Abstract
In this paper, chelating agents were introduced as standalone fluids for enhancing the oil recovery from carbonate and sandstone reservoirs. Chelating agents such as glutamic acid di-acetic acid (GLDA), ethylene-diamine-tetra acetic acid (EDTA), and hydroxyl-ethylethylene-diamine-tri-acetic acid (HEDTA) were used. Chelating agents can be found in different forms such as sodium, potassium, or calcium salts. There is a significant gap in the literature about the influence of salt type on the hydrocarbon recovery from carbonate and sandstone reservoirs. In this study, the impact of the salt type of GLDA chelating agent on the oil recovery was investigated. Potassium-, sodium-, and calcium-based high-pH GLDA solutions were used. Coreflooding experiments were conducted at high-pressure high-temperature (HPHT) conditions using carbonate and sandstone cores. The used samples had porosity values of 15–18%, and permeability values were between 10 and 75 mD. Seawater was injected as a secondary recovery process. Thereafter, a GLDA solution was injected in tertiary mode, until no more oil was recovered. In addition to the recovery experiments, the collected effluent was analyzed for cations concentrations such as calcium, magnesium, and iron. Moreover, dynamic adsorption, interfacial tension, and contact angle measurements were conducted for the different forms of GLDA chelating agent solutions. The results of this study showed that incremental oil recovery between 19% and 32% of the Original Oil in Place (OOIP) can be achieved, based on the salt type and the rock lithology. Flooding carbonate rocks with the calcium-based GLDA chelating agent yielded the highest oil recovery (32% of OOIP), followed by that with potassium-based GLDA chelating agent, and the sodium-based GLDA chelating agent yielded the lowest oil recovery. The reason behind that was the adsorption of the calcium-based GLDA on the rock surface was the highest without reducing the rock permeability, which was indicated by the contact angle, dynamic adsorption, and flooding experiments. The outcome of this study will help in maximizing the oil recovery from carbonate and sandstone reservoirs by suggesting the most suitable salt type of chelating agents.
1. Introduction
Enhanced Oil Recovery (EOR) techniques are applied to enhance hydrocarbon recovery utilizing several methods such as gas injection, thermal treatments, and chemical flooding [,]. EOR methods are implemented after the primary production, in which the natural reservoir energy is utilized to produce hydrocarbon. Various chemicals are injected into a reservoir to enhance the hydrocarbon flow by controlling the oil movement and minimizing interfacial tension (IFT) [,,,]. The most effective mechanisms during EOR treatments are wettability alteration, IFT reduction, and viscosity reduction. Common chemicals used for EOR treatments are surfactants, polymers or a combination of them [,,]. Recently, chelating agents have been introduced as new chemicals for improving the oil production from carbonate and sandstone formations by lowering the IFT or altering the rock wettability [,,,,]. Glutamic acid di-acetic acid (GLDA), hydroxyl-ethylethylene-diamine-tri-acetic acid (HEDTA), and ethylene-diamine-tetra acetic acid (EDTA) are used for EOR treatments [,,,,].
Mahmoud and Abdelgawad [] studied the oil recovery from carbonate and sandstone reservoirs during HEDTA, DTPA, and EDTA. The efficacy of chelating solutions was assessed using interfacial tension, coreflood, and zeta-potential measurements. They concluded that an injection of a 5 wt % chelating agent can enhance the oil recovery by around 20% for carbonate and sandstone rocks. In addition, they reported that no formation damage was induced during the chelating agent flooding. Instead, rock dissolution took place in the carbonate samples due to the chemical reaction between the carbonate matrix and injected fluids. Analyzing the produced effluent from coreflooding experiments using inductively coupled plasma (ICP) analysis showed high concentrations of calcium, iron, and magnesium ions.
Hassan and Al-Hashim [,] studied the efficiency of EDTA, a chelating agent for enhancing the hydrocarbon recovery from carbonate reservoirs. An optimization study was conducted to estimate the best fluid volume and concentration that can minimize the treatment cost and increase the oil production. They concluded that injecting 3 wt % of an EDTA solution can increase the recovery by around 20% with a minimum operational cost. Shafiq et al. [] examined the efficacy of several chelating agents’ solutions for changing the wettability conditions. Flooding tests were carried out at reservoir conditions to estimate the oil recovery during GLDA, HEDTA, and EDTA flooding. In addition, the impact of chemical injection on the rock porosity system was studied using computed tomography (CT) scans and NMR analyses. HEDTA showed the highest impact on the rock properties for dolomite and sandstone; the rock porosity and permeability were increased significantly after the HEDTA flooding.
Hassan and Al-Hashim [,] investigated the alterations in carbonate rocks during chelating agent flooding. The impacts of chemical concentration, injected volume, system pH, and brine salinity on the hydrocarbon system were studied. Zeta potential, ions concentration, spontaneous imbibition, coreflooding, and NMR experiments were conducted. After the treatment, the carbonate wettability was changed to less oil-wet status, and more oil was produced consequently. In addition, the capillary pressure was reduced considerably due to the chemical flooding, resulting in better flow conditions for oil. Among all tested chemicals, the highest oil recovery was obtained using GLDA as the chelating agent. Indicating that GLDA can outperform other chelating agents for enhancing the oil recovery at the same injected volume and concentration.
Chelating agents can be used as standalone fluids for enhancing the oil recovery from carbonate and sandstone formations [,,]. However, the effectiveness of chelating agent solutions depends on different factors such as acid concentration, salt type, and injected volume. Extensive work was conducted to assess the performance of several chelating agents under different reservoir and treatment conditions [,,]. The impacts of chemical concentration, chelating agent type, solution pH, and rock type were investigated [,,,]. However, there is a gap in the literature about the influence of salt type on the hydrocarbon recovery from carbonate and sandstone reservoirs. Therefore, the objective of this work is to study the influence of the salt type of chelating agent on the oil recovery. The performances of the calcium-based GLDA chelating agent (Ca2GLDA), the potassium-based chelating agent (K4GLDA), and the sodium-based chelating agent (Na4GLDA) in recovering oil from sandstone and carbonate rocks are examined. The three salts used in this study are readily available in the market and can be secured easily compared to other chelating agents types. In addition, GLDA was selected in this work, because it is the most environmentally friendly chelating agent compared to all chelating agents. Results of coreflooding, dynamic adsorption, interfacial tension, and contact angle measurements are discussed in this paper.
2. Materials and Equipment
2.1. Materials
This work investigates the impact of salt type on oil recovery from sandstone and carbonate rocks. Potassium-, sodium-, and calcium-based high-pH GLDA solutions were used. Rock samples from Indiana limestone and Berea sandstone formations were used. The rock properties were evaluated by measuring the core porosity and permeability. The limestone cores had an average porosity of 15% and a permeability of 10 mD, and the sandstone cores had an average porosity of 18% and a permeability of 75 mD. Table 1 lists the rock properties, pore volume (PV), initial water saturation (Swi), and the used chemicals for each experiments.

Table 1.
Rock properties and the used fluids during core flooding experiments.
Arabian medium oil with an API (the American Petroleum Institute) gravity of 32 was used in this study. The composition of this crude oil is provided in Table 2. In addition, Gulf seawater (SW) with a total dissolved solid (TDS) of 57.3 g/L was used to recover the oil during the secondary recovery process, and formation brine (FB) with TDS of 213.7 g/L was utilized for saturating the rock samples and establishing the initial water saturation. Table 3 lists the ionic composition of the synthetic SW and FB used in this work. All used brines were clear, and no precipitation was observed, to avoid the plugging of the rock samples during the flooding experiments. Moreover, the calcium-based GLDA chelating agent (Ca2GLDA), the potassium-based chelating agent (K4GLDA), and the sodium-based chelating agent (Na4GLDA) were used. All GLDA solutions had the same concentration of 5 wt % and pH of 11, which minimized the experimental uncertainty, and the impact of salt type on the oil recovery can be reasonably assessed.

Table 2.
Oil composition.

Table 3.
Composition of synthetic seawater (SW) and formation brine (FB).
2.2. Equipment
In this work, different experiments were conducted including coreflooding, contact angle, interfacial tension, and dynamic adsorption measurements. The coreflooding experiments were conducted using 6-inch-length and 1.5-inch-diameter core samples. All experiments were conducted at 100 °C and a 500 psi backpressure and at an injection rate of 0.5 cm3/min. The coreflooding setup (Figure 1) consists of an oven, a core holder, an injection pump, a pressure controller, and transfer cylinders. The core holder and the injection pump are positioned horizontally to minimize the gravity effect. The coreflooding experiments were conducted by first saturating the rocks with the formation brine and then the core samples were flooded with oil. The flooding setup was used to inject oil into the rock samples displacing the brine out of the cores, and the produced effluents were monitored. The maximum oil saturation was achieved, once no more brine is coming out of the rocks, indicating the rock samples were at the irreducible water saturation and maximum oil saturation. Thereafter, the samples were aged at high-pressure and high-temperature conditions to ensure reasonable reservoir conditions. The samples were aged at 100 °C and 2500 psi for two weeks to ensure that the rock samples are saturated with the oil. The flooding experiments were started by injecting SW as a secondary recovery process, until no more oil was produced. Thereafter, a GLDA solution was injected in a tertiary mode, until no more oil was recovered.
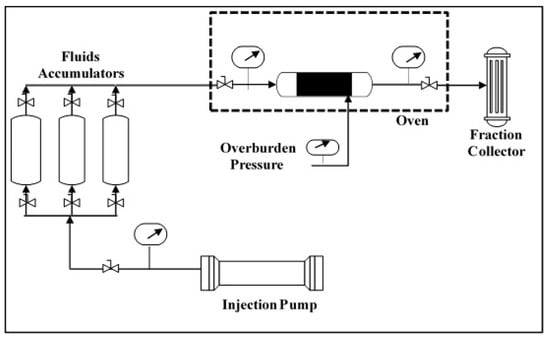
Figure 1.
Coreflooding setup.
In addition, contact angle measurements were also conducted to evaluate the wettability condition for the rock/fluid system. First, the baseline was defined by measuring the contact angle for the rock/brine/oil system, and then, the impact of introducing different types of GLDA solutions on the wettability status was examined. All contact angle measurements were performed at 100 °C and 1000 psi for 24 h. In addition, IFT experiments were conducted to assess the impact of adding different types of chelating agents to the brine–oil interface. The effects of using Ca2GLDA, Na4GLDA, and K4GLDA on the IFT reduction were determined. Finally, dynamic adsorption measurements were conducted to understand the adsorption behavior of GLDA types on the rock surfaces. All experiments were conducted at a temperature of 100 °C.
3. Results and Discussion
This work investigates the performances of different salt types of GLDA chelating agents for enhancing the oil recovery from carbonate and sandstone formations. The results of coreflooding, interfacial tension, contact angle, and adsorption measurements are thoroughly discussed in this section.
3.1. Coreflooding
In this work, the oil recovery from carbonate and sandstone rocks was evaluated using coreflooding experiments. First, the performance of SW flooding to increase the oil recovery was studied by injecting SW bine of 57.3 g/L TDS into the carbonate and sandstone cores. Figure 2 shows the percentage of recovered oil using SW flooding from six core samples. It should be noted that all samples were prepared and treated using the same experimental conditions; hence, the changes in oil recovery can be attributed mainly to the rock types and the petrophysical properties. All the carbonate samples (ILS1–ILS3) showed oil recovery between 37% and 39% of the Original Oil in Place (OOIP), while flooding the sandstone samples (BSS1–BBS3) with SW showed higher oil recovery of around 45–47% of the OOIP. The petrophysical measurements showed that the sandstone samples had a higher rock permeability compared to the carbonate samples; hence, higher oil recovery was obtained for the sandstone samples. The average rock permeabilities were 10 and 75 mD for the carbonate and sandstone samples, respectively. Overall, SW flooding showed higher oil recovery from the sandstone samples compared to that from the carbonate samples, due to the high rock permeability of the sandstone samples.
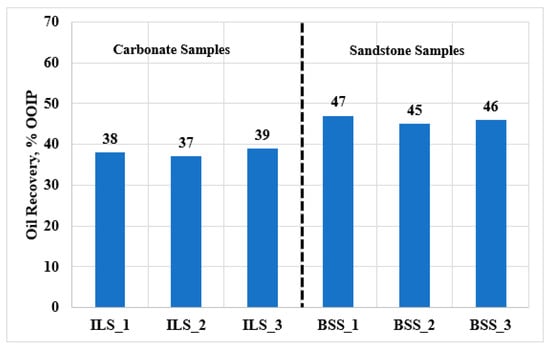
Figure 2.
Oil recovery during SW flooding for carbonate (ILS) and sandstone (BSS) samples.
After SW flooding, the chelating agent solutions were injected into the carbonate and sandstone samples by utilizing the same experimental conditions. Figure 3 shows the volume to breakthrough of GLDA solutions during the chemical flooding. In general, a lower volume of the GLDA solution was required for treating the sandstone samples compared to for treating the carbonate samples, which can be attributed mainly to rock permeability. The breakthrough behavior is affected by the rock permeability and heterogeneity. In this work, the rock heterogeneity was minimized by using rock samples from the same formations, then, the breakthrough behavior was controlled mainly by the rock permeability. Higher rock permeability can result in faster breakthrough due to the less flow resistance, and thus a less chemical volume was used. The average PVs to breakthrough were 0.57 and 0.67 PV for the sandstone and carbonate samples, respectively. In addition, in both the sandstone and carbonate samples, Na4GLDA and K4GLDA showed faster breakthrough compared to Ca2GLDA, indicating that higher oil recovery would be achieved using calcium-based GLDA. A faster breakthrough can lead to a poorer sweep efficiency and thus will reduce the oil recovery. Injecting Ca2GLDA into the carbonate and sandstone samples showed the highest volume to breakthrough, and thus, higher oil recovery would be achieved using Ca2GLDA. The injected fluid spread along the rock surface and displaced a higher volume of oil, resulting in late breakthrough and better oil recovery.
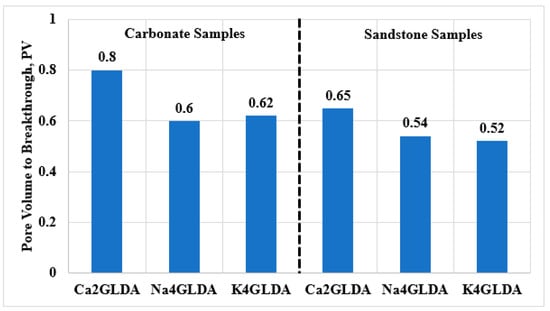
Figure 3.
Pore volume to breakthrough during glutamic acid di-acetic acid (GLDA) flooding in carbonate and sandstone samples.
Also, the oil recovery during injection of GLDA solutions into carbonate and sandstone samples was determined, as shown in Figure 4. Higher oil recovery was achieved from the carbonate samples compared to from the sandstone samples, indicating that all types of GLDA solutions work better in a carbonate reservoir. The additional oil recovery varied between 26% and 32% for the carbonate samples, while around 19% to 21% of the OOIP was recovered from the sandstone samples. This could be attributed to the reaction behavior between GLDA solutions and the rock matrix. It is well known that carbonate rocks are more reactant compared to sandstone rocks. GLDA is a weak acid that can dissolve part of the carbonate matrix, similar to the HCl reaction with a slower reaction rate. Therefore, a higher chemical reaction rate will be induced in carbonate rocks compared to in sandstone, leading to higher oil recovery from carbonate formations. Furthermore, Ca2GLDA showed the highest oil recovery among all tested GLDA types, for both sandstone and carbonate samples. The maximum oil recovery of 32% was obtained by flooding carbonate samples with Ca2GLDA solutions. It should be noted that all coreflooding experiments were conducted at the same conditions and similar chemical concentrations and pH were used for all GLDA types. However, the chelating agent salt was changed during the coreflooding experiments. Hence, the additional oil recovery can be attributed mainly to the GLDA salt type; the best chelating agent type provides the highest oil recovery. Overall, the coreflooding experiments indicated that under the same conditions Ca2GLDA can outperform the other GLDA types and results in the highest oil recovery from carbonate rocks.
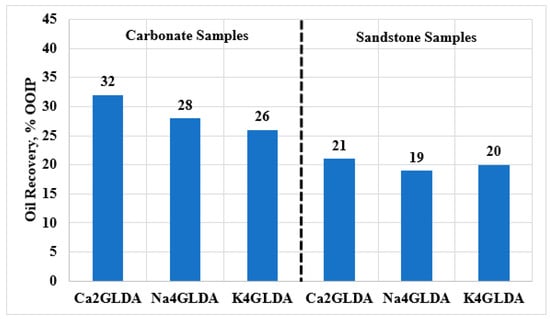
Figure 4.
Oil recovery during the injection of different types of GLDA solutions into sandstone and carbonate rocks.
Figure 5 shows the oil recovery profiles from the sandstone and carbonate samples during SW and Ca2GLDA flooding. The total oil recovery of 70% of the OOIP was obtained for the carbonate sample, while around 68% of the OOIP was produced from the sandstone sample, indicating that relatively higher oil recovery can be achieved from carbonate reservoirs. During SW flooding, the oil recovery from the sandstone sample was higher compared to that from the carbonate sample, which is correlated mainly to the higher rock permeability for sandstone rocks. An average permeability of 75 mD was obtained for the sandstone sample, while the carbonate sample had an average permeability of 10 mD. On the other hand, higher oil recovery was produced from the carbonate sample during the chelating agent flooding, indicating that GLDA solutions can work better in carbonate reservoirs compared to in sandstone formations.
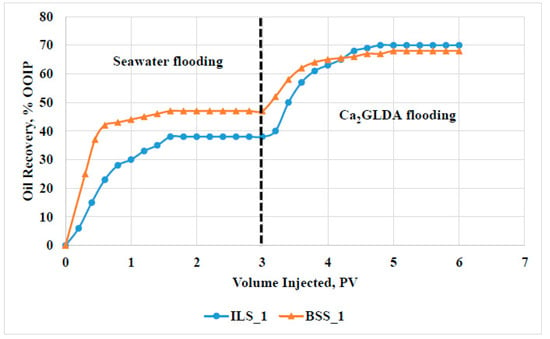
Figure 5.
Oil recovery profiles from carbonate (ILS1) and sandstone (BSS1) rocks during SW and Ca2GLDA flooding.
In addition, the pressure drop was measured for all experiments. Figure 6 shows the pressure drop profiles for the three salt types in limestone rocks. As expected, the pressure profiles were different, because the GLDA breakthrough times were different and depended on the salt type. The SW injection period was very similar in the three experiments, because the breakthrough times were almost the same and the recovered oil amounts were the same. In the case of the GLDA injection period, the breakthrough time was different for the calcium-based GLDA compared to for the sodium- and potassium-based GLDA. In addition, the pressure drop values were higher for both K4GLDA and Na4GLDA compared to for Ca2GLDA, because the oil recovery was higher in the case of calcium-based GLDA compared to both sodium- and potassium-based GLDA. Overall, at the end of the flooding experiments, Ca2GLDA showed the lowest pressure drop values, indicating that the flow conditions were improved.
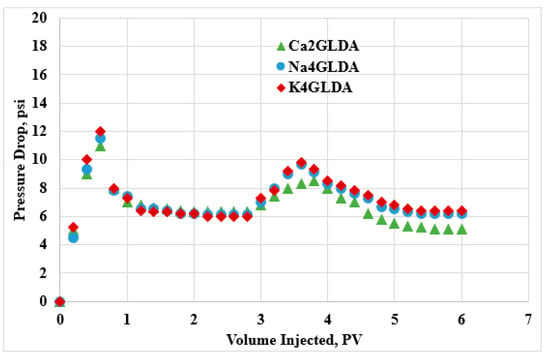
Figure 6.
Pressure drop profiles during the injection of SW followed by Ca2GLDA, NA4GLDA, and K4GLDA.
3.2. Interfacial Tension
IFT at the oil/chemical contact is one of the crucial factors that affect oil recovery [,]. Reducing the IFT at the oil/brine interface can lead to improving the displacement efficiency, and hence, more oil can be produced. Introducing the chelating agent into the oil/brine interface can reduce the interfacial forces, leading to increasing the oil recovery [,]. In this work, the reduction in interfacial tension at the oil–brine interface due to the injection of chelating agents was examined. Figure 7 shows the IFT for oil/SW, oil/K4GLDA, oil/Na4GLDA, and oil/Ca2GLDA systems. The injection of GLDA solutions to the oil–brine system reduced the interfacial tension from 15.54 to less than 4 dynes/cm. Among all tested GLDA types, calcium-based solutions (Ca2GLDA) showed the lowest IFT value of 1.5 dynes/cm. Compared to the other GLDA types, Ca2GLDA can reduce the interfacial tension by around 57%, under the same conditions, revealing that more oil can be recovered by injecting Ca2GLDA solutions due to the reduction in capillary pressure. However, it should be noted that the IFT reduction by chelating agent may help for EOR, but the reduction in IFT and enhancement in capillary number are far from those obtained in surfactant-based EOR.
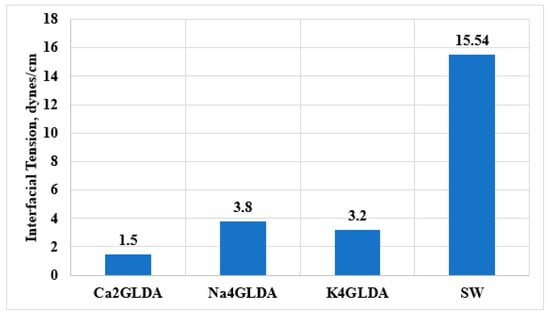
Figure 7.
The interfacial tension between oil and SW as well as those between oil and different types of GLDA salts.
The changes in capillary conditions due to chemical flooding can be evaluated by determining the capillary number (Nc). Equation (1) can be used to estimate the capillary number [,] and was written as:
where is the capillary number, is the fluid viscosity, is the velocity, and is the IFT at the fluid–oil contact. In general, the oil movement can be enhanced by increasing the capillary number; consequently, less oil saturation would be achieved, and more oil can be recovered [,].
The impact of injecting chelating solutions on reducing the capillary pressure forces was estimated by calculating the capillary number before and after introducing the GLDA solutions. All parameters were kept unchanged except the interfacial tension. The interfacial tension experiments indicated that the IFT can be reduced by around 90% using GLDA chelating agent. The IFT reduction will result in raising the capillary number as described by Equation (1), thereby improving the hydrocarbon flow. Figure 8 shows the capillary numbers for the oil/SW, oil/K4GLDA, oil/Na4GLDA, and oil/Ca2GLDA systems. The injection of GLDA chelating agents can raise the capillary number (Nc) by a factor of 10.5 compared to the conventional SW flooding. As observed, Nc was increased from 1.96 × 10−8 to 2.03 − 10–7 by using GLDA injection. The increase in capillary number is mainly due to the reduction in IFT. The injected chelating agents created a small layer at the oil/GLDA contact, and this layer was generated due to the deprotonation of some acid components present in the crude oil. The generated layer led to inducing a surface-active zone in the form of soap, thus lowering the IFT in a way similar to surfactant flooding [,].
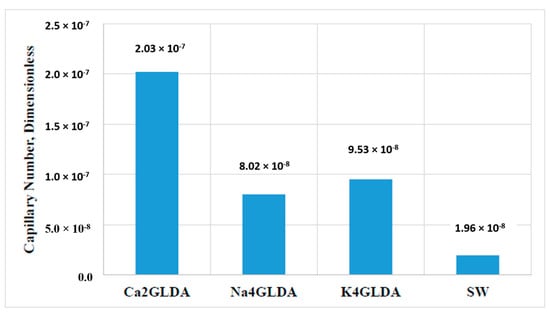
Figure 8.
The capillary numbers for oil and SW as well as oil and different types of GLDA salts systems.
3.3. Contact Angle
Rock wettability plays an important role during the oil recovery process. Altering the wettability condition to less oil-wet reservoirs can lead to increasing the oil production. In this work, the wettability conditions for the sandstone and carbonate samples were studied before and after adding chelating agents [,]. The results are provided in Figure 9. In general, higher values of contact angle (above 90°) indicate oil-wet conditions, and contact angles less than 90° indicate water-wet systems [,]. For carbonate samples, the contact angle was 106° during the SW flooding. Most of the carbonate reservoirs are oil-wet due to the adsorption of carboxylic hydrocarbon on the carbonate surface. However, introducing chelating agents to the carbonate/oil/SW system showed a significant reduction in the contact angle values, indicating that the carbonate wettability was changed from oil-wet to water-wet status. The lowest value for contact angles was obtained by using Ca2GLDA, revealing that the calcium-based GLDA outperforms other types of chelating agents in altering the rock wettability, thereby improving the oil recovery and utilizing the wettability alteration mechanism. On the other hand, all sandstone samples are water-wet as indicated by the contact angle values less than 90°. Then, adding the calcium-based GLDA to the brine oil system led to reducing the contact angle from 70° to 43°, i.e., more water-wet conditions. Overall, using Ca2GLDA can change the wettability conditions for the carbonate and sandstone samples to less oil-wet conditions, resulting in more oil recovery.
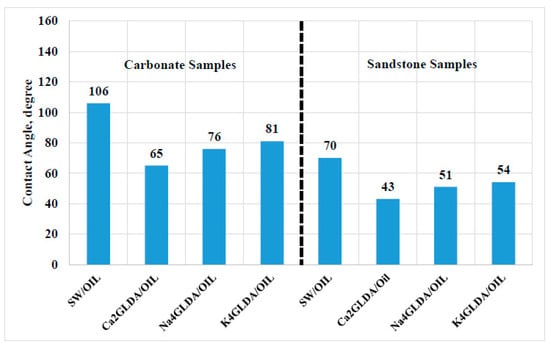
Figure 9.
Contact angle results for SW and chelating agent solutions for sandstone and carbonate rocks.
3.4. Adsorption Behavior
During chemical flooding, the injected fluids can be adsorbed on the rock surface, leading to considerable changes such as wettability alterations or hydrocarbon liberations. However, the adsorption of injected chemicals on the rock surface can lead to reducing the effectiveness of the injected chemicals. Therefore, the adsorption capacity is preferred to be within an acceptable level that alters the rock wettability without sacrificing a great deal of the injected chemicals. In this work, the impact of chelating agent type on the adsorption behavior was studied. Figure 10 shows the adsorption profiles for different types of GLDA solutions. The adsorption experiment was conducted at a temperature of 100 °C using carbonate rock with a 10.6 mD permeability, a 16% porosity, and a 6-inch size. The same concentrations and volumes of Ca2GLDA, Na4GLDA, and K4GLDA were injected, and the concentrations of the produced fluids from the rock samples were analyzed. The lower the concentration, the higher the chemical adsorption. During the adsorption experiment, the fluid concentration was determined as a function of the injected PV. Overall, high concentration during the adsorption experiment indicated poor performance for the injected fluids, and low chemical efficiency was achieved. Among the tested types, the calcium-based GLDA showed the best adsorption behavior with the minimum fluid concentration. Ca2GLDA was the best salt type because of the high adsorption capacity on the rock surface. It should be noted that Ca2GLDA was adsorbed on the rock surface in a form of a very thin film around the grains, leading to the alteration of the work wettability without considerable reductions in the rock porosity and permeability, as confirmed by the pressure drop profiles provided in Figure 6.
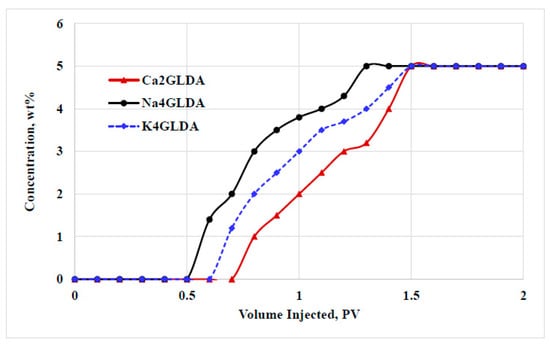
Figure 10.
Adsorption profiles for different types of GLDA at a temperature of 100 °C.
Combining the coreflooding, interfacial tension, contact angle, and adsorption measurements, it was shown that the calcium-based GLDA can outperform all the other GLDA types under the same conditions. Ca2GLDA had the highest adsorption capacity that led to the alteration of the wettability conditions to more water-wet status and the reduction of the IFT at the oil–fluid interface. Consequently, higher oil recovery can be achieved using Ca2GLDA compared to by using the other GLDA types.
4. Conclusions
This study investigated the impact of the salt type of GLDA chelating agent on oil recovery. Several measurements were conducted including coreflooding, interfacial tension, contact angle, and dynamic adsorption. The following conclusions can be drawn based on this work:
- Incremental oil recovery between 19% and 32% of the OOIP can be achieved, based on the salt type and rock lithology.
- Flooding carbonate rocks with the calcium-based GLDA chelating agent yielded the highest oil recovery (32% of OOIP), followed by that with the potassium-based GLDA chelating agent, and the sodium-based GLDA chelating agent yielded the lowest oil recovery.
- The adsorption behavior of the calcium-based GLDA on the rock surface was the highest, which was indicated by the contact angle and dynamic adsorption experiments.
- This study will help in maximizing the oil recovery from carbonate and sandstone reservoirs by suggesting the most suitable salt type of chelating agents.
Author Contributions
Conceptualization, M.M. and S.P.; methodology, M.M.; validation, M.M., A.H. and S.P.; formal analysis, M.M., A.H. and S.P.; investigation, M.M., A.H. and S.P.; resources, M.M.; data curation, M.M.; writing—original draft preparation, A.H.; writing—review and editing, M.M. and S.P.; visualization, M.M.; supervision, M.M. and S.P.; project administration, M.M. and S.P.; funding acquisition, M.M. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The College of Petroleum and Geoscience at King Fahd University of Petroleum & Minerals is acknowledged for the support and permission to publish this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Green, D.W.; Willhite, G.P. Enhanced oil recovery. In Richardson, TX: Henry L. Doherty Memorial Fund of AIME; Society of Petroleum Engineers: Tulsa, Oklahoma, 1998. [Google Scholar]
- Drexler, S.; Hoerlle, F.; Godoy, W.; Boyd, A.; Couto, P. Wettability Alteration by Carbonated Brine Injection and Its Impact on Pore-Scale Multiphase Flow for Carbon Capture and Storage and Enhanced Oil Recovery in a Carbonate Reservoir. Appl. Sci. 2020, 10, 6496. [Google Scholar] [CrossRef]
- Donaldson, E.C.; Chilingarian, G.V.; Yen, T.F. Enhanced Oil Recovery, II: Processes and Operations; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Lake, L.W.; Johns, R.; Rossen, B.; Pope, G.A. Fundamentals of Enhanced Oil Recovery; Society of Petroleum Engineers: Richardson, TX, USA, 2014; Volume 1, p. 1. [Google Scholar]
- Riswati, S.S.; Bae, W.; Park, C.; Permadi, A.K.; Novriansyah, A. Nonionic Surfactant to Enhance the Performances of Alkaline–Surfactant–Polymer Flooding with a Low Salinity Constraint. Appl. Sci. 2020, 10, 3752. [Google Scholar] [CrossRef]
- Aljuboori, F.A.; Lee, J.H.; Elraies, K.A.; Stephen, K.D. Using Low Salinity Waterflooding to Improve Oil Recovery in Naturally Fractured Reservoirs. Appl. Sci. 2020, 10, 4211. [Google Scholar] [CrossRef]
- Thomas, S. Chemical EOR: The past–does it have a future. Soc. Pet. Eng. Disting. Lect. Ser. 2006, 108828, 2005–2006. [Google Scholar]
- Dandekar, A.Y. Petroleum Reservoir Rock and Fluid Properties; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Shakeel, M.; Pourafshary, P.; Hashmet, M.R. Hybrid Engineered Water–Polymer Flooding in Carbonates: A Review of Mechanisms and Case Studies. Appl. Sci. 2020, 10, 6087. [Google Scholar] [CrossRef]
- Mahmoud, M.; AbdelGawad, K.Z. Chelating-Agent Enhanced Oil Recovery for Sandstone and Carbonate Reservoirs. SPE J. 2015, 20, 483–495. [Google Scholar] [CrossRef]
- Hassan, A.M.; S Al-Hashim, H. Cost effective chelating agent EOR fluid system for carbonate reservoirs. In SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition; Society of Petroleum Engineers: Tulsa, Oklahoma, 2016. [Google Scholar]
- Hassan, A.M.; Al-Hashim, H.S. Optimization of Chelating Agent EOR Solutions for Carbonate Reservoirs. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 23–26 April 2018. [Google Scholar]
- Mahmoud, A.A.; Al-Hashim, H. Insight into the mechanism for oil recovery using EDTA chelating agent solutions from clayey sandstone rocks. J. Pet. Sci. Eng. 2018, 161, 625–635. [Google Scholar] [CrossRef]
- Almubarak, T.; Ng, J.H.; Ramanathan, R.; Nasr-El-Din, H.A. Chelating agents for oilfield stimulation: Lessons learned and future outlook. J. Pet. Sci. Eng. 2021, 205, 108832. [Google Scholar] [CrossRef]
- Attia, M.; Mahmoud, M.; Al-Hashim, H.S.; Sultan, A. Shifting to a New EOR Area for Sandstone Reservoirs with High Recovery, No Damage, and Low Cost. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 31 March–2 April 2014. [Google Scholar]
- Mahmoud, M.; Attia, M.; Al-Hashim, H. EDTA chelating agent/seawater solution as enhanced oil recovery fluid for sandstone reservoirs. J. Pet. Sci. Eng. 2017, 152, 275–283. [Google Scholar] [CrossRef]
- Hassan, A.; Mahmoud, M.; Bageri, B.S.; Aljawad, M.S.; Kamal, M.S.; Barri, A.A.; Hussein, I.A. Applications of Chelating Agents in the Upstream Oil and Gas Industry: A Review. Energy Fuels 2020, 34, 15593–15613. [Google Scholar] [CrossRef]
- Hassan, A.M.; Al-Hashim, H.S. Oil Recovery Mechanisms during Sequential Injection of Chelating Agent Solutions Into Carbonate Rocks. J. Energy Resour. Technol. 2019, 142, 012903. [Google Scholar] [CrossRef]
- Soleimani, H.; Shahsavani, B.; Riazi, M. Evaluation of EDTA as an EOR Agent in Carbonate Rocks. Insight into the Mechanisms. In Proceedings of the 82nd EAGE Annual Conference & Exhibition; European Association of Geoscientists & Engineers: Houten, The Netherlands, 2020; Volume 2020, pp. 1–5. [Google Scholar]
- Shafiq, M.U.; Ben Mahmud, H.K.; Zahoor, M.K.; Shahid, A.S.A.; Rezaee, R.; Arif, M. Investigation of change in different properties of sandstone and dolomite samples during matrix acidizing using chelating agents. J. Pet. Explor. Prod. Technol. 2019, 9, 2793–2809. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.M.; Al-Hashim, H.S. Surface charge study of EDTA interaction with carbonate rock during chelating agent flooding. J. Pet. Sci. Eng. 2020, 191, 107163. [Google Scholar] [CrossRef]
- Mahmoud, M.A.N.E.-D. Effect of Chlorite Clay-Mineral Dissolution on the Improved Oil Recovery from Sandstone Rocks During Diethylenetriaminepentaacetic Acid Chelating-Agent Flooding. SPE J. 2018, 23, 1880–1898. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Al-Hashim, H. Evaluating the integrity of clayey sandstone rocks flooded with high pH EDTA chelating agent solutions. J. Pet. Sci. Eng. 2019, 177, 614–623. [Google Scholar] [CrossRef]
- Chatzis, I.; Morrow, N.R. Correlation of Capillary Number Relationships for Sandstone. Soc. Pet. Eng. J. 1984, 24, 555–562. [Google Scholar] [CrossRef]
- Bashiri, A.; Kasiri, N. Properly Use Effect of Capillary Number on Residual Oil Saturation. In Proceedings of the Nigeria Annual International Conference and Exhibition, Abuja, Nigeria, 30 July–3 August 2011. [Google Scholar]
- Delshad, M.; Bhuyan, D.; Pope, G.A.; Lake, L.W. Effect of Capillary Number on the Residual Saturation of a Three-Phase Micellar Solution. In Proceedings of the SPE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 20–23 April 1986. [Google Scholar]
- Delshad, M.; Najafabadi, N.F.; Anderson, G.; Pope, G.A.; Sepehrnoori, K. Modeling Wettability Alteration by Surfactants in Naturally Fractured Reservoirs. SPE Reserv. Eval. Eng. 2009, 12, 361–370. [Google Scholar] [CrossRef]
- Chen, P.; Mohanty, K.K. Wettability Alteration in High Temperature Carbonate Reservoirs. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Tiab, D.; Donaldson, E.C. Petrophysics: Theory and Practice of Measuring Reservoir Rock and Fluid Transport Properties; Elsevier: Amsterdam, The Netherlands; Gulf Professional Publishing: Oxford, UK, 2015. [Google Scholar]
- Levitt, D.; Bourrel, M. Adsorption of EOR Chemicals under Laboratory and Reservoir Conditions, Part III: Chemical Treatment Methods. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).