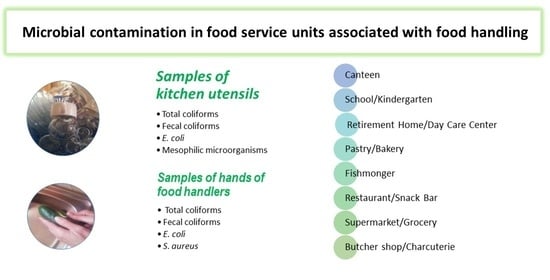
Microbiological Contamination in Different Food Service Units Associated with Food Handling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting and Data Collection
2.2. Microbiological Parameters Assessed
2.3. Data Processing
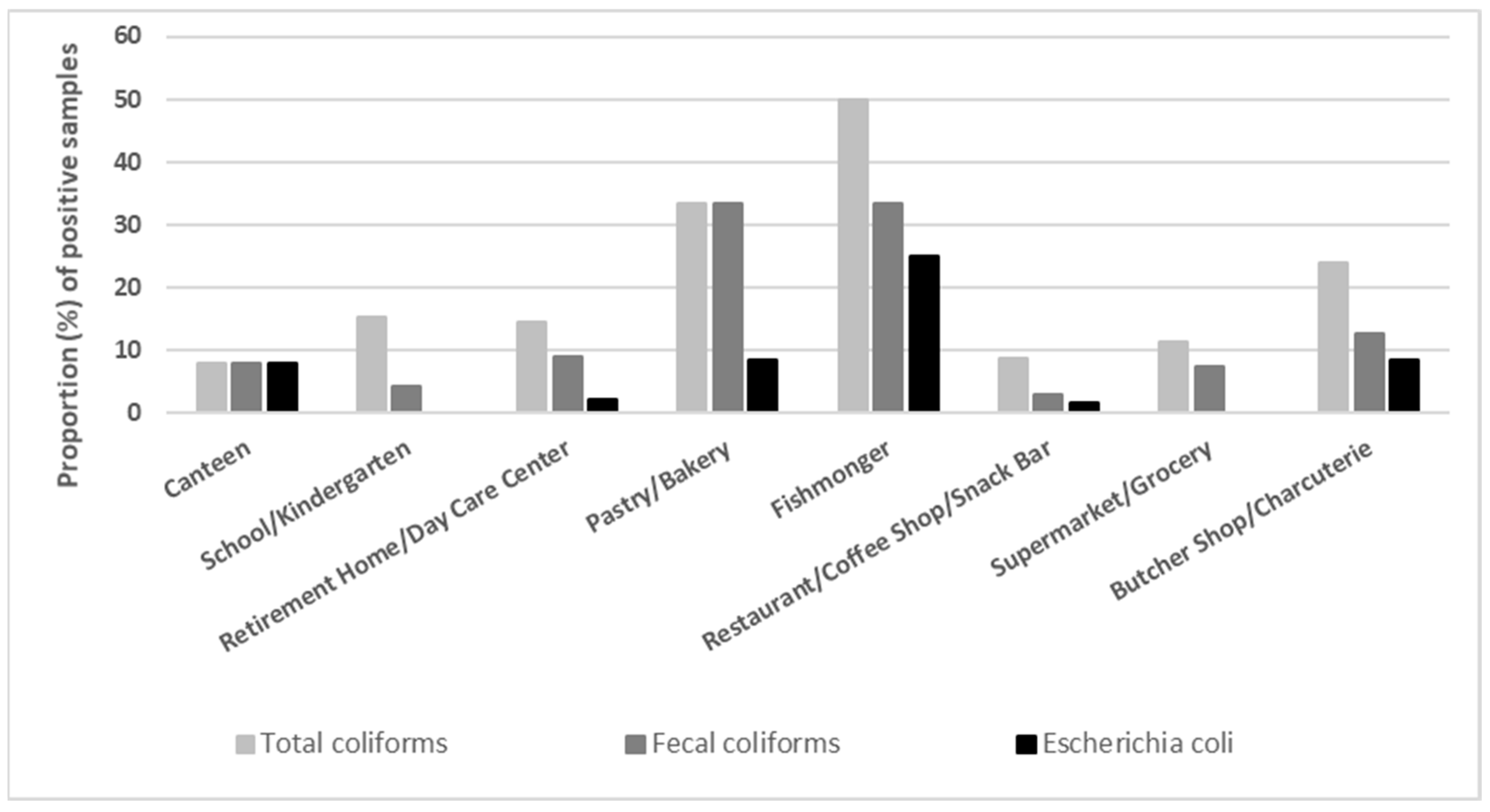
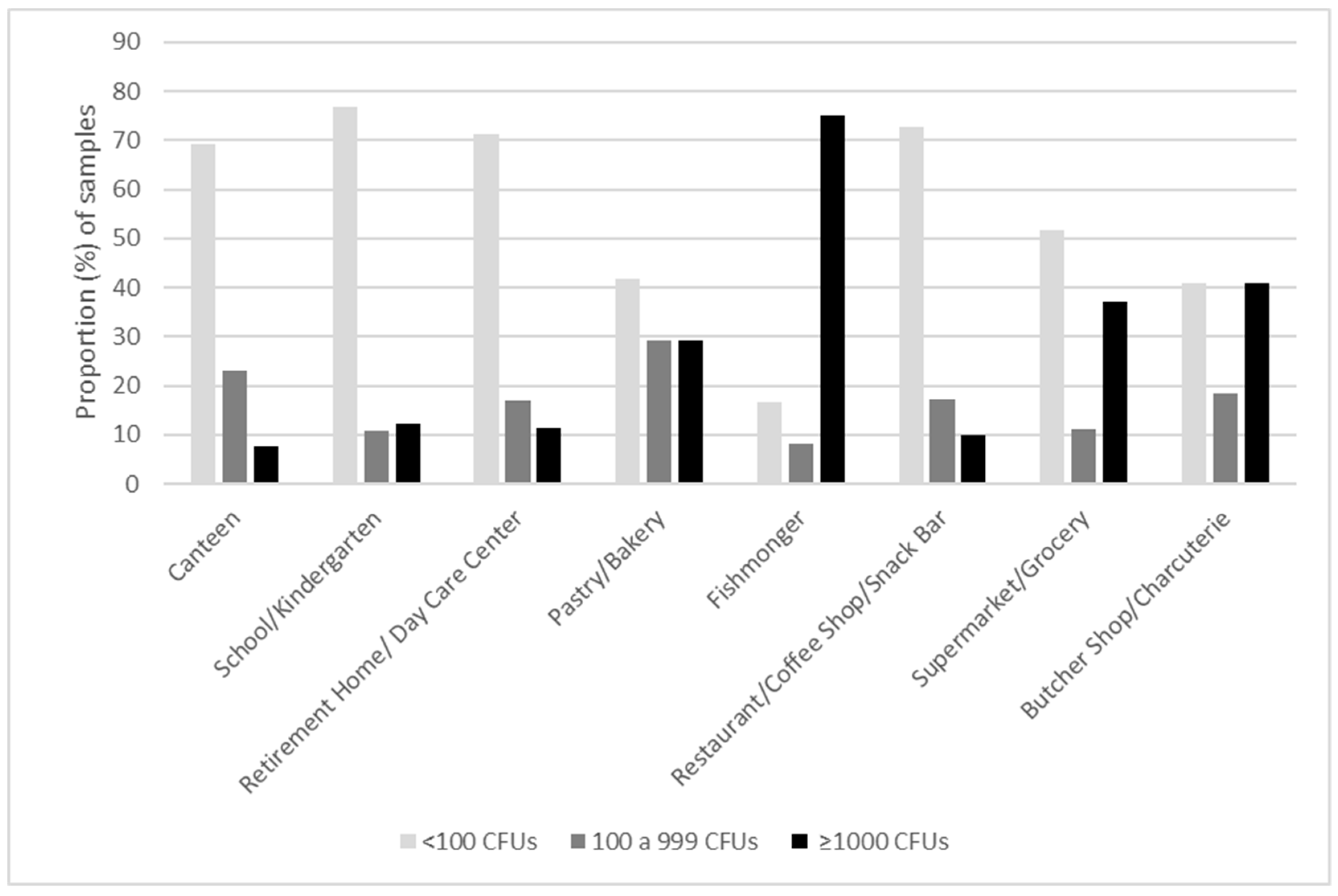
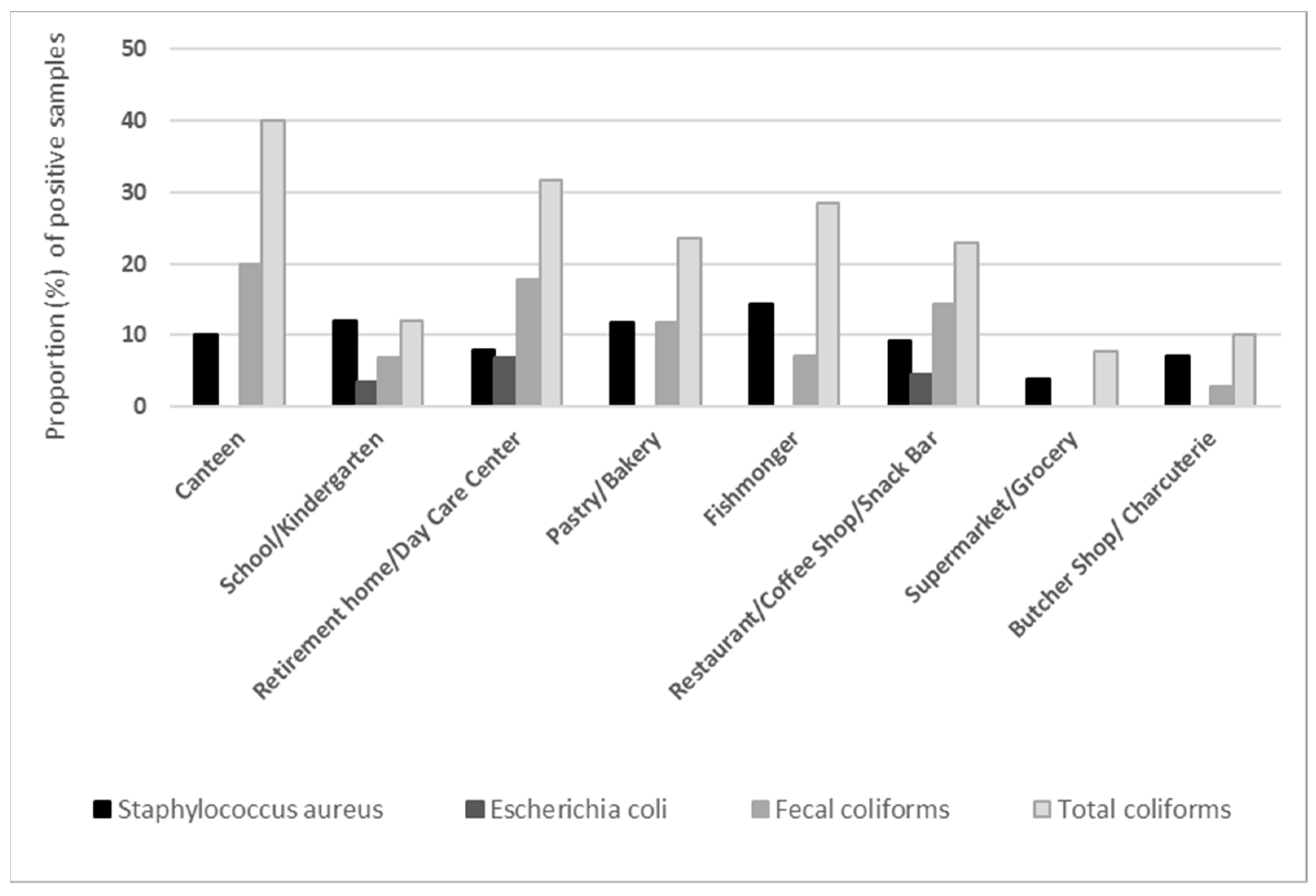
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viegas, S. Alterações do Estado de Saúde Associadas à Alimentação—Contaminação Microbiológica dos Alimentos. 2010. Available online: http://hdl.handle.net/10400.18/143 (accessed on 18 June 2021).
- WHO. WHO Estimates of the Global Burden of Foodborne Diseases: Executive Summary. WHO Executive Summary, 257. 2015. Available online: http://www.who.int/foodsafety/publications/foodborne_disease/fergreport/en/ (accessed on 18 June 2021).
- Fung, F.; Wang, H.-S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Valero, A.; Rodríguez, M.-Y.; Posada-Izquierdo, G.D.; Pérez-Rodríguez, F.; Carrasco, E.; García-Gimeno, R.M. Risk Factors Influencing Microbial Contamination in Food Service Centers. In Significance, Prevention and Control of Food Related Diseases; IntechOpen Limited: London, UK, 2016; Available online: https://dx.doi.org/10.5772/63029 (accessed on 18 June 2021).
- Augustin, J.-C.; Kooh, P.; Bayeux, T.; Guillier, L.; Meyer, T.; Silva, N.J.-D.; Villena, I.; Sanaa, M.; Cerf, O.; on Behalf of the Anses Working Group on Consumer Information on Foodborne Biological Risks. Contribution of Foods and Poor Food-Handling Practices to the Burden of Foodborne Infectious Diseases in France. Foods 2020, 9, 1644. [Google Scholar] [CrossRef] [PubMed]
- Dantas, S.T.A.; Rossi, B.F.; Bonsaglia, E.C.R.; Castilho, I.G.; Hernandes, R.T.; Fernandes, A.; Rall, V.L.M.; Júnior, A.F. Cross-Contamination and Biofilm Formation by Salmonella enterica Serovar Enteritidis on Various Cutting Boards. Foodborne Pathog. Dis. 2018, 15, 81–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.Y.H.; Nishibuchi, M.; Nakaguchi, Y.; Ghazali, F.M.; Saleha, A.A.; Son, R. Transfer of Campylobacter jejuni from raw to cooked chicken via wood and plastic cutting boards. Lett. Appl. Microbiol. 2011, 52, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Garayoa, R.; Abundancia, C.; Díez-Leturia, M.; Vitas, A.I. Essential tools for food safety surveillance in catering services: On-site inspections and control of high risk cross-contamination surfaces. Food Control 2017, 75, 48–54. [Google Scholar] [CrossRef]
- Byrd-Bredbenner, C.; Berning, J.; Martin-Biggers, J.; Quick, V. Food Safety in Home Kitchens: A Synthesis of the Literature. Int. J. Environ. Res. Public Health 2013, 10, 4060–4085. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, M.J.; Ferreira, V.; Truninger, M.; Maia, R.; Teixeira, P. Cross-contamination events of Campylobacter spp. in domestic kitchens associated with consumer handling practices of raw poultry. Int. J. Food Microbiol. 2021, 338, 108984. [Google Scholar] [CrossRef]
- Adhikari, U.; Esfahanian, E.; Mitchell, J.; Charbonneau, D.; Song, X.; Lu, Y. Quantitation of Risk Reduction of E. coli Transmission after Using Antimicrobial Hand Soap. Pathogens 2020, 9, 778. [Google Scholar] [CrossRef]
- Yap, M.; Chau, M.L.; Hartantyo, S.H.P.; Oh, J.Q.; Aung, K.T.; Gutiérrez, R.A.; Ng, L.C. Microbial Quality and Safety of Sushi Prepared with Gloved or Bare Hands: Food Handlers’ Impact on Retail Food Hygiene and Safety. J. Food Prot. 2019, 82, 615–622. [Google Scholar] [CrossRef]
- Ho, J.; Boost, M.; O’Donoghue, M. Sustainable reduction of nasal colonization and hand contamination with Staphylococcus aureus in food handlers, 2002–2011. Epidemiol. Infect. 2015, 143, 1751–1760. [Google Scholar] [CrossRef]
- Castro, A.; Santos, C.; Meireles, H.; Silva, J.; Teixeira, P. Food handlers as potential sources of dissemination of virulent strains of Staphylococcus aureus in the community. J. Infect. Public Health 2016, 9, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Mahros, M.A.; Abd-Elghany, S.M.; Sallam, K.I. Multidrug-, methicillin-, and vancomycin-resistant Staphylococcus aureus isolated from ready-to-eat meat sandwiches: An ongoing food and public health concern. Int. J. Food Microbiol. 2021, 346, 109165. [Google Scholar] [CrossRef]
- Kroning, I.S.; Iglesias, M.A.; Sehn, C.P.; Valente Gandra, T.K.; Mata, M.M.; da Silva, W.P. Staphylococcus aureus isolated from handmade sweets: Biofilm formation, enterotoxigenicity and antimicrobial resistance. Food Microbiol. 2016, 58, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.D.; Walsh, K.A.; Gould, L.H. Foodborne disease outbreaks caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus—United States, 1998–2008. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 57, 425–433. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004. On the Hygiene of Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2004/852/oj (accessed on 20 July 2021).
- Osimani, A.; Aquilanti, L.; Tavoletti, S.; Clementi, F. Evaluation of the HACCP System in a University Canteen: Microbiological Monitoring and Internal Auditing as Verification Tools. Int. J. Environ. Res. Public Health 2013, 10, 1572–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duthoo, E.; Krings, S.; Daube, G.; Leroy, F.; Taminiau, B.; Heyndrickx, M.; De Reu, K. Monitoring of Hygiene in Institutional Kitchens in Belgium. J. Food Prot. 2020, 83, 305–314. [Google Scholar] [CrossRef]
- Atnafie, B.; Paulos, D.; Abera, M.; Tefera, G.; Hailu, D.; Kasaye, S.; Amenu, K. Occurrence of Escherichia coli O157:H7 in cattle feces and contamination of carcass and various contact surfaces in abattoir and butcher shops of Hawassa, Ethiopia. BMC Microbiol. 2017, 17, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F.; et al. Food-borne diseases—The challenges of 20years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139, S3–S15. [Google Scholar] [CrossRef]
- Wilson, D.; Dolan, G.; Aird, H.; Sorrell, S.; Dallman, T.J.; Jenkins, C.; Robertson, L.; Gorton, R. Farm-to-fork investigation of an outbreak of Shiga toxin-producing Escherichia coli O157. Microb. Genom. 2018, 4, e000160. [Google Scholar] [CrossRef]
- Gil, A.I.; Lanata, C.F.; Hartinger, S.M.; Mäusezahl, D.; Padilla, B.; Ochoa, T.J.; Lozada, M.; Pineda, I.; Verastegui, H. Fecal contamination of food, water, hands, and kitchen utensils at the household level in rural areas of Peru. J. Environ. Health 2014, 76, 102. [Google Scholar]
- Simões, J.A.; Augusto, G.F.; Fronteira, I.; Hernández-Quevedo, C. Portugal: Health system review. Health Syst. Transit. 2017, 19, 1–184. Available online: https://www.euro.who.int/__data/assets/pdf_file/0007/337471/HiT-Portugal.pdf (accessed on 30 July 2021).
- INSA. Interpretação de Resultados de Ensaios Microbiológicos em Alimentos Prontos Para Consumo e em Superfícies do Ambiente de Preparação e Distribuição Alimentar: Valores Guia; INSA: Lisboa, Portugal, 2019. [Google Scholar]
- European Parliament and Council. Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002. Laying down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying down Procedures in Matters of Food Safety. Available online: https://eur-lex.europa.eu/eli/reg/2002/178/oj (accessed on 20 July 2021).
- INSA. Monitorização do Estado Higiénico de Louças e Talheres em Contacto com Alimentos; INSA: Porto, Portugal, 2001. [Google Scholar]
- INSA. Monitorização do Estado Higiénico de Mãos e Mãos com Luvas; INSA: Porto, Portugal, 2001. [Google Scholar]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Salmela, S.P.; Fredriksson-Ahomaa, M.; Hatakka, M.; Nevas, M. Microbiological contamination of sheep carcases in Finland by excision and swabbing sampling. Food Control 2013, 31, 372–378. [Google Scholar] [CrossRef]
- Bakhtiary, F.; Sayevand, H.R.; Remely, M.; Hippe, B.; Hosseini, H.; Haslberger, A.G. Evaluation of Bacterial Contamination Sources in Meat Production Line. J. Food Qual. 2016, 39, 750–756. [Google Scholar] [CrossRef]
- Wang, S.; Fu, L.; Chen, G.; Xiao, H.; Pan, D.; Shi, R.; Yang, L.; Sun, G. Multisite survey of bacterial contamination in ready-to-eat meat products throughout the cooking and selling processes in urban supermarket, Nanjing, China. Food Sci. Nutr. 2020, 8, 2427–2435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoslavskij, A.; Terentjeva, M.; Eizenberga, I.; Valciņa, O.; Bartkevičs, V.; Bērziņš, A. Major foodborne pathogens in fish and fish products: A review. Ann. Microbiol. 2016, 66, 1–15. [Google Scholar] [CrossRef]
- Kalimuddin, S.; Chen, S.; Lim, C.T.K.; Koh, T.H.; Tan, T.Y.; Kam, M.; Wong, C.W.; Mehershahi, K.S.; Chau, M.L.; Ng, L.C.; et al. 2015 Epidemic of Severe Streptococcus agalactiae Sequence Type 283 Infections in Singapore Associated with the Consumption of Raw Freshwater Fish: A Detailed Analysis of Clinical, Epidemiological, and Bacterial Sequencing Data. Clin. Infect. Dis. 2017, 64, S145–S152. [Google Scholar] [CrossRef] [Green Version]
- Kotzekidou, P. Microbiological examination of ready-to-eat foods and ready-to-bake frozen pastries from university canteens. Food Microbiol. 2013, 34, 337–343. [Google Scholar] [CrossRef]
- Sato, Y.; Ishihara, M.; Nakamura, S.; Fukuda, K.; Kuwabara, M.; Takayama, T.; Hiruma, S.; Murakami, K.; Fujita, M.; Yokoe, H. Comparison of Various Disinfectants on Bactericidal Activity Under Organic Matter Contaminated Environments. Biocontrol Sci. 2019, 24, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Mumma, J.; Simiyu, S.; Aseyo, E.; Anderson, J.; Czerniewska, A.; Allen, E.; Dreibelbis, R.; Baker, K.K.; Cumming, O. The Safe Start trial to assess the effect of an infant hygiene intervention on enteric infections and diarrhoea in low-income informal neighbourhoods of Kisumu, Kenya: A study protocol for a cluster randomized controlled trial. BMC Infect. Dis. 2019, 19, 1066. [Google Scholar] [CrossRef] [Green Version]
| Samples Taken from Kitchen Utensils/Crockery n = 649 | Samples Taken from Hands of Food Handlers n = 471 | ||||||
|---|---|---|---|---|---|---|---|
| Type of Food Service | Total | Unsatisfactory | Bad | p-Value | Total | Unsatisfactory | p-Value |
| n | n (%) | n (%) | n | n (%) | |||
| Canteen | 13 | 3 (23.1) | 1 (7.7) | <0.001 * | 10 | 4 (40.0) | 0.026 ǂ |
| School/Kindergarten | 73 | 17 (23.3) | 3 (4.1) | 58 | 14 (24.1) | ||
| Retirement Home/Day Care Center | 147 | 31 (21.1) | 13 (8.8) | 101 | 38 (37.6) | ||
| Pastry/Bakery | 24 | 6 (25.0) | 8 (33.3) | 17 | 5 (29.4) | ||
| Fishmonger | 12 | 4 (50.0) | 6 (33.3) | 14 | 4 (28.6) | ||
| Restaurant/Coffee Shop/Snack Bar | 282 | 75 (26.6) | 8 (2.8) | 175 | 48 (27.4) | ||
| Supermarket/Grocery | 27 | 11 (40.7) | 2 (7.4) | 26 | 3 (11.5) | ||
| Butcher Shop/Charcuterie | 71 | 32 (45.1) | 10 (14.1) | 70 | 10 (14.3) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, A.; Viveiros, C.; Lopes, J.; Nogueira, A.; Pires, B.; Afonso, A.F.; Teixeira, C. Microbiological Contamination in Different Food Service Units Associated with Food Handling. Appl. Sci. 2021, 11, 7241. https://doi.org/10.3390/app11167241
Alves A, Viveiros C, Lopes J, Nogueira A, Pires B, Afonso AF, Teixeira C. Microbiological Contamination in Different Food Service Units Associated with Food Handling. Applied Sciences. 2021; 11(16):7241. https://doi.org/10.3390/app11167241
Chicago/Turabian StyleAlves, Ana, Cristina Viveiros, Jéssica Lopes, António Nogueira, Bruno Pires, Andrea F. Afonso, and Cristina Teixeira. 2021. "Microbiological Contamination in Different Food Service Units Associated with Food Handling" Applied Sciences 11, no. 16: 7241. https://doi.org/10.3390/app11167241
APA StyleAlves, A., Viveiros, C., Lopes, J., Nogueira, A., Pires, B., Afonso, A. F., & Teixeira, C. (2021). Microbiological Contamination in Different Food Service Units Associated with Food Handling. Applied Sciences, 11(16), 7241. https://doi.org/10.3390/app11167241