Abstract
This research aimed to investigate a new fabrication of poly-(2-dimethyl(aminoethyl) methacrylate)-co-styrene in a porous polymer monolithic morphology. Poly-(2-dimethyl(aminoethyl) methacrylate) (PDMAEMA) is responsive to changes in pH while styrene remains unresponsive to external stimuli. IR, UV/Vis, and SEM were employed to determine that the proposed porous polymer (PPM) monolith can present pH-initiated stimuli response while remaining intact. The copolymerization of PDMAEMA with styrene has not been previously documented in a PPM morphology. It is important to demonstrate that the PPM retains tunable capabilities without destruction to the material. The utility of PDMAEMA copolymers is wide-reaching and this new adaptation of its tunability in a resilient PPM can serve as a distinct preface to original applications in fields such as surface modification, membrane technologies and stationary phases. To further the groundwork of this communication, dynamic studies on the interactions of small molecules with the pores of the monolith will be essential and accomplished via capillary electrochromatography.
1. Introduction
Poly-(2-dimethyl(aminoethyl) methacrylate), or PDMAEMA, is a stimuli-responsive material that was introduced in the 1990s [1,2,3]. It exhibits a strong morphological response to changes in environmental pH (as well as temperature) [4]. When PDMAEMA is subjected to a low pH environment, the amino group of the monomer is protonated. This protonation causes repulsion along the bonding backbone of the polymer chain, which results in elongation due to the electrostatic repulsion of the protonated monomers [5]. Conversely, when PDMAEMA is exposed to high pH, very few of the amino groups are protonated. With fewer protonated amino groups, less repulsion occurs and a more compact polymer chain is observed [5].
This pH-dependent tunability has made PDMAEMA a material of great interest across multiple fields and applications. It has been studied for its utility in gene delivery, as a sensor, and a mediator for drug encapsulation and release [6,7,8,9,10,11,12,13]. This methacrylate polymer is usually polymerized into self-assembled moieties, brushes, dendrimers, and star-like forms [7,14,15]. It is also combined with common monomers such as styrene and polyethylene glycol to make for more flexible and expanded usage [10,11,16]. This includes surface modifications as well as stand-alone structures [14,17,18].
One way that is not present in the literature is the attempt to crosslink PDMAEMA with a compatible monomer into a porous polymer monolith (PPM) morphology. The present group utilizes PPM morphologies to study the mass transport mechanisms of analytes at the solid-liquid interface via capillary electrochromotography [19,20]. The tunability of PDMAEMA presents an interesting choice for surface modifications. However, a fundamental understanding of the small molecule dynamic interactions should be pursued to fully understand the potential of this material. To attempt the study of small molecule dynamics at the solid–liquid interface, an attempt to form a monolithic co-block polymer of PDMAEMA-co-styrene and assess the sturdiness of the monolith under changing environments was made. While polystyrene is not a stimuli-responsive material, it is a strong vinyl polymer ubiquitous in everyday materials. The goal of the present experiment was to create a monolith that retained the structural tunability of the PDMAEMA that also possessed the stability and strength of the polystyrene and proved resistant to degradation. A copolymer with a pH-dependent structural response and a resistance to degradation over time could contribute greatly to work on analyte capture and release, drug delivery, separation science, sensors, and more. The present study attempts to confirm the presence of the tunable features of the co-block polymer in PPM form while remaining intact.
2. Materials and Methods
DMAEMA:Styrene Polymers: Representative polymers A, B, and C were selected as representative samples (Figure S1). The casting solutions contained 10 mL of 2.5 mM sodium phosphate buffer (at pH 8.8–8.9), 30 mL of propanol, 60 mL of acetonitrile, and 250 mg of both 2,2′-azobisisobutyronitrile (AIBN) as the free-radical polymerization initiator, and acrylamido-2-methyl-1-propanesulfonic acid (AMPS) to support electroosmotic flow initiators. All three preparations consisted of 3.5 mL of the casting solution, which contained either a dibasic (A/B) or monobasic (C) 2.5 mM sodium phosphate buffer, and 1.7 mL of DMAEMA:Styrene in predetermined ratios. Polymer A was 75:25 (v:v) DMAEMA:Styrene; Polymers B and C were 85:15 (v:v) DMAEMA:Styrene. Polymers A and C received 1.00 mL DI water to the polymerization solution; Polymer B received 0.75 mL of DI water to the polymerization solution. The solutions were sonicated for 10 min to degas and ensure homogeneity. The various polymer solutions were then sealed in air-tight scintillation vials and submerged in a 90 °C water bath for a period of 48 h.
Spectroscopic Sample Preparation: IR spectra (Nicolet iS10, Thermo Scientific Smart iTR, Madison, USA) and UV/Vis spectra (Varian 50 Bio UV/Vis, Cary Instruments, Palo Alto, USA) were taken of the 0.01 M HCl (pH 2.0) and 0.01 M NaOH (pH 11.9) solutions that contained submerged portions of the polymer samples (A, B, and C). Spectra of the exposed solutions were obtained every other hour, over the course of seven hours. IR spectra were also collected for solid samples of the polymers.
Scanning Electron Microscope: The untreated polymer samples were air-dried, transferred onto a conductive and adhesive carbon tape mounted on an aluminum stage. The treated polymer samples were rewetted with either a drop of 0.1 M HCl (pH 1.1) or 0.1 M NaOH (pH 12.6) on top of the dried polymer. The samples were then allowed air dry for at least four hours.
Morphological characterization of the samples was performed using SEM (Phenom XL, Nanoscience Instruments, Waltham, USA) working in the high vacuum mode at 1 Pa. Viewing was performed at a working distance of 10 mm, with a voltage of 15 kV and a spot size of 5.
3. Results
3.1. Synthesis
The polymer solutions prepared were clear and had the viscosity of water. The polymerization of these solutions resulted in opaque, white solids that were tacky and malleable.
3.2. Spectroscopic Analysis
3.2.1. IR Analysis
The IR spectra collected show that all three polymers remain stable when submerged in both acid (0.01 M HCl, pH 2.0) and base (0.01 M NaOH, pH 11. 9) solution. This can be seen in Figure 1, where the spectra remain mostly unchanged over seven hours of exposure.
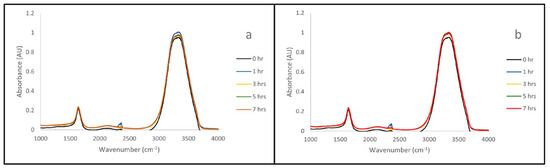
Figure 1.
IR Time Lapse of B with 0.01 M HCl (a) and 0.01 M NaOH (b). Zero, one-, three-, five- and seven-hour IR time lapse of polymer B with exposure to 0.01 M HCl (a) and 0.01 M NaOH (b). The overlay of the spectra suggests that the polymer does not significantly degrade over the course of exposure.
3.2.2. UV/Vis Analysis
The UV/Vis spectra collected also show that all three polymers remain stable when submerged in both acid (0.01 M HCl, pH 2.0) and base (0.01 M NaOH, pH 11.9) solution. This can be seen in Figure 2, where the spectra remain mostly unchanged over the seven hours of exposure.
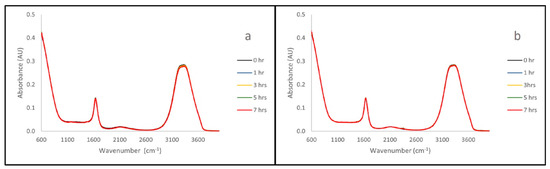
Figure 2.
UV/Vis Time Lapse of B with 0.1 M HCl (a) and 0.1 M NaOH (b). Zero, one-, three-, five- and seven-hour UV-Vis time lapse of polymer B with exposure to 0.1 M HCl. The overlay of the spectra suggests that the polymer does not degrade over the course of the exposure.
3.3. SEM
After the air-drying process, the samples were no longer tacky and had lost most of their malleability. To the naked eye, the acid and base exposure did not cause any changes to these polymers.
SEM images of the unexposed air-dried polymers, the acid exposed polymers (0.1 M HCl, pH 1.1), and the base exposed polymers (0.1 M NaOH, pH 12.6) showed distinct changes in the morphology of the polymers (Figure 3). When compared to the unexposed air-dried polymer, the acid-exposed polymer samples had a relaxed structure, and the base-exposed polymer samples had constrictions that appear pinched. This corresponds to the expected behavior of pure PDMAEMA. When it is exposed to acidic environments, the lone polymer will swell. When exposed to basic environments, the lone polymer will constrict. This is seen in the bulk behavior of the co-polymer presented in Figure 3.
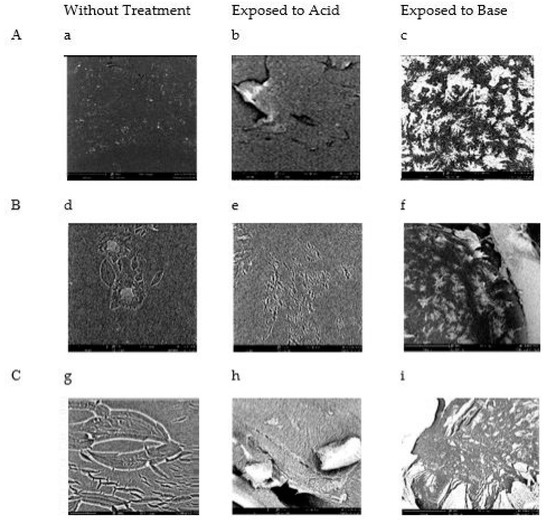
Figure 3.
Comparative SEM images of polymers (A–C) that have been air dried (a,d,g), exposed to 0.1 M HCl (pH 1.1) solution (b,e,h), and exposed to 0.1 M NaOH (pH 12.6) solution (c,f,i).
4. Discussion
This analysis aims to study DMAEMA in a co-block PPM with styrene for the first time. DMAEMA has been shown to exhibit very strong morphological changes in response to altered pH environments [21,22,23]. The amino groups of the monomers are protonated in low pHs which results in repulsion along the polymer chain. This is observed as swelling behavior of the polymer in acidic environments [5]. Conversely, in high pHs, the polymer chain does not have the electrostatic repulsion due to the absence of protons and collapses upon itself [5].
To understand this phenomenon when incorporated into a PPM morphology, three successful fabrications of the co-block PPM (A, B, and C) were tested in extreme acidic and basic environments. SEM images were taken of these with and without exposure to solutions with adjusted pHs. These images showed a consistent behavior that is reliant on environmental pH. These PPMs are reacting in a uniform manner in response to stimuli, which gives them a unique range of potential.
Initial efforts to characterize this PPM were performed via SEM. The images collected from the air-dried samples show a distinct and pH-reliant behavior (Figure 3). These were all white, opaque, and flexible solids (SI Figure S1). It can be observed across all three polymers that the polymers exposed to acid appear to relax the solid surface (Figure 3b,e,h). This is especially apparent in polymer B (Figure 3e). A contrasting effect can be seen in the samples exposed to a basic solution, as the polymer appears to constrict and tighten in pockets and veins (Figure 3c,f,i). This illustrates the expected swelling (in acid) and shrinking (in base) behavior of PDMAEMA. However, it is the first time that a PPM fabrication of this copolymer has been reported.
IR and UV/Vis readings were taken of HCl and NaOH solutions that had been exposed to the polymers of interest (A, B, C) over the course of seven hours. These measurements were performed to ensure that the exposure of the PPM to the adjusted pH solutions does not alter, or degrade, the PPM. The representative spectra for sample B are shown here (Figure 1 and Figure 2), as this was the sample that appeared to undergo the most change during SEM analysis. Representative spectra for samples A and C can be seen in Supplemental Information Figures S2–S5. PDMAEMA characteristically shows IR stretches at 1180 cm−1 (C-O), 1650 cm−1 (N-H) and 1710 cm−1 (C=O) [24]. These stretches do not appear in Figure 1. This is a clear indication that the polymer is not disintegrating in the acid/base solutions. In a parallel manner, no additional stretches appear in the UV/Vis spectra (Figure 2). This is a clear indication that the polymer is not disintegrating in the acid/base solutions. The time-lapse shows that, although there are small variances present, they do not result in the appearance of the DMAEMA monomer in the solution over time (Figure 1 and Figure 2). Peaks characteristic to the polymers were identified and used for comparison to the time-lapse spectra to check for polymer degradation. When comparing the IR spectra of the polymers themselves (Figures S6–S8) to that of the acid/base solution spectra, polymer-specific peaks at 1180 cm−1 (C-O), 1650 cm−1 (N-H) and 1710 cm−1 (C=O) are not present in the solution spectra [24]. This confirms that the polymer is not degrading into solution in acidic or basic environments, and therefore the PPM remains structurally intact. This leads us to believe that the change observed under SEM is not chemically destructive, but simply a behavior in the morphology of the solids.
Various DMAEMA:Styrene co-block polymers were observed in this study. It was shown that the co-block polymer can be formed into a monolithic morphology and retain the stimuli-responsive features of DMAEMA. Exposure to acid and base resulted in a distinct change in the appearance of the sample. The changes mimic those seen in strand morphologies while remaining in a monolith. The pH sensitivity observed indicates a level of environmental interactions that impact the physical morphology of the solids without impairing their chemical integrity. These features had not been observed and characterized in this manner previously. This is exciting information that has brought on new questions and ideas for future experimentation. Future efforts aim to analyze the transport behavior of analytes within the PPM [19,20].
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11157097/s1, Supplemental Information Figure S1. Successful polymerization of representative samples A, B, and C. Figure S2. IR A Time Lapse with 0.01 M HCl and 0.01 M NaOH. Figure S3. IR C Time Lapse with 0.01 M HCl, and 0.01 M NaOH. Figure S4. UV-Vis Time Lapse of A with 0.1 M HCl and 0.1 M NaOH. Figure S5. UV-Vis Time Lapse of C with 0.1 M HCl and 0.1 M NaOH. Figure S6. IR Spectra of Solid Sample Polymer A. Figure S7. IR Spectra of Solid Sample Polymer B. Figure S8. IR Spectra of Solid Sample Polymer C.
Author Contributions
Conceptualization, C.R.D.; methodology, M.H., A.S. and C.A.; validation, M.H., A.S. and W.D.; formal analysis, M.H., A.S. and C.R.D.; investigation, M.H.; data curation, M.H. and A.S.; writing—original draft preparation, M.H. and C.R.D.; writing—review and editing, M.H., A.S. and C.R.D.; supervision, C.R.D.; project administration, C.R.D.; funding acquisition, C.R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the American Chemical Society Petroleum Research Fund, grant number 57814-UNI7 and Northern Kentucky University CINSAM Research Grants.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to university policy.
Acknowledgments
The authors wish to acknowledge Lauren Rigg, Eric Milner, and Peyton Hayes for their contributions to this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beadle, P.M.; Rowan, L.; Mykytiuk, J.; Billingham, N.C.; Armes, S.P. Synthesis and characterization of sterically stabilized colloidal dispersions of polypyrrole using novel tailor-made water-soluble block copolymers of narrow molecular weight distribution. Polymer 1993, 34, 1561–1563. [Google Scholar] [CrossRef]
- Aggour, Y.A. Preparation, characterization and thermal stability of poly[2-(dimethylamino)ethylacrylate]. J. Therm. Anal. 1994, 42, 1185–1191. [Google Scholar] [CrossRef]
- Oh, J.; Lee, H.; Shim, H.; Choi, S. Synthesis and surface activity of novel ABA type triblock cataionic amphiphiles. Polym. Bull. 1994, 32, 149–154. [Google Scholar] [CrossRef]
- Li, F.M.; Chen, S.J.; Du, F.S.; Wu, Z.Q.; Li, Z.C. Stimuli-Responsive Behavior of N,N-Dimethylaminoethyl Methacrylate Polymers and Their Hydrogels. In Field Responsive Polymers: Electroresponsive, Photoresponsive, and Responsive Polymers in Chemistry and Biology; Khan, I.M., Harrison, J.S., Eds.; American Chemical Society Distributed by Oxford University Press: Washington, DC, USA, 1999; pp. 266–276. [Google Scholar]
- Mohammadi, M.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Golshan, M. Effect of molecular weight and polymer concentration on the triple temperature/pH/ionic strength-sensitive behavior of poly(2-(dimethylamino)ethyl methacrylate). Int. J. Polym. Mater. 2016, 66, 455–461. [Google Scholar] [CrossRef]
- Bitoque, D.B.; Rosa da Costa, A.; Silva, G.A. Insights on the intracellular trafficking of PDMAEMA gene therapy vectors. Mater. Sci. Eng. C 2018, 93, 277–288. [Google Scholar] [CrossRef]
- Ren, M.J.; McKenzie, T.G.; Fu, Q.; Wong, E.H.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiau, G.G. Star Polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef]
- Reifarth, M.; Hoeppener, S.; Schubert, U.S. Uptake and Intracellular Fate of Engineered Nanoparticles in Mammalian Cells: Capabilities and Limitations of Transmission Electron Microscopy—Polymer-Based Nanoparticles. Adv. Mater. 2018, 30, 1703704. [Google Scholar] [CrossRef]
- Tan, J.K.Y.; Choi, J.L.; Wei, H.; Schellinger, J.G.; Pun, S.H. Reducible, dibromomaleimide-linked polymers for gene delivery. Biomater. Sci. 2015, 3, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Werfel, T.A.; Jackson, M.A.; Kavanaugh, T.E.; Kirkbride, K.C.; Miteva, M.; Giorgio, T.D.; Duvall, C. Combinatorial optimization of PEG architecture and hydrophobic content improves ternary siRNA polyplex stability, pharmacokinetics, and potency in vivo. J. Control. Release 2017, 255, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Lou, B.; De Beuckelaer, A.; Dakwar, G.R.; Remaut, K.; Grooten, J.; Braeckmans, K.; De Geest, B.G.; Mastrobattista, E.; De Koker, S.; Hennink, W.E. Post-PEGylated and crosslinked polymeric RNA nanocomplexes as adjuvants targeting lymph nodes with increased cytolytic T cell inducing properties. J. Control. Release 2018, 284, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.R.; Zhao, B.; Anson, F.; Fernandez, A.; Singh, K.; Homyak, C.; Canakci, M.; Vachet, R.W.; Thayumanavan, S. Matrix Metalloproteinase-9-Responsive Nanogels for Proximal Surface Conversion and Activated Cellular Uptake. Biomacromolecules 2018, 19, 860–871. [Google Scholar] [CrossRef]
- Uchida, S.; Kataoka, K. Design concepts of polyplex micelles for in vivo therapeutic delivery of plasmid DNA and messenger RNA. J. Biomed. Mater. Res. Part A 2018, 107, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Teper, P.; Sotirova, A.; Mitova, V.; Oleszko-Torbus, N.; Utrata-Wesolek, A.; Koseva, N.; Kowalczuk, A.; Mendrek, B. Antrimicrobial Activity of Hybrid Nanomaterials Based on Star and Linear Polymers of N,N’-Dimethylaminoethyl Methacrylate with In Situ Produced Silver Nanoparticles. Materials 2020, 13, 3037. [Google Scholar] [CrossRef]
- Kafetzi, M.; Pispas, S. Effects of Hydrophobic Modifications on the Solution Self-Assembly of P(DMAEMA-co-QDMAEMA)-b- POEGMA Random Diblock Copolymers. Polymers 2021, 13, 338. [Google Scholar] [CrossRef]
- Bitoque, D.B.; Simão, S.; Oliveira, A.V.; Machado, S.; Duran, M.R.; Lopes, E.; Rosa da Costa, A.M.; Silva, G.A. Efficiency of RAFT-synthesized PDMAEMA in gene transfer to the retina. J. Tissue Eng. Regen. Med. 2014, 11, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, M.; Li, S.; Li, A.; An, H.; Ye, H.; Zhang, Y. Aggregation and supreamolecular chirality of 5,10,15,20-tetrakis-(4-sulfonatophenyl)-porphyrin on an achiral poly(2-(dimethylamino)ethyl methylacrylate)-grafted ethylene-vinyl alcohol membrane. J. Mater. Chem. C 2015, 3, 3650–3658. [Google Scholar] [CrossRef]
- Mushtaq, S.; Ahmad, N.M.; Mahmood, A.; Iqbal, M. Antibacterial Amphiphilic Copolymers of Dimethylamino Ethyl Methacrylate and Methyl Methacrylate to Control Biofilm Adhesion for Antifouling Applications. Polymers 2021, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Daniels, C.R.; Waguespack, B.L.; Hodges, S.A.; Bushey, M.M. Temperature effects on retention and efficiency of butyl and lauryl acrylate porous polymer monoliths in capillary electrochromatography. J. Sep. Sci. 2019, 42, 3703–3711. [Google Scholar] [CrossRef]
- Daniels, C.R.; Li, S.Y.; Zhao, Y.; Kuklinski, N.; Bushey, M.M. A thermodynamic study of capillary electrochromatographic retention of aromatic hydrocarbons on a lauryl acrylate porous polymer monolithic column with measured phase ratio. J. Sep. Sci. 2021. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Qian, Y.; Yu, Y.; Jiang, Y.; Zhang, G.; Liu, S. Cytoplasmic Reactive Cationic Amphiphiles for Efficient Intracellular Delivery and Self-Reporting Smart Release. Macromolecules 2015, 48, 5959–5968. [Google Scholar] [CrossRef]
- Lu, B.; Tarn, M.D.; Pamme, N.; Georgiou, T.K. Fabrication of tailorable pH responsive cationic amphiphilic microgels on a microfluidic device for drug release. J. Polym. Sci. Part. A Polym. Chem. 2017, 56, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Välimäki, S.; Khakalo, A.; Ora, A.; Johansson, L.; Rojas, O.J.; Kostiainen, M.A. Effect of PEG-PDMAEMA Block Copolymer Architecture on Polyelectrolyte Complex Formation with Heparin. Biomacromolecules 2016, 17, 2891–2900. [Google Scholar] [CrossRef]
- Veuillet, M.; Ploux, L.; Roucoules, V.; Gourbeyre, Y.; GAudichet-Maurin, E. Bacterial adhesion driven by mechanical properties of DMAEMA plasma polymer coatings. In Proceedings of the 2015 22nd International Symposium on Plasma Chemistry, Antwerp, Belgium, 5–10 July 2015; pp. 1–3. Available online: https://www.ispc-conference.org/ispcproc/ispc22/O-16-1.pdf (accessed on 24 June 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).