Enhanced Oil Recovery with Size-Dependent Interactions of Nanoparticles Surface-Modified by Zwitterionic Surfactants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanoparticle Dispersions
2.3. Characterization
2.4. Evaluation of Emulsification
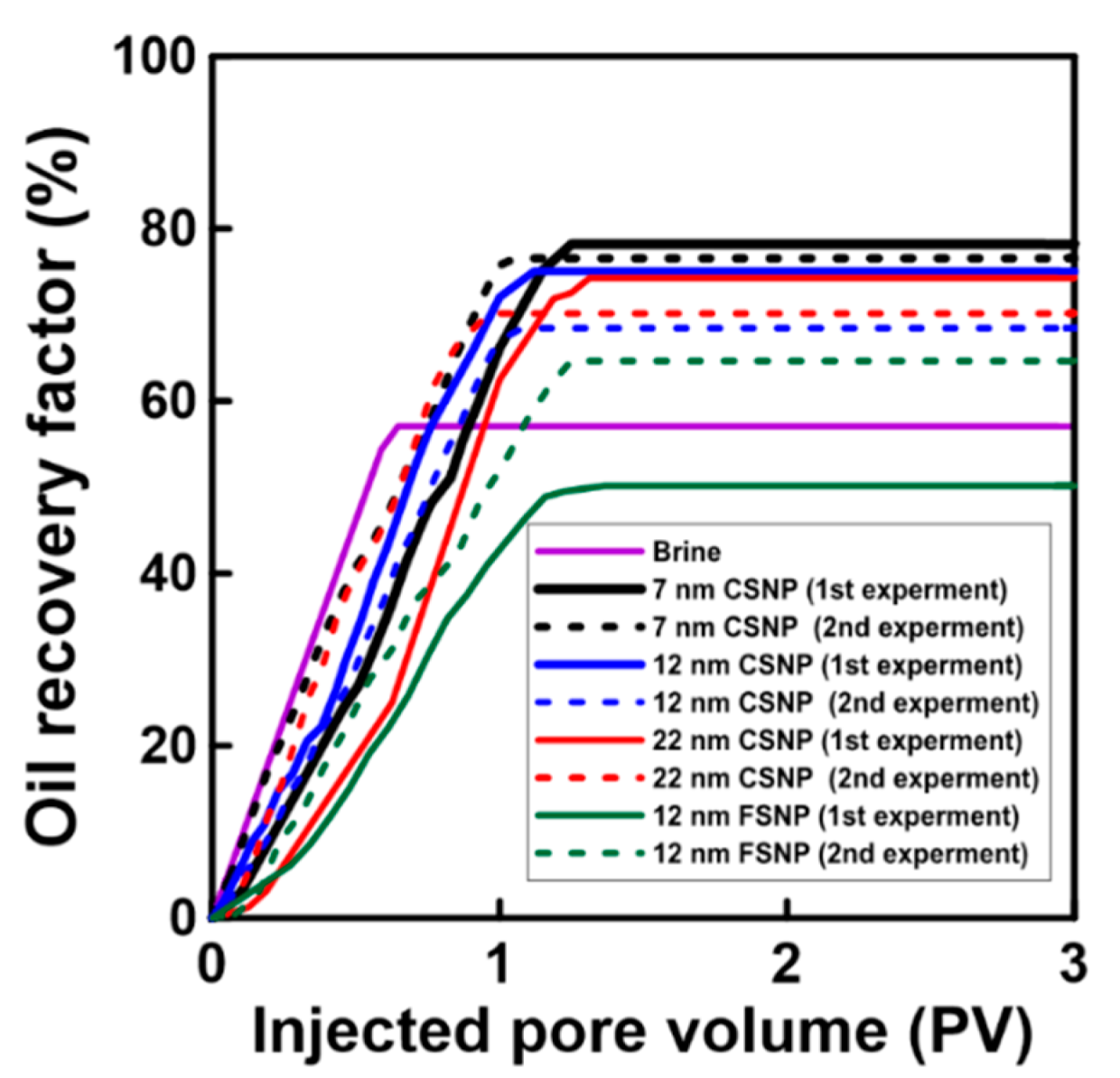
2.5. Core Flooding Experiments
3. Results and Discussion
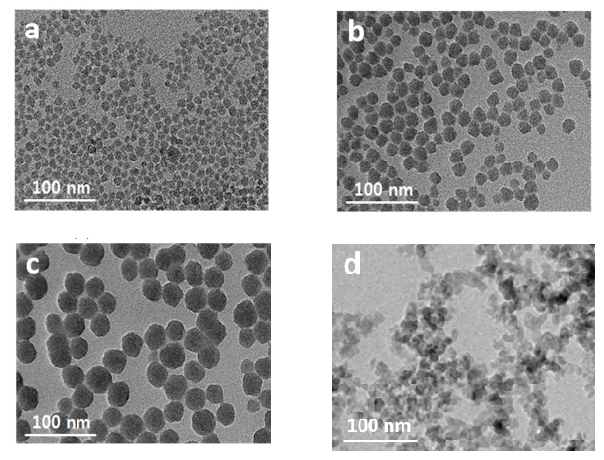
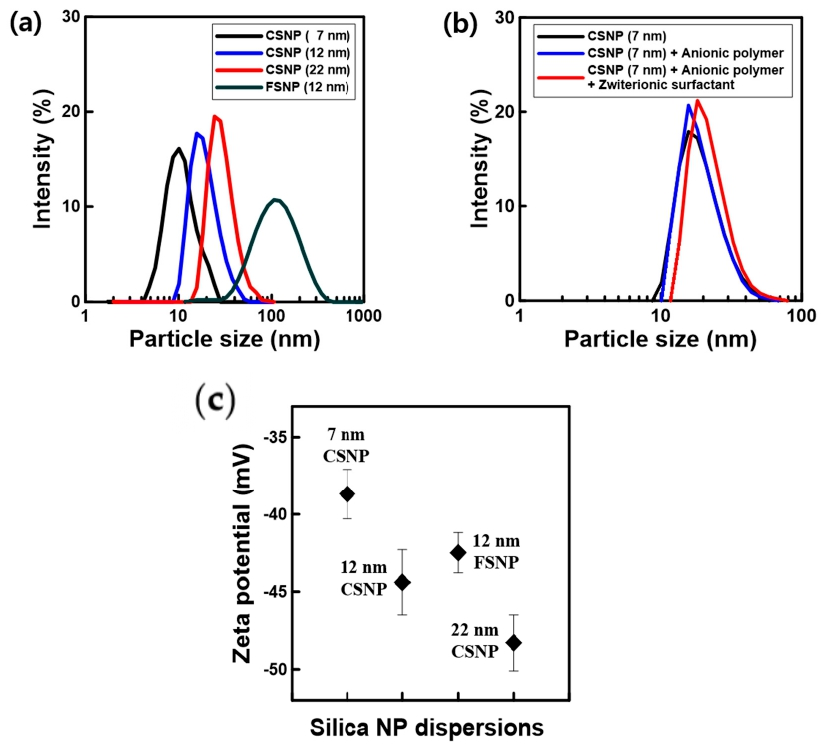
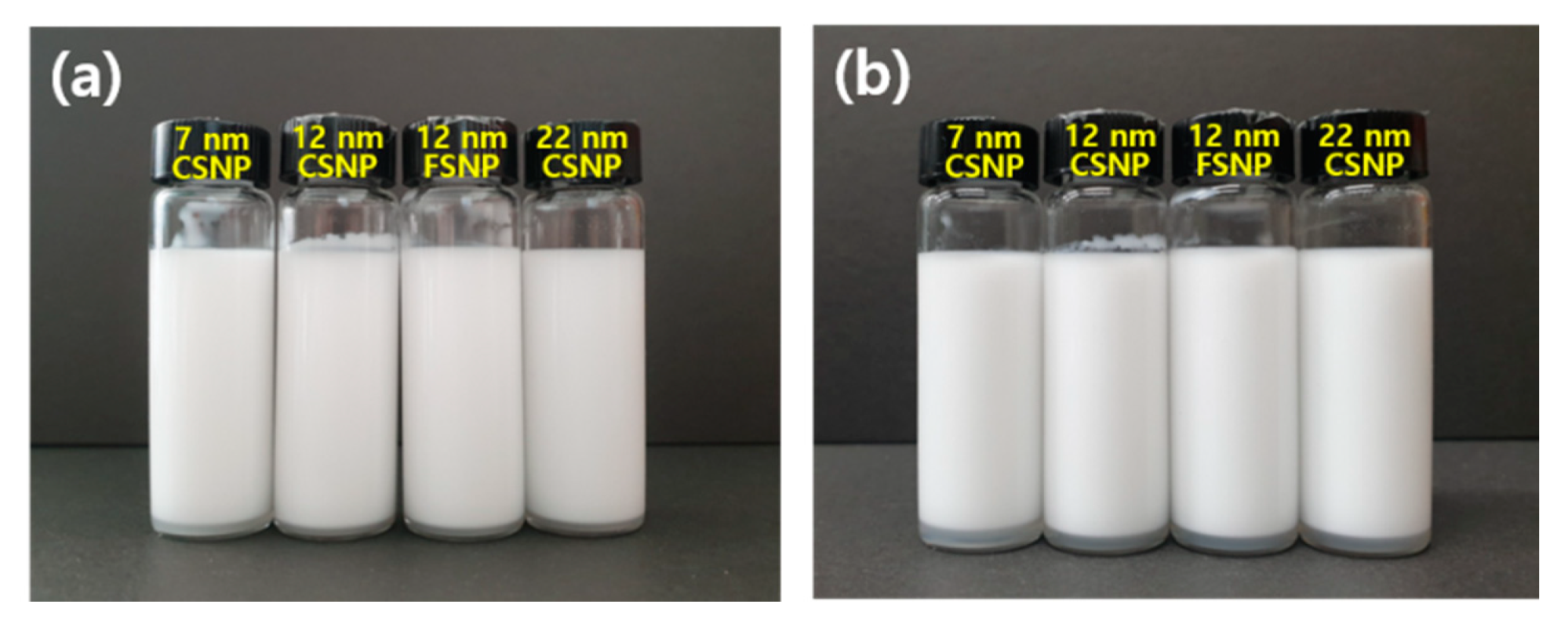
3.1. Dispersion of Silica NPs in Aqueous Solutions
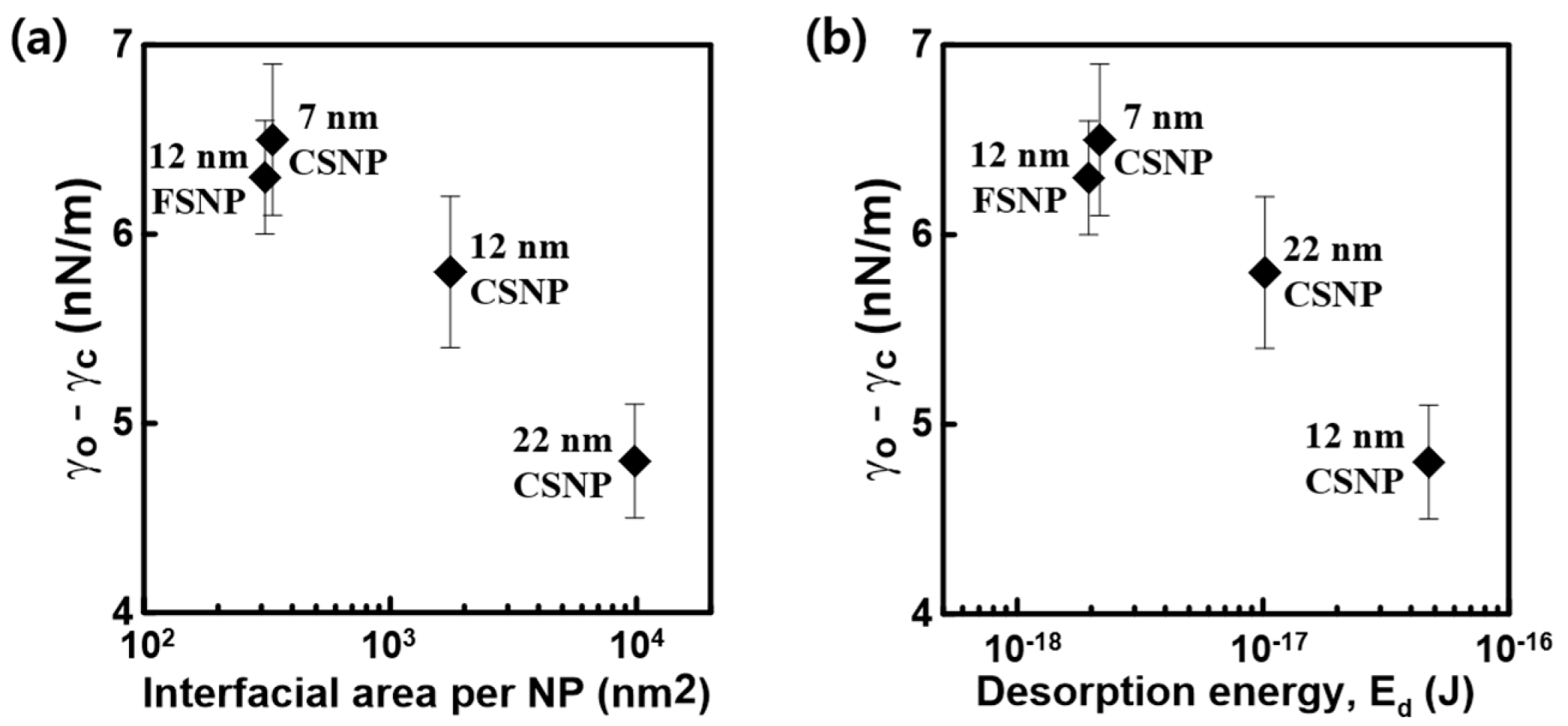
3.2. Interfacial Stability of NPs at the Oil–Water Interface
3.3. Effect of NP Number Density on the Oil–Water IFT
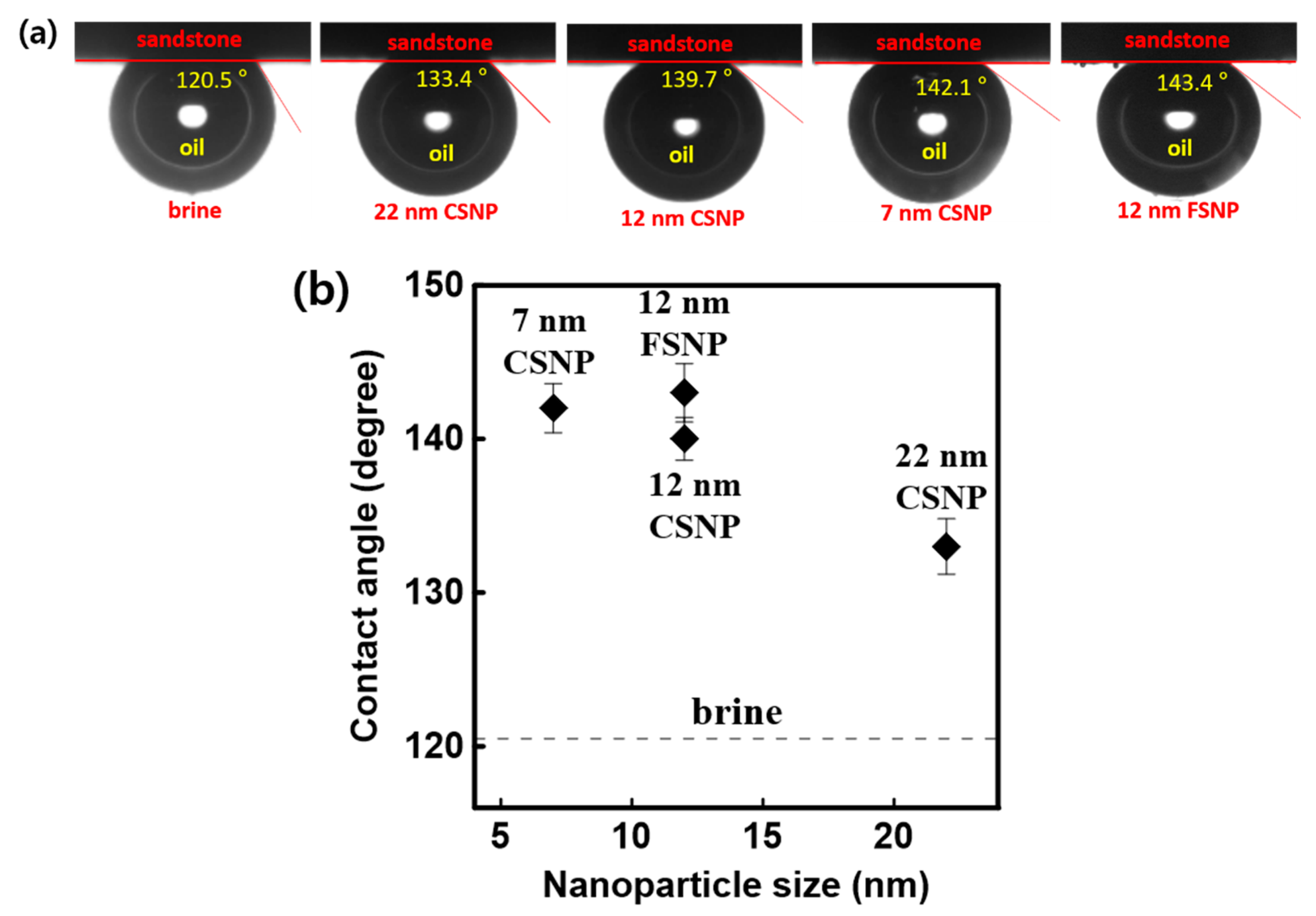
3.4. Contact Angles According to NP Size in the Three-Phase Rock–Oil–Aqueous System
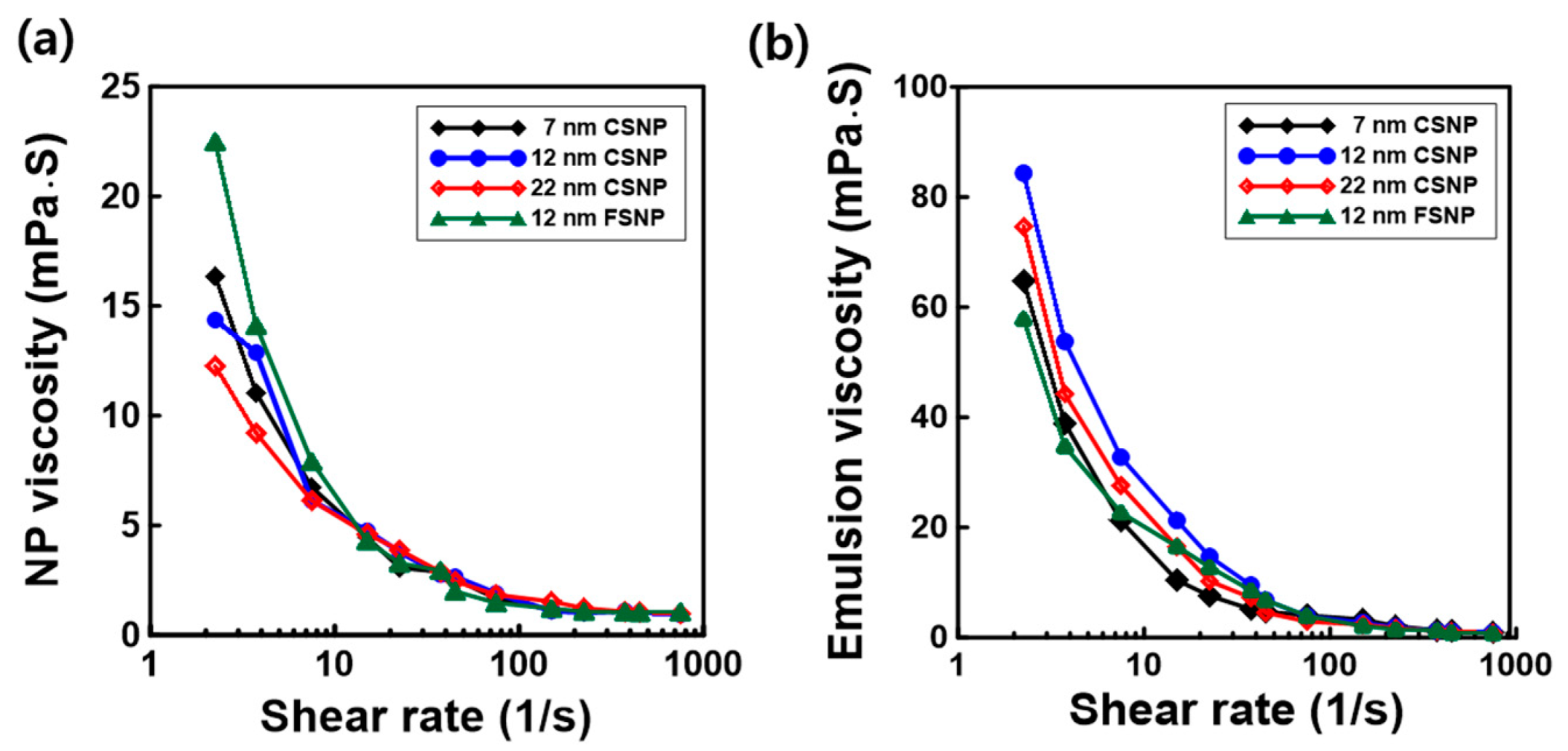
3.5. Interfacial Rheological Behaviors of Complex Colloidal Dispersions
3.6. Effect of NP Number Density on the Oil–Water IFT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ju, B.; Fan, T. Experimental study and mathematical model of nanoparticle transport in porous media. Powder Technol. 2009, 192, 195–202. [Google Scholar] [CrossRef]
- Son, H.A.; Yoon, K.Y.; Lee, G.J.; Cho, J.W.; Choi, S.K.; Kim, J.W.; Im, K.C.; Kim, H.T.; Lee, K.S.; Sung, W.M. The potential applications in oil recovery with silica nanoparticle and polyvinyl alcohol stabilized emulsion. J. Pet. Sci. Eng. 2015, 126, 152–161. [Google Scholar] [CrossRef]
- Xu, K.; Zhu, P.; Huh, C.; Balhoff, M.T. Microfluidic investigation of nanoparticles’ role in mobilizing trapped oil droplets in porous media. Langmuir 2015, 31, 13673–13679. [Google Scholar] [CrossRef]
- Yu, H.; He, Y.; Li, P.; Li, S.; Zhang, T.; Rodriguez-Pin, E.; Du, S.; Wang, C.; Cheng, S.; Bielawski, C.W. Flow enhancement of water based nanoparticle dispersion through microscale sedimentary rocks. Sci. Rep. 2015, 5, 8702–8709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, C.A.; Zabala, R.; Cortés, F.B. Nanotechnology aplied to the enhancement of oil and gas productivity and recovery of Colombian fields. J. Pet. Sci. Eng. 2017, 157, 39–55. [Google Scholar] [CrossRef]
- Li, Y.; Dai, C.; Zhou, H.; Wang, X.; Lv, W.; Wu, Y.; Zhao, M. A novel nanofluid based on fluorescent carbon nanoparticles for enhanced oil recovery. Ind. Eng. Chem. Res. 2017, 56, 12464–12470. [Google Scholar] [CrossRef]
- Nwidee, L.N.; Al-Anssari, S.; Barifcani, A.; Sarmadivaleh, M.; Lebedev, M.; Iglauer, S. Nanoparticles influ-ence on wetting behaviour of fractured limestone formation. J. Pet. Sci. Eng. 2017, 149, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhu, X.; Wang, L.; Yangm, H.; Fu, M. Experimental study of effective amphiphilic graphene oxide flooding for an ultralow-permeability reservoir. Energy Fuels 2018, 32, 11269–11278. [Google Scholar] [CrossRef]
- Karimi, A.; Fakhroueian, Z.; Bahramian, A.; Pour Khiabani, N.; Darabad, J.B.; Azin, R.; Arya, S. Wettability alteration in carbonates using zirconium oxide nanofluids: EOR implications. Energy Fuels 2012, 26, 1028–1036. [Google Scholar] [CrossRef]
- Hu, Z.; Siddeequah, M.A.; Ghulam, R.; Paul, W.J.G.; Dongsheng, W. Nanoparticle-assisted water-flooding in Berea sandstone. Energy Fuels 2016, 30, 2791–2804. [Google Scholar] [CrossRef]
- Choi, S.K.; Son, H.A.; Kim, H.T.; Kim, J.W. Nanofluid enhanced oil recovery using hydrophobically associ-ative zwitterionic polymer coated silica nanoparticles. Energy Fuels 2017, 31, 7777–7782. [Google Scholar] [CrossRef]
- Eltoum, H.; Yang, Y.L.; Hou, J.R. The effect of nanoparticle on reservoir wettability alteration: A critical review. Pet. Sci. 2021, 18, 136–153. [Google Scholar] [CrossRef]
- Fan, H.; Striolo, A. Nanoparticle effects on the water-oil interfacial tension. Phys. Rev. E 2012, 86, 051610. [Google Scholar] [CrossRef] [Green Version]
- Hendraningrat, L.; Li, S.; Torsaeter, O. A coreflood investigation nanofluid enhanced oil recovery. J. Petrol. Sci. Eng. 2013, 111, 128–138. [Google Scholar] [CrossRef]
- Zhang, H.; Nikolov, A.; Wasan, D. Enhanced oil recovery (EOR) using nanoparticle dispersions: Underly-ing mechanism and imbibition experiments. Energy Fuels 2014, 28, 3002–3009. [Google Scholar] [CrossRef]
- Sofla, S.J.D.; James, L.A.; Zhang, Y. Insight into stability of hydrophilic silica nanoparticles in seawater for enhanced oil recovery implications. Fuel 2018, 216, 559–571. [Google Scholar] [CrossRef]
- Zhong, X.; Li, C.; Pu, H.; Zhou, Y.; Zhao, J.K. Enhanced oil recovery in high salinity and elevated tempera-ture conditions with a zwitterionic surfactant and silica nanoparticles acting in synergy. Energy Fuels 2020, 34, 2893–2902. [Google Scholar] [CrossRef]
- Kim, I.; Taghavy, A.; DiCarlo, D.; Huh, C. Aggregation of silica nanoparticles and its impact on particle mobility under high-salinity conditions. J. Pet. Sci. Eng. 2015, 133, 376–383. [Google Scholar] [CrossRef]
- Druetta, P.; Picchioni, F. Polymer and nanoparticles flooding as a new method for enhanced oil recovery. J. Petrol. Sci. Eng. 2019, 177, 479–495. [Google Scholar] [CrossRef]
- Binks, B.P.; Rodrigues, J.A. Enhanced stabilization of emulsions due to surfactant-induced nanoparticle floculation. Langmuir 2007, 23, 7436–7439. [Google Scholar] [CrossRef]
- Binks, B.P.; Kirkland, M.; Rodrigues, J.A. Origin of stabilisation of aqueous foams in nanoparticle–surfactant mixtures. Soft Matter 2008, 4, 2373–2382. [Google Scholar] [CrossRef]
- Binks, B.P.; Rodrigues, J.A.; Frith, W.J. Synergistic interaction in emulsions stabilized by a mixture of silica nanoparticles and cationic surfactant. Langmuir 2007, 23, 3626–3636. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Son, H.A.; Choi, S.K.; Kim, J.W.; Sung, W.M.; Kim, H.T. Core flooding of complex nanoscale colloidal dispersions for enhanced oil recovery by in-situ formation of stable oil-in-water Pickering emulsions. Energy Fuels 2016, 30, 2628–2635. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Dai, C.; Huang, Y.; Zhao, M.; He, L.; Jiao, B. Reducing surfactant adsorption on rock by silica nanoparticles for enhanced oil recovery. J. Petrol. Sci. Eng. 2017, 153, 283–287. [Google Scholar] [CrossRef]
- Yang, G.C.C.; Tu, H.C.; Hung, C.H. Stability of nanoiron slurries and their transport in the subsurface environment. Sep. Pulif. Technol. 2007, 58, 166–172. [Google Scholar] [CrossRef]
- Bagaria, H.G.; Neilson, B.M.; Worthen, A.J.; Xue, Z.; Yoon, K.Y.; Cheng, V.; Lee, J.H.; Velagala, S.; Huh, C.; Bryant, S.L.; et al. Adsorption of iron oxide nanoclusters stabilized with sul-fonated copolymers on silica in concentrated NaCl and CaCl2 Brine. J. Colloid. Interface. Sci. 2013, 398, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Son, H.A.; Ahn, T. Effect of capillary number on the residual saturation of colloidal dispersions stabilized by a zwitterionic surfactant. Appl. Sci. 2021, 11, 524. [Google Scholar] [CrossRef]
- Wasan, D.; Nikolov, A. Spreading of nanofluids on solids. Nature 2003, 423, 156–159. [Google Scholar] [CrossRef]
- Wasan, D.; Nikolov, A.; Kondiparty, K. The wetting and spreading of nanofluids on solids: Role of the structural disjoining pressure. Curr. Opin. Colloid Interface Sci. 2011, 16, 344–349. [Google Scholar] [CrossRef]
- Al-Anssari, S.; Wang, S.; Barifcani, A.; Lebedev, M.; Iglauer, S. Effect of temperature and SiO2 nanoparticle size on wettability alteration of oil-wet cakcute. Fuel 2017, 206, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Tanvir, S.; Qiao, L. Surface tension of nanofluid-type fuels containing suspended nanomaterials. Na-Noscale Res. Lett. 2012, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.A.; Duyckaerts, N.; Redondo, A.B.; Jordan, I.; Nolting, F.; Kleibert, A.; Ammann, M.; Wörner, H.J.; van Bokhoven, J.A.; Abbas, Z. Effect of surface charge density on the affinity of oxide nanoparticles for the vapor–water interface. Langmuir 2013, 29, 5023–5029. [Google Scholar] [CrossRef]
- Kutuzov, S.; He, J.; Tangirala, R.; Emrick, T.; Russell, T.P.; Boker, A. On the kinetics of nanoparticle self-assembly at liquid/liquid interfaces. Phys. Chem. Chem. Phys. 2007, 9, 6351–6358. [Google Scholar] [CrossRef] [PubMed]
- Manga, M.S.; Hunter, T.N.; Cayre, O.J.; York, D.W.; Reichert, M.D.; Anna, S.L.; Walker, L.M.; Williams, R.A.; Biggs, S.R. Measurements of submicron particle adsorption and particle film elasticity at oil-water interfaces. Langmuir 2016, 32, 4125–4133. [Google Scholar] [CrossRef] [Green Version]
- Júnior, J.A.A.; Baldo, J.B. The behavior of zeta potential of silica suspensions. New J. Glass Ceram. 2014, 4, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Bink, B.P.; Yin, D. Pickering emulsions stabilized by hydrophilic nanoparticles: In situ surface modification by oil. Soft Matter 2016, 12, 6858–6867. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Glogowski, E.; Emrick, T.; Russell, T.P.; Dinsmore, A.D. Adsorption energy of nano- and microparticles at liquid-liquid interfaces. Langmuir 2010, 26, 12518–12522. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Li, K.; Horne, R. A mechanism study of wettability and interfacial tension for EOR using silica nanoparticles. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 9–11 October 2017; Society of Petroleum Engineers: Richardson, TX, USA, 2017. [Google Scholar] [CrossRef]
- Kopanichuk, I.V.; Vanin, A.A.; Brodskaya, E.N. Disjoining pressure and structure of a fluid confined be-tween nanoscale surfaces. Colloids Surf. A Phys. Eng. Aspects. 2017, 527, 42–48. [Google Scholar] [CrossRef]
- Lim, S.; Wasan, D. Structural disjoining pressure induced solid particle removal from solid substrates using nanofluids. J. Colloid Interface Sci. 2017, 500, 96–104. [Google Scholar] [CrossRef]
- Jiao, K.; Feng, Q.; Davarpanah, A. Effect of anionic and non-anionic surfactants on the adsorption density. Pet. Sci. Technol. 2021, 39, 362–372. [Google Scholar] [CrossRef]
- Davarpanah, A. Parametric study of nanoparticles-assisted injectivity performance for axisymmetric two-phase flow in EOR processes. Nanomaterials 2020, 10, 1818. [Google Scholar] [CrossRef] [PubMed]

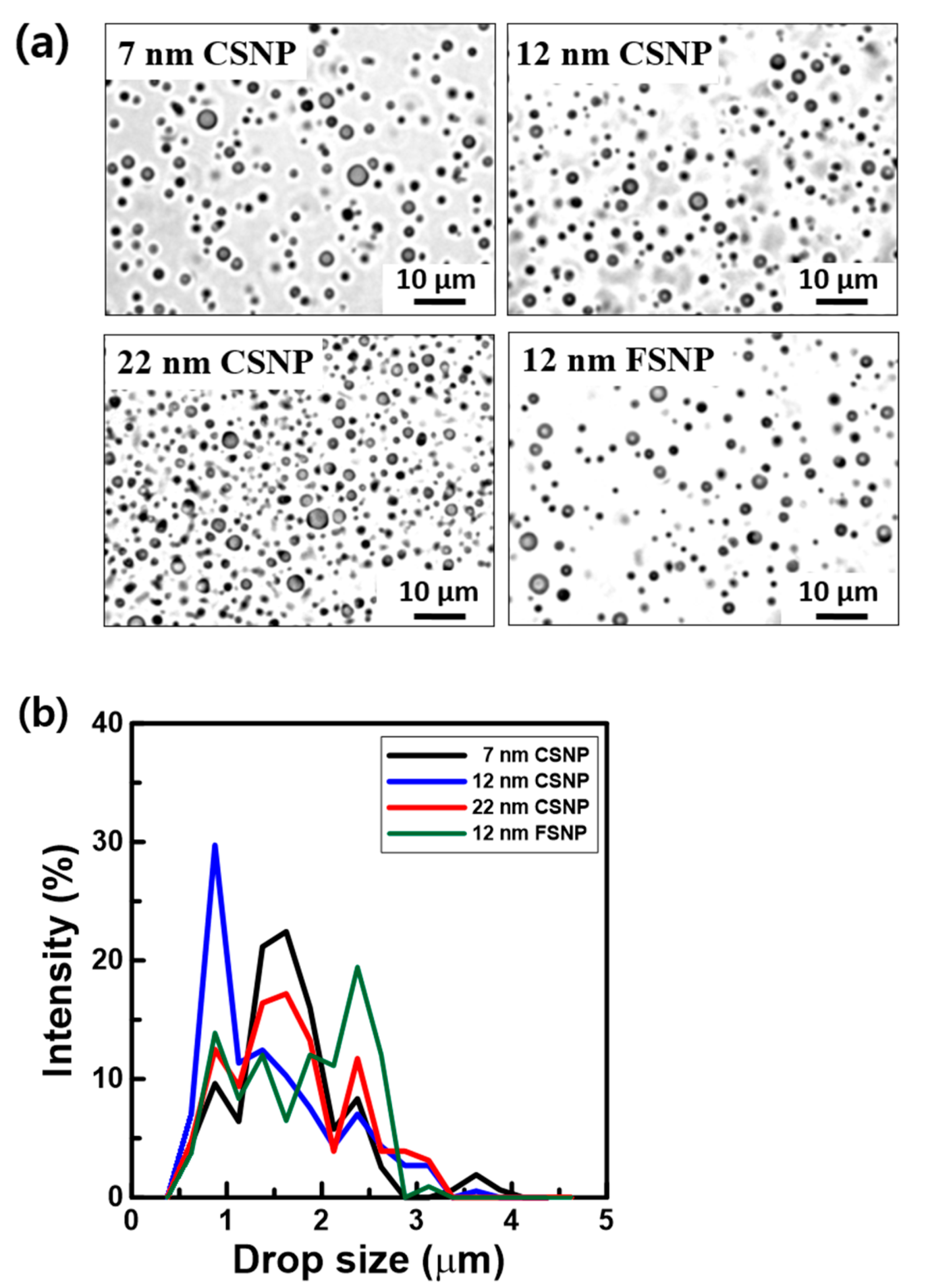
| 7 nm CSNP | 12 nm CSNP | 22 nm CSNP | 12 nm FSNP | |
|---|---|---|---|---|
| Mean droplet diameter (Ddrop.), µm | 1.63 | 1.47 | 1.66 | 1.76 |
| Mean surface area of droplet (Saver. drop. = π × Ddrop.2, μm2) | 8.34 | 6.78 | 8.66 | 9.73 |
| Mean droplet volume (Vdrop. = 1/6 π × Ddrop.3), μm3 | 2.26 | 1.66 | 2.39 | 2.85 |
| Total oil volume in emulsion (Vtot. oil vol.), µm3 | 4.0 × 1012 | |||
| Total number of emulsion droplets (Ntot. drop. = Vtot. oil vol./Vdrop.) | 1.76 × 1012 | 2.40 × 1012 | 1.67 × 1012 | 1.40 × 1012 |
| Total surface area of emulsion droplets (Stot. drop. = Ntot. drop. Saver. drop.), μm3 | 1.47 × 1013 | 1.63 × 1013 | 1.44 × 1013 | 1.36 × 1013 |
| 7 nm CSNP | 12 nm CSNP | 22 nm CSNP | 12 nm FSNP | |
|---|---|---|---|---|
| Total mass of bare NPs used for emulsion formation (M tot. NP), g | 0.0199 (0.5 wt% in the aqueous phase) | |||
| Density of bare NPs (ρ NP), g/nm3 | 2.50 × 10−21 | 2.36 × 10−21 | 2.42 × 10−21 | Approximately 0.50 × 10−22 |
| Total volume of NPs in emulsion (V tot. NP = M tot. NP/ρ NP), nm3 | 7.93 × 1018 | 8.41 × 1018 | 8.19 × 1018 | 3.98 × 1019 |
| Mean diameter of bare NPs (D NP), nm | 7 | 12 | 22 | 12 |
| Volume of bare NPs (VNP = 1/6 π × D NP3), nm3 | 179.59 | 904.77 | 5575.27 | 904.77 |
| Total number of NPs in emulsion (N tot. NP = V tot. NP/VNP) | 4.42 × 1016 | 9.30 × 1015 | 1.46 × 1015 | 4.39 × 1016 |
| Number of NPs per unit surface area (N sur. NP = N tot. NP/S tot. drop.), 1/μm2 | 3002 | 569 | 101 | 3225 |
| Interfacial area covered per NP (A inter. NP = Stot. drop./ N tot. NP), nm2 | 333.10 | 1754.51 | 9836.82 | 309.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, H.A.; Lee, T. Enhanced Oil Recovery with Size-Dependent Interactions of Nanoparticles Surface-Modified by Zwitterionic Surfactants. Appl. Sci. 2021, 11, 7184. https://doi.org/10.3390/app11167184
Son HA, Lee T. Enhanced Oil Recovery with Size-Dependent Interactions of Nanoparticles Surface-Modified by Zwitterionic Surfactants. Applied Sciences. 2021; 11(16):7184. https://doi.org/10.3390/app11167184
Chicago/Turabian StyleSon, Han Am, and Taehun Lee. 2021. "Enhanced Oil Recovery with Size-Dependent Interactions of Nanoparticles Surface-Modified by Zwitterionic Surfactants" Applied Sciences 11, no. 16: 7184. https://doi.org/10.3390/app11167184
APA StyleSon, H. A., & Lee, T. (2021). Enhanced Oil Recovery with Size-Dependent Interactions of Nanoparticles Surface-Modified by Zwitterionic Surfactants. Applied Sciences, 11(16), 7184. https://doi.org/10.3390/app11167184






