over 4 weeks (28 days). Cell viability was evaluated by optical fluorescent microscopy; cells were marked with Calcein-AM 2 h before analysis. Pictures of living cells were collected systematically at each time point at 4 × magnification.
2.6.3. Antimicrobial Activity–Kirby Bauer Test
Antimicrobial activity evaluation against two strains of bacteria (Gram+ and Gram−) was assessed. Multi-drug resistant Staphilococcus aureus (ATCC 25923) and Pseudomonas aeruginosa (ATCC 27853) were employed. The diffusion disk method (or the Kirby–Bauer technique) was adopted and was modified ad hoc in order to assay the 3D-printed chitosan-based scaffolds.
VitE0.1 and VitE1 scaffolds were cut with circular 6 mm diameter stamps (same size as antibiogram discs). Samples were disinfected as reported in Section 2.3.3 and were subsequently stored in sterile water at 4 °C prior to the start of the experiment.
Bacteria were seeded in pure culture and inoculated in a Mueller Hinton Broth at 37 °C in aerobic conditions for 1–2 h (0.5 McFarland). The bacterial suspension was seeded through the use of sterile tampons on a Mueller Hinton Agar terrain (carefully covering the entire Petri dish). At this time, the scaffolds could be applied using sterile forceps. Each test was conducted in duplicate. The positive control consisted of a Petri dish seeded with bacteria but that free of scaffolds, while the negative control consisted of a non-seeded Petri dish incubated with the scaffolds in order to verify their potential contamination. Finally, all of the plates were incubated at 37 °C for 18–24 h. Result evaluation was based on inhibition ring presence/absence followed by its determination in terms of diameter. Bacterial sensibility to antimicrobial activity is dependent from inhibition ring dimensions.
2.6.4. Statistical Analysis and Graphs
Statistical analysis was performed with Microsoft Excel for Mac ver. 16. The obtained data were compared using a t-test by setting the significance as p < 0.05. Graphs were prepared with Kaleida Graph 4.5.2 (Synergy software).
3. Results
3.1. Vitamin E Stability Study throughout Whole Production Process of Scaffolds
The basic material, which consisted of the chitosan solution in acetic acid, presents acidic characteristics. On the opposite hand, the step next of 3D printing implies the exposition of the scaffold to an alkaline environment. The inclusion of VitE in the acid chitosan solution (pH 5) could lead to the degradation of the active principle. Its stability was thus investigated in a preliminary way, settling on a system simulating the formulation conditions in terms of pH. The stability of VitE in an acidic (pH 2.4) environment was tested over 24 h with no significant changes in the initial concentration (98 ± 2%). This result confirmed that the inclusion of VitE in the ink would not damage the molecule. Afterwards, alkaline degradation tests were conducted to assess if gelation step could affect VitE stability; the results showed that VitE was stable (99.7 ± 3.3%) at a basic pH (11.5) up to one hour after exposure to ammonia vapors, followed by a very fast degradation. On the basis of these results, a gelation time of 1 h under ammonia vapors was chosen to be suitable for 3D scaffold preparation.
A disinfection step was also taken into consideration as a possible passage affecting VitE scaffold content. The stability study of VitE under the disinfection process with ethanol (70%, v/v) indicated that the compound is released over time due to its solubility in organic solvents (log p = 12.2). The VitE concentration decreased linearly by 50 ± 1% within 6 h, while amount of vitamin E was not significantly reduced I the timer period leading up to one hour: for this reason, one hour of disinfection in 70% v/v ethanol was adopted as standard procedure.
Chitosan/vitamin E dispersions did not show any evident separation phase within 24 h, suggesting adequate stability in this period, which was confirmed by quantitative analysis performed over 48 h, indicating dosage uniformity (103 ± 5% and 98 ± 2%, respectively). To confirm that homogeneity was maintained throughout the process, an estimation of the VitE content and distribution was performed directly on the printed and disinfected scaffolds. In particular, scaffolds divided in quarters showed that their concentrations were not statistically different among the 12 samples that were tested, confirming the homogeneity of the formulation. Repeatability was also statistically confirmed since, as reported in Section 2.6.3, as a whole, the scaffolds that were analyzed in this experiment were not shown to be significantly different among each other.
3.2. Chemico-Physical Characterization
3.2.1. Scaffold Rehydration Capability
The total water content of scaffolds after gelation was calculated, resulting in 90.1 ± 0.5% and 88.7 ± 0.6% for the VitE 0.1 and VitE 1 formulations, respectively. By comparing the rehydration statuses of the VitE 1 scaffolds (Figure 1a), no statistical differences were observed independently from their initial dehydration status, with the exception of those that had been rehydrated from 11 ± 0.6% (p < 0.05), which reached a maximum rehydration of 82 ± 2.6%.
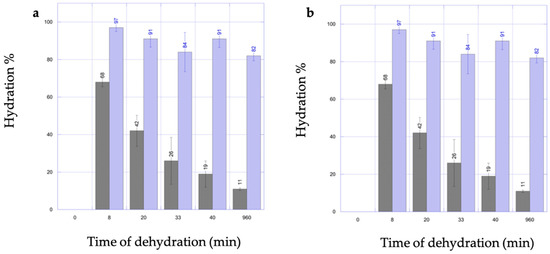
Figure 1.
Graphs showing VitE 1 (a) or VitE 0.1 (b) scaffold rehydration capability. Blue bars indicate hydration status after 1 h of soaking in UP water. Time refers to dehydration in oven. Gray bars = starting dehydration level; blue bars re-hydration level.
As for thee VitE 0.1 scaffolds, no statistical differences were observed in terms of maximum rehydration independently from the initial hydration state, as reported in Figure 1b.
Scaffolds of both formulations demonstrated the capability of being rehydrated at very high water percentages (over 80%). These results were achievable after a very short rehydration time (1 h), even when starting the process from very low hydration statuses (19–26%).
3.2.2. Elasticity
The VitE 0.1 and VitE 1 scaffolds showed analogue elasticity average values corresponding to 0.1 MPa ± 0.005 MPa and 0.1 MPa ± 0.02 MPa, respectively. Figure 2 shows the mechanical behavior of the scaffolds under longitudinal traction. The trend is completely linear for all of the scaffolds, indicating totally elastic behavior until the yield point.
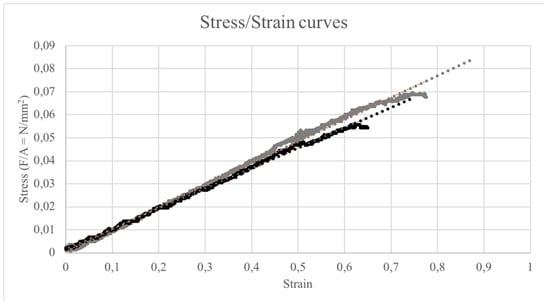
Figure 2.
Stress/Strain curves of VitE 0.1 (black dots) and VitE 1 (gray dots) under longitudinal traction.
3.2.3. Scanning Electron Microscopy (SEM) Characterization
The SEM analysis highlighted the presence of a porous network both on the surface area and in the cross section. In particular, the analysis of these images according to the method described (Section 2.5.2) showed that the surface area of the VitE 0.1 scaffolds was characterized by the pores having sizes between 4 and 14 µm, while for the VitE 1 scaffolds, the pore size was within 3 and 57 µm. In the cross section, the VitE 0.1 scaffolds showed pores in the range between 5 and 40 µm, while the VitE 1 scaffolds, similar to the characteristics of the pores observed on the surface, displayed a bigger pore size range, which was between 10 and 57 µm (Figure 3).
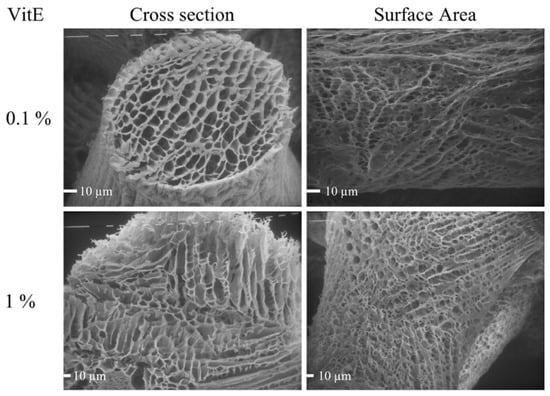
Figure 3.
SEM photographs of freeze-dried VitE 0.1 and VitE 1 scaffolds. View of surface area and cross section. VitE 0.1 Mag: 360×; VitE 1 Mag 640×.
During scaffold preparation for SEM analysis, a visible volumetric reduction was noticed after the freeze-drying process. It was calculated that the scaffold volume, once anhydrous, was reduced to 41.7 ± 3.2 %; it is reasonable to assume that the pores in the network were subject to an analogue reduction. The estimated real size ranges concerning surface area were 6–20 µm and 4–81 µm for VitE 0.1 and VitE 1, respectively, while the recalculated cross section ranges were between 7 and 57 µm and 14 and 81 µm for VitE 0.1 and VitE 1, respectively. Cumulative pore size distribution is shown in Figure 4.
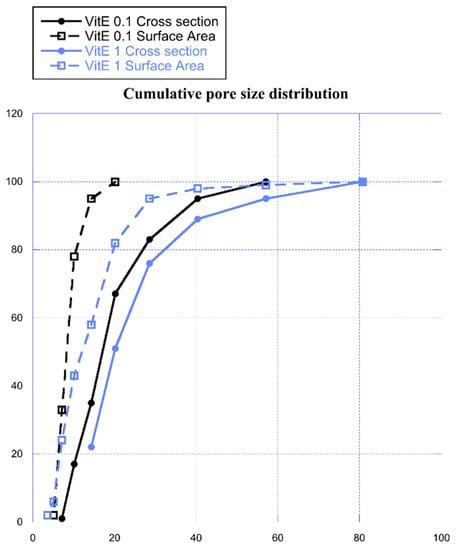
Figure 4.
Cumulative pore size distribution in cross section (dot) and on the surface (empty square) of VitE 0.1 (black) and VitE 1 (light blue) scaffolds.
From graph in Figure 4, it is clearly observable that the VitE 1 scaffolds have bigger pores compared to VitE 0.1. This result is evident both on the surface area and in the internal structure of the scaffold in the cross section.
As seen in Figure 5, the overall morphology and topography of the porous scaffold is maintained. A three-dimensional grid of orthogonally overlapping parallel strands form a micro-patterned gauze. In particular, measurements taken for strand sizes and holes (n = at least 10/each) indicated mean values of 180.7 µm ± 11.2 µm and 164.9 µm ± 16.5 µm, respectively. The volume correction made on the pore measurements was also applied here. An estimation of these measurements in their fully hydrated state would result in 256.1 µm ± 15.9 µm for strands and 233.7 µm ± 23.3 µm for the holes formed by the grid. Only data related to scaffolds VitE 1 are reported since the topographic parameters were exactly the same as for the VitE 0.1 set. Theoretical CAD model values were fixed at 260 µm for strand size and at 200 µm for holes, as described in the methods Section 2.3.1.
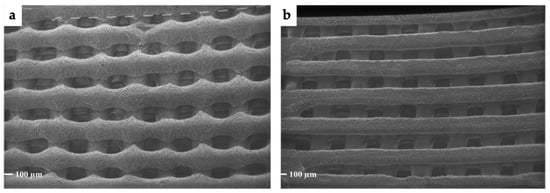
Figure 5.
SEM micrographs representing (a) the top of the 3D VitE 1 scaffold and (b) the bottom of the 3D VitE 1 scaffold. Magnification 40×. Magnification 40×.
3.3. Biocompatibility and Pharmacological Activity
3.3.1. Antioxidant Activity
In this work, the scavenging activity of VitE tested through DPPH assay demonstrated the active antioxidant capacity of the compound included in chitosan hydrogels. The scavenging activity of the VitE 0.1 samples were discrete, ranging from 35.5% after 5 min to a maximum of 39.1% at 47 min and did not show appreciable changes over time (Figure 6).
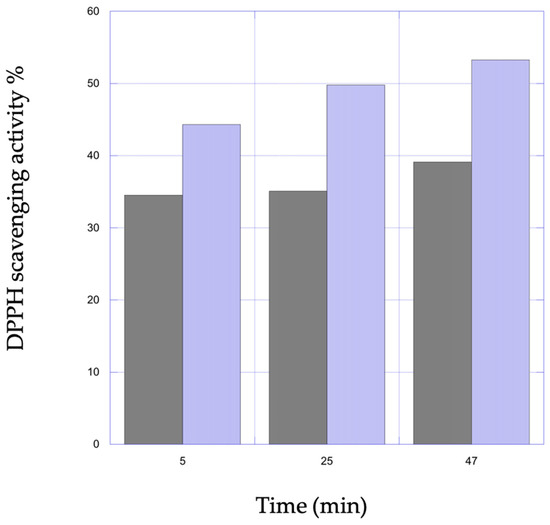
Figure 6.
Representation of percent scavenging activity on DPPH as a function of time of VitE 0.1 (black) and VitE 1 (light blue) scaffolds.
Antioxidant action for the VitE 1 scaffolds resulted in steady increase over time from the minimum value of 44.3% to a maximum of 53.3%. The VitE scaffolds loaded with a higher concentration suggested that scavenging activity could be dose dependent.
3.3.2. In Vitro Cell Cultures
The biocompatibility of the VitE/chitosan scaffolds was tested on human fibroblast cultures (Figure 7). Cells grown up to 28 days maintained the typical spindle morphology and remained close to confluence independently from the presence of VitE and from its concentration. By specifically observing images taken at day 7, it is possible to state that cells on the VitE 1 scaffolds proliferated with a certain delay with respect to the VitE 0.1 scaffolds and the control. At day 14, fluorescent clusters of cells started to be visible in all of the samples within the macro-pores. After day 21, confluence was achieved in all of the scaffold types, and the cell density was higher than previous sampling points, demonstrating high biocompatibility and excluding the hypothesis of toxic effects.
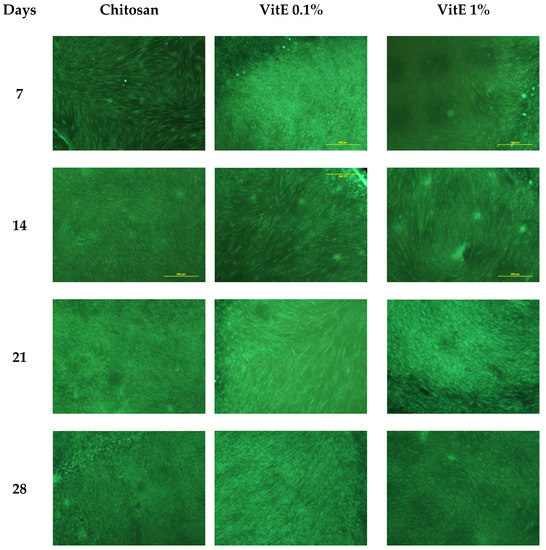
Figure 7.
Fluorescent microscopy (magnification 4×) of human fibroblasts cultured with 3D chitosan scaffolds (control) on VitE 0.1 and VitE 1, respectively.
3.3.3. Antimicrobial Activity
The efficacy of the disinfection procedure on the scaffolds was proved by the negative controls described in the experimental section.
Antimicrobial activity was assessed on multi-drug resistant species of Staphilococcus aureus (ATCC 25923) and Pseudomonas aeruginosa (ATCC 27853). The inhibition ring measured 8 mm for all of the tested samples. According to Zone Diameter Interpretive Standards (French Society of Microbiology 2001, Paris, France), these results would indicate no bacteria susceptibility of the VitE 1 and VitE 0.1 scaffolds (inhibition ring with an average ≥ 15 mm for susceptibility). However, no bacteria grew under the area covered by the scaffolds, independent of VitE concentration (Figure 8).
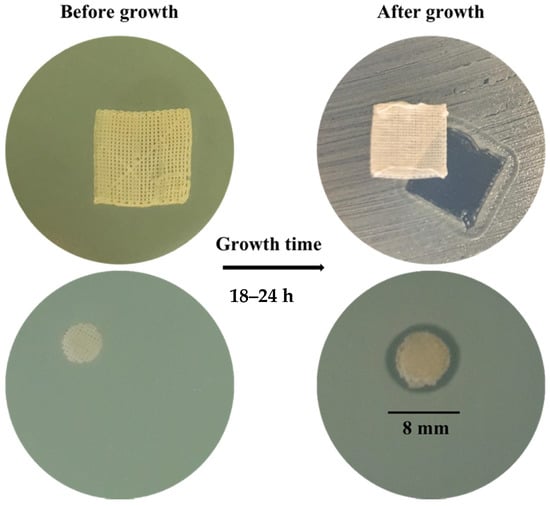
Figure 8.
Photographs representing test samples (entire scaffold); VitE 1 on Staphilococcus aureus.
4. Discussion
4.1. Stability Evaluation of VitE through Scaffold Production and Disinfection
This work aimed to set up a scaffold formulation procedure through 3D printing in order to obtain three-dimensionally structured chitosan hydrogels loaded with the antioxidant VitE at defined dosages. Liquid formulations based on chitosan were already successfully employed for the production of 3D hydrogels that resulted in hydrogels that are potentially employable for skin tissue regeneration purposes from in vitro and in vivo tests [27]. The modification of the material used as ink for 3D printing by its enrichment with biologically active compounds, such as supplementation with VitE, might ameliorate the general effectiveness of the scaffold by acting on multiple fronts. Moreover, the intrinsic antimicrobial activity of chitosan-based systems represents a desirable feature for prototypes of devices that can be easily contaminated in clinical settings by multi-drug resistant bacteria.
The preparation of dispersions intended for pharmaceutical purposes generally implies the conduction of investigations on their physical and chemical stability over time. In particular, the dispersions developed as inks in this work were conceived to be prepared by avoiding the use of surfactants as stabilizing agents since the majority of them often show toxic effects when in contact with human cells [33]. Gel viscosity was the only element counteracting the coalescence of the droplets: for this reason, the developed materials were only intended for use as pre-formulations for scaffold production through 3D printing to be used within 24 h of their preparation.
The scaffold production process implies the exposition of VitE to strong acidic and alkaline environments: these potentially critical chemical conditions needed to be investigated before its inclusion in the chitosan inks. In particular, acid degradation of VitE was assayed in order to evaluate its compatibility with the acidic chitosan solution. The obtained results indicated that VitE was chemically stable over 24 h even at a pH lower (pH 2.4) than the pH of the chitosan solution (pH 5). Afterwards, alkaline degradation tests showed that VitE was stable for up to one hour after exposure to ammonia vapors followed by a very fast degradation: for this reason, 60 min was selected as the maximum gelation time. The chosen time had already been demonstrated to be sufficient in order to obtain reproducible chitosan hydrogels with the right properties to be employed as scaffolds for soft tissue regeneration (i.e., biocompatibility, water content, elastic modulus) [32].
The overall stability and total drug content in the ink formulations of VitE 1 and VitE 0.1 were evaluated over a period of 48 h to ensure the physical stability and the homogeneity of the dispersion for a sufficient time for usage. VitE was homogeneously dispersed in the ink for 48 h in both formulations, and the VitE content was homogeneous in the scaffolds after the disinfection process.
Disinfection was also taken into consideration as a possible passage affecting the VitE content of the scaffolds. Due to the sensitivity of VitE to light, common sterilization under UV light could not be exploited, so disinfection using ethanol aqueous solutions was explored. VitE, as is the case with most oils, is soluble in alcohols, and given this fact, VitE content could be significantly lost. Stability tests demonstrated that after one hour, drug loss was not statistically significant compared to the drug content at time 0.
4.2. Chemico-Physical Characterization
The hydration state of a hydrogel is crucial for its effectiveness in terms of its capability to stimulate cell proliferation, absorb fluids, and maintain moisture [34]. The capability of a hydrogel to swell starting from different dehydration percentages is a desirable property in order to potentially use scaffolds at different wound stages depending on the need to keep the underlying tissue more moisturized or, on the opposite hand, to absorb large amounts of exudate while maintaining ambient moisture [34]. After the production/disinfection process, the scaffolds contained around 90% water, which is considered to be a high water content and is suitable for the biomimetic use of scaffolds directly on tissues. Interestingly, all of the scaffold types could be rehydrated by over 90% starting from hydration levels of 19–26%. Furthermore, a three-dimensional architecture could be restored perfectly. High percentages of water (around 80%) could be re-taken up even from harder dehydration conditions (10–11% of hydration), but in this case, it was noticed that the 3D structure was damaged and did not return back to a fully turgid state. This can constitute a limitation in view of a potential scale up of the dressing, for which a fully dehydrated state could be preferrable for packaging.
Materials that are clinically employed for skin regeneration purposes require a certain elasticity, both to favor their application and their adaptability in situ. For all of the conducted tests, the elastic modulus indicated that the material was significantly more elastic (about ten times higher) than human skin, that is, by about 1 MPa [35].
Porosity is widely reported to be one out of the five most important requirements for a hydrogel scaffold for tissue regeneration [36]. An accurate analysis of SEM micrographs revealed that an interconnected porous network was present both on the surface and in the inner part of the scaffolds. Interconnected pores separated by thin membranes, as observable in Figure 3, can easily permit gas and small nutrient diffusion. Pores were shown to have dimension ranges compatible with human cell attachment and proliferation [37]. In particular, the cumulative pore size distribution reported in Figure 4 clearly shows that the VitE 1 pores were bigger on an average and presented wider size ranges compared to the VitE 0.1 scaffolds. This phenomenon could be explained by the higher concentration of the material inside the VitE 1 scaffolds. In fact, this means a lower thermic conductivity, and thus, a slower freezing rate. Since it is known in literature that freezing velocity has a direct impact on the formation of ice crystals and on their dimensions [37], provided that the pores are formed by ice formation, it is possible to conclude that an higher concentration of VitE may lead to the formation of slightly bigger pores.
Three-dimensionally structured chitosan-based hydrogels, as reported in the previously work mentioned [27], might boost human fibroblast proliferation on/through the scaffold compared to bi-dimensional objects of the same material. As a consequence, the maintenance of the three-dimensional structure compared to the CAD model becomes of primary importance for the prediction of cell behavior. Data reported in Section 3.2.3 show evidence that the correspondence between the hydrogel and the model was quite well obtained and also depict the high reproducibility of the technique used as well as its fidelity in creating three-dimensionally structured chitosan-based hydrogels. Moreover, it is reported in the literature that three-dimensional patterned hydrogels with similar holes sizes stimulated angiogenesis and the formation of new blood vessels in vivo [37].
4.3. Biocompatibility and Pharmaceutical Activity
Toxicity is among the one most common issues related to the fabrication of biomimetics, particularly if they are designed to be left in situ for long periods [38]. Cell cultures with human fibroblasts were designed to evaluate the potential cytotoxicity of VitE 0.1 and VitE 1 scaffolds. Cytotoxicity evaluation usually takes 48 h maximum; in this case, both the proliferation through the scaffolds and the possible cell toxicity over long periods of time were taken into consideration. Excellent biocompatibility was overall demonstrated, and in fact, no signs of toxicity were evidenced over the 28 days of cultures in any of the tested scaffolds. Interestingly, the cells started to adhere to the 3D scaffold surfaces with a little delay in the VitE 1 scaffolds, and, in general, all of the scaffold types seemed to adhere more and more over time on the substrate (Figure 7). A short delay in terms of attachment followed by boosted proliferation was also already observed and measured in the previously work referenced [27].
VitE, apart from showing no toxicity, was demonstrated to be a good radical scavenger. In fact, a discrete antioxidant charge was observed after 5 min in all of the samples. In particular, VitE 1 increased its antioxidant activity over 47 min of analysis, while VitE 0.1 activity remained constant. The idea that the wider reservoir of VitE in the scaffolds might lead to a more prolonged antioxidant activity should not be excluded. Moreover, organic solvents were used in this experimental model to accelerate the reaction and to measure it in a reliable way; however, the scaffolds would not be applied in a similar environment. Indeed, VitE quickly dissolves in organic solvents, favoring its release from the scaffolds. In accordance with dissolution in ethanol, it is quite probable that in less than one hour, only a small amount of the drug would have been released, suggesting that once applied in situ, being that the release much slower, the antioxidant activity could be yielded in a lower amount and over a longer time. The demonstrated antioxidant activity of the VitE scaffolds might create a favorable environment for the development of re-growing tissue in wounds.
The test was conducted using scaffolds as large as antibiogram disks and clearly showed that no bacteria grew under the scaffold area. Inhibition ring measurements were only 2 mm wider than the diameter of the disks, probably due to the poor solubility of VitE in the culture medium. Indeed, the VitE scaffolds were not conceived as systems intended to freely release the compound but rather were intended as systems incorporating it. This approach is compatible with intrinsic chitosan scaffold degradability over long periods. Since chitosan is the agent that is supposed to provide antimicrobial activity and since its biodegradation velocity due to enzymes present in vivo is too complicated to be generally defined [39], its actual antimicrobial activity should be measured in an environment that is more representative of chronic wounds. Good signs of antimicrobial potential for the chitosan-based scaffolds containing VitE were still evident from the experimental results.
5. Conclusions
In this work, two types of chitosan-based inks with VitE were formulated for the 3D printing of scaffolds with antioxidant activity to help wound healing in chronic wounds. The production process did not affect VitE stability and led to the manufacturing of scaffolds possessing adequate physico-chemical and mechanical characteristics to be applied to damaged skin. The dehydration of scaffolds could not be pushed to 100% without partially altering their structure, but dehydration up to 80% guaranteed a full recovery of the shape with the potential to absorb wound fluids. From a pharmacological point of view, efficient antioxidant activity was provided through the presence of vitamin E together with long-term biocompatibility. Antimicrobial activity was moderate, but this could be potentially improved by the addition of further active compounds thanks to the flexibility of 3D printing technology, provided that their stability in the manufacturing process is demonstrated.
Author Contributions
Conceptualization, L.E. and C.B.; validation, A.B. and G.R.; formal analysis, C.B., C.M. and F.Z.; investigation, C.B., G.R., C.M., F.Z. and M.C.O.; resources, F.B. and R.B.; writing—original draft preparation, C.B. and A.B.; writing—review and editing, A.B. and L.E.; supervision, L.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alexiadou, K.; Doupis, J. Management of diabetic foot ulcers. Diabetes Ther. 2012, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, D.; Trepman, E.; Embil, J.M. Health-related quality of life in diabetic patients with foot ulcers: Literature review. J. Wound Ostomy Cont. Nurs. 2005, 32, 368–377. [Google Scholar] [CrossRef]
- Bergqvist, D.; Lindholm, C.; Nelzén, O. Chronic leg ulcers: The impact of venous disease. J. Vasc. Surg. 1999, 29, 752–755. [Google Scholar] [CrossRef]
- Ojeh, N.; Pastar, I.; Tomic-Canic, M.; Stojadinovic, O. Stem Cells in Skin Regeneration, Wound Healing, and Their Clinical Applications. Int. J. Mol. Sci. 2015, 16, 25476–25501. [Google Scholar] [CrossRef]
- Ammons, M.C.B.; Morrissey, K.; Tripet, B.P.; Leuven, J.T.V.; Han, A.; Lazarus, G.S.; Zenilman, J.M.; Stewart, P.S.; James, G.A.; Copié, V. Biochemical Association of Metabolic Profile and Microbiome in Chronic Pressure Ulcer Wounds. PLoS ONE 2015, 10, e0126735. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.C. The causes of skin damage and leg ulceration in chronic venous disease. Int. J. Low Extrem. Wounds 2006, 5, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Dissemond, J.; Goos, M.; Wagner, S.N. The role of oxidative stress in the pathogenesis and therapy of chronic wounds. Hautarzt 2002, 53, 718–723. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharm. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Derm. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Rasik, A.M.; Shukla, A. Antioxidant status in delayed healing type of wounds. Int. J. Exp. Pathol. 2000, 81, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Musalmah, M.; Fairuz, A.H.; Gapor, M.T.; Ngah, W.Z.W. Effect of vitamin E on plasma malondialdehyde, antioxidant enzyme levels and the rates of wound closures during wound healing in normal and diabetic rats. Asia Pac. J. Clin. Nutr. 2002, 11 (Suppl. S7), S448–S451. [Google Scholar] [CrossRef] [PubMed]
- Musalmah, M.; Nizrana, M.Y.; Fairuz, A.H.; NoorAini, A.H.; Azian, A.L.; Gapor, M.T.; Wan Ngah, W.Z. Comparative effects of palm vitamin E and alpha-tocopherol on healing and wound tissue antioxidant enzyme levels in diabetic rats. Lipids 2005, 40, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Ziegler, K.; Görl, R.; Effing, J.; Ellermann, J.; Mappes, M.; Otten, S.; Kapp, H.; Zoellner, P.; Spaeth, D.; Smola, H. Reduced cellular toxicity of a new silver-containing antimicrobial dressing and clinical performance in non-healing wounds. Ski. Pharm. Physiol. 2006, 19, 140–146. [Google Scholar] [CrossRef]
- Bianchera, A.; Catanzano, O.; Boateng, J.; Elviri, L. The Place of Biomaterials in Wound Healing. In Therapeutic Dressings and Wound Healing Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 337–366. [Google Scholar]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Ding, Z.Z.; Ma, J.; He, W.; Ge, Z.L.; Lu, Q.; Kaplan, D.L. Simulation of ECM with Silk and Chitosan Nanocomposite Materials. J. Mater. Chem. B Mater. Biol. Med. 2017, 5, 4789–4796. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Bilgic, M.B.; Lacin, N.T.; Berber, H.; Mansuroglu, B. In vitro evaluation of alpha-tocopherol loaded carboxymethylcellulose chitosan copolymers as wound dressing materials. Mater. Technol. 2019, 34, 386–393. [Google Scholar] [CrossRef]
- Nasef, S.M.; Khozemy, E.E.; Kamoun, E.A.; El-Gendi, H. Gamma radiation-induced crosslinked composite membranes based on polyvinyl alcohol/chitosan/AgNO3/vitamin E for biomedical applications. Int. J. Biol. Macromol. 2019, 137, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R.; et al. 3D-printed chitosan-based scaffolds: An in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Elviri, L.; Foresti, R.; Bergonzi, C.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R. Highly defined 3D printed chitosan scaffolds featuring improved cell growth. Biomed. Mater. 2017, 12, 045009. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials–Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef]
- Singh, T.; Kumar, S.; Sehgal, S. 3D printing of engineering materials: A state of the art review. Mater. Today Proc. 2020, 28, 1927–1931. [Google Scholar] [CrossRef]
- Bettini, R.; Romani, A.A.; Morganti, M.M.; Borghetti, A.F. Physicochemical and cell adhesion properties of chitosan films prepared from sugar and phosphate-containing solutions. Eur. J. Pharm. Biopharm. 2008, 68, 74–81. [Google Scholar] [CrossRef]
- Bergonzi, C.; Di Natale, A.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R.; Elviri, L. Study of 3D-printed chitosan scaffold features after different post-printing gelation processes. Sci. Rep. 2019, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Karasulu, H.Y. Microemulsions as novel drug carriers: The formation, stability, applications and toxicity. Expert Opin. Drug Deliv. 2008, 5, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Okan, D.; Woo, K.; Ayello, E.A.; Sibbald, G. The role of moisture balance in wound healing. Adv. Ski. Wound Care 2007, 20, 39–53, quiz 53–55. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, R.; Sherratt, M.J.; Cruickshank, J.K.; Derby, B. Characterizing the elastic properties of tissues. Mater. Today 2011, 14, 96–105. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Lim, T.C.; Chian, K.S.; Leong, K.F. Cryogenic prototyping of chitosan scaffolds with controlled micro and macro architecture and their effect on in vivo neo-vascularization and cellular infiltration. J. Biomed. Mater. Res. A 2010, 94, 1303–1311. [Google Scholar] [CrossRef]
- Kang, B.-C.; Kang, K.-S.; Lee, Y.-S. Biocompatibility and long-term toxicity of InnoPol implant, a biodegradable polymer scaffold. Exp. Anim. 2005, 54, 37–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).