Thermal and Physical Properties of Crude Palm Oil with Higher Oleic Content
Abstract
:1. Introduction
2. Material and Methods
2.1. Physicochemical Characterization
2.2. Physical and Thermal Properties
3. Results and Discussion
3.1. Chemical Characterization of the Oils
3.2. Physicochemical Properties
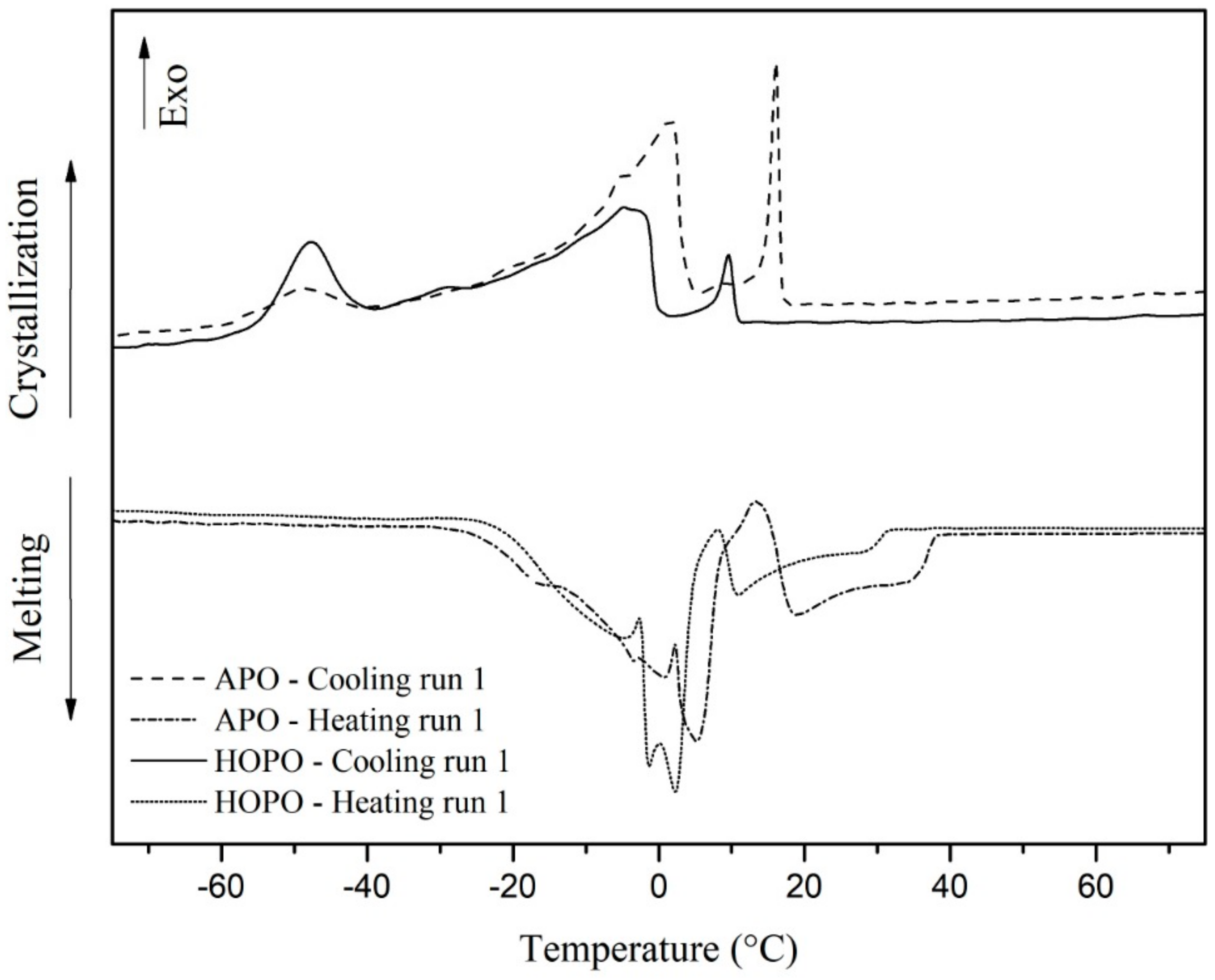
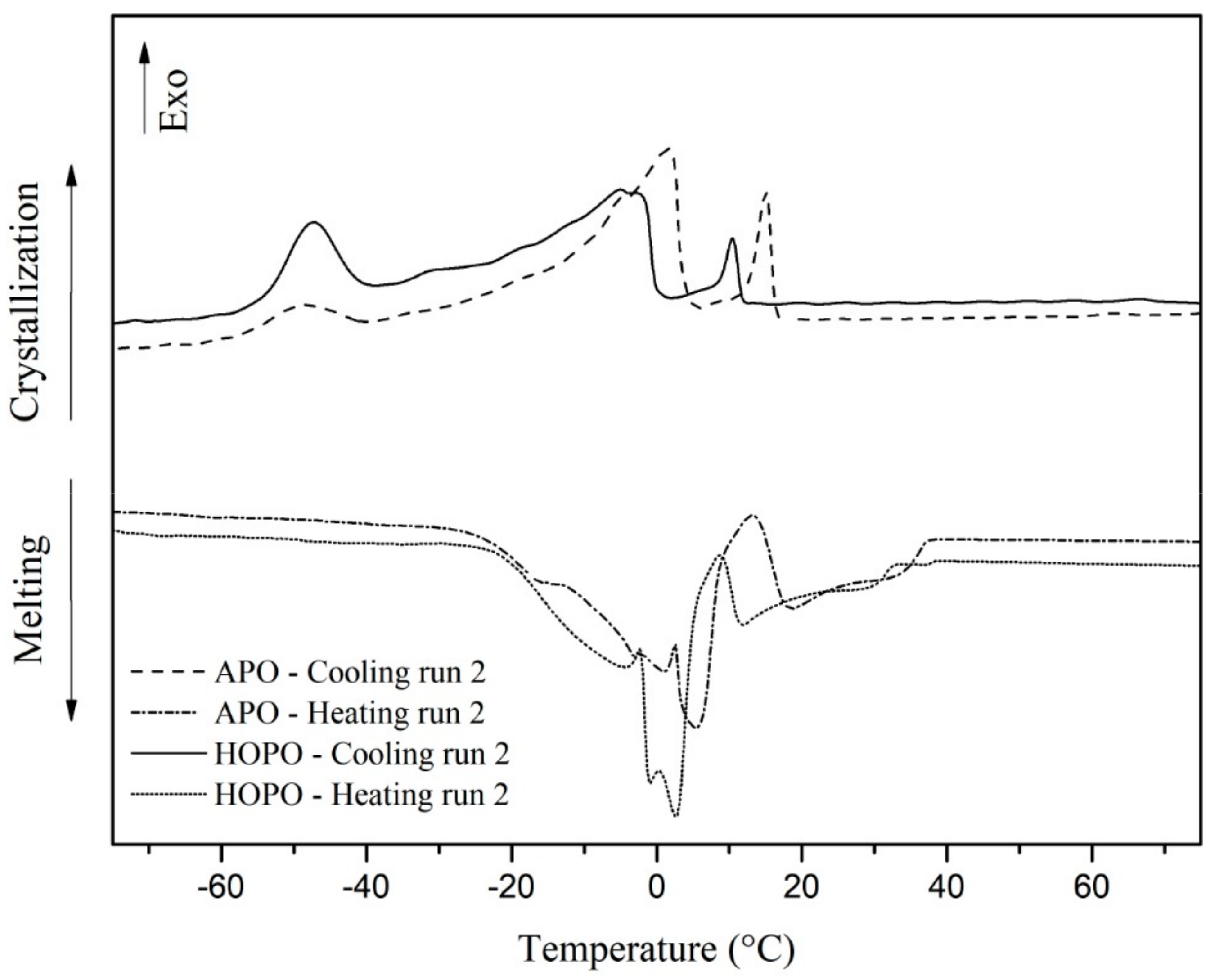
3.3. Thermal Analysis of the Oils
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture (USDA). Oilseeds: World Markets and Trade. Available online: https://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf (accessed on 5 January 2021).
- Oosterveer, P. Sustainability of Palm Oil and Its Acceptance in the EU. J. Oil Palm Res. 2020, 32, 365–376. [Google Scholar] [CrossRef]
- Sampaio, K.A.K.A.; Ayala, J.V.; Van Hoed, V.; Monteiro, S.; Ceriani, R.; Verhé, R.; Meirelles, A.J. Impact of Crude Oil Quality on the Refining Conditions and Composition of Nutraceuticals in Refined Palm Oil. J. Food Sci. 2017, 82, 1842–1850. [Google Scholar] [CrossRef]
- Pirker, J.; Mosnier, A.; Kraxner, F.; Havlík, P.; Obersteiner, M. What Are the Limits to Oil Palm Expansion? Glob. Environ. Chang. 2016, 40, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Mozzon, M.; Pacetti, D.; Lucci, P.; Balzano, M.; Frega, N.G. Crude Palm Oil from Interspecific Hybrid Elaeis Oleifera × Elaeis Guineensis: Fatty Acid Regiodistribution and Molecular Species of Glycerides. Food Chem. 2013, 141, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.A.A.; Almeida, E.S.; Neto, B.A.D.; Abdelnur, P.V.; Monteiro, S. Identification of Carotenoid Isomers in Crude and Bleached Palm Oils by Mass Spectrometry. LWT Food Sci. Technol. 2018, 89, 631–637. [Google Scholar] [CrossRef]
- Mozzon, M.; Pacetti, D.; Frega, N.G.; Lucci, P. Crude Palm Oil from Interspecific Hybrid Elaeis Oleifera × E. Guineensis: Alcoholic Constituents of Unsaponifiable Matter. J. Am. Oil Chem. Soc. 2015, 92, 717–724. [Google Scholar] [CrossRef]
- Buscato, M.H.M.; Zaia, B.G.; De Godoi, K.R.R.; Ribeiro, A.P.B.; Kieckbusch, T.G. Modification of Palm Oil Crystallization by Phytosterol Addition as a Tool for Structuring a Low Saturated Lipid Blend. Braz. J. Chem. Eng. 2018, 35, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Vidoca, L.P.; Almeida, E.S.; Cardoso, M.F.; Otavio, L.; Valadares, L.F.; Monteiro, S. Extraction of Carotene from Crude Hybrid Palm Oil Using Polymeric Resin. J. Food Eng. 2020, 278, 109944. [Google Scholar] [CrossRef]
- Hardon, J.J. Interspecific Hybrids in the Genus Elaeis II. Vegetative Growth and Yield of F1 Hybrids E. Guineensis × E. Oleifera. Euphytica 1969, 18, 380–388. [Google Scholar] [CrossRef]
- Montoya, C.; Lopes, R.; Flori, A.; Cros, D.; Cuellar, T.; Summo, M.; Espeout, S.; Rivallan, R.; Risterucci, A.M.; Bittencourt, D.; et al. Quantitative Trait Loci (QTLs) Analysis of Palm Oil Fatty Acid Composition in an Interspecific Pseudo-Backcross from Elaeis Oleifera (H.B.K.) Cortés and Oil Palm (Elaeis Guineensis Jacq.). Tree Genet. Genomes 2013, 9, 1207–1225. [Google Scholar] [CrossRef] [Green Version]
- Mozzon, M.; Foligni, R.; Mannozzi, C. Current Knowledge on Interspecific Hybrid Palm Oils as Food and Food Ingredient. Foods 2020, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Basiron, Y. Palm Oil. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; John Wiley & Son: New York, NY, USA, 2005; Volume 2, pp. 333–430. [Google Scholar]
- Che Man, Y.B.; Haryati, T.; Ghazali, H.M.; Asbi, B.A. Composition and Thermal Profile of Crude Palm Oil and Its Products. J. Am. Oil Chem. Soc. 1999, 76, 237–242. [Google Scholar] [CrossRef]
- Hishamuddin, E.; Nagy, Z.K.; Stapley, A.G.F. Thermodynamic Analysis of the Isothermal Fractionation of Palm Oil Using a Novel Method for Entrainment Correction. J. Food Eng. 2020, 273, 109806. [Google Scholar] [CrossRef]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef]
- West, R.; Rousseau, D. Tripalmitin-Driven Crystallization of Palm Oil: The Role of Shear and Dispersed Particles. JAOCS J. Am. Oil Chem. Soc. 2020, 97, 989–999. [Google Scholar] [CrossRef]
- Gibon, V.; Danthine, S. Systematic Investigation of Co-Crystallization Properties in Binary and Ternary Mixtures of Triacylglycerols Containing Palmitic and Oleic Acids in Relation with Palm Oil Dry Fractionation. Foods 2020, 9, 1891. [Google Scholar] [CrossRef] [PubMed]
- Wan Nik, W.B.; Ani, F.N.; Masjuki, H.H. Thermal Stability Evaluation of Palm Oil as Energy Transport Media. Energy Convers. Manag. 2005, 46, 2198–2215. [Google Scholar] [CrossRef] [Green Version]
- Ceriani, R.; Paiva, F.R.; Goncalves, C.B.; Batista, E.A.C.; Meirelles, A.J.A. Densities and Viscosities of Vegetable Oils of Nutritional Value. J. Chem. Eng. Data 2008, 53, 1846–1853. [Google Scholar] [CrossRef]
- Freitas, S.V.D.; e Silva, F.A.; Pastoriza-Gallego, M.J.; Piñeiro, M.M.; Lima, A.S.; Coutinho, J.A.P. Measurement and Prediction of Densities of Vegetable Oils at Pressures up to 45 MPa. J. Chem. Eng. Data 2013, 58, 3046–3053. [Google Scholar] [CrossRef]
- AOCS. Methods and Recommended Practices of the American Oil Chemists’ Society; America Oil Chemists’ Society: Champaign, IL, USA, 1998. [Google Scholar]
- Siew, W.L.; Tang, T.S.; Tan, Y.A. PORIM: Test Methods; Palm Oil Research Institute of Malaysia: Kuala Lumpur, Malaysia, 1995. [Google Scholar]
- Ansolin, M.; de Souza, P.T.; de Almeida Meirelles, A.J.; Batista, E.A.C. Tocopherols and Tocotrienols: An Adapted Methodology by UHPLC/MS Without Sample Pretreatment Steps. Food Anal. Methods 2017, 10, 2165–2174. [Google Scholar] [CrossRef]
- Vigli, G.; Philippidis, A.; Spyros, A.; Dais, P. Classification of Edible Oils by Employing 31P and 1H NMR Spectroscopy in Combination with Multivariate Statistical Analysis. A Proposal for the Detection of Seed Oil Adulteration in Virgin Olive Oils. J. Agric. Food Chem. 2003, 51, 5715–5722. [Google Scholar] [CrossRef] [PubMed]
- De Graef, V.; Dewettinck, K.; Verbeken, D.; Foubert, I. Rheological Behavior of Crystallizing Palm Oil. Eur. J. Lipid Sci. Technol. 2006, 108, 864–870. [Google Scholar] [CrossRef]
- España, M.D.; Mendonça, S.; Carmona, P.A.O.; Guimarães, M.B.; da Cunha, R.N.V.; Souza, M.T.; Cunha, R.N.V.; Souza, M.T. Chemical Characterization of the American Oil Palm from the Brazilian Amazon Forest. Crop Sci. 2018, 58, 1982–1990. [Google Scholar] [CrossRef]
- Braipson-Danthine, S.; Gibon, V. Comparative Analysis of Triacylglycerol Composition, Melting Properties and Polymorphic Behavior of Palm Oil and Fractions. Eur. J. Lipid Sci. Technol. 2007, 109, 359–372. [Google Scholar] [CrossRef]
- Gibon, V.; De Greyt, W.; Kellens, M. Palm Oil Refining. Eur. J. Lipid Sci. Technol. 2007, 109, 315–335. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond Tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codex Alimentarius. Codex Standard For Named Vegetable Oils CXS 210-1999. Available online: http://www.fao.org/3/y2774e/y2774e04.htm (accessed on 5 January 2021).
- Danthine, S.; De Clercq, N.; Dewettinck, K.; Gibon, V. Monitoring Batch Lipase Catalyzed Interesterification of Palm Oil and Fractions by Differential Scanning Calorimetry. J. Therm. Anal. Calorim. 2014, 115, 2219–2229. [Google Scholar] [CrossRef]
- De Oliveira, G.M.; Badan Ribeiro, A.P.; dos Santos, A.O.; Cardoso, L.P.; Kieckbusch, T.G. Hard Fats as Additives in Palm Oil and Its Relationships to Crystallization Process and Polymorphism. LWT Food Sci. Technol. 2015, 63, 1163–1170. [Google Scholar] [CrossRef]
- Kodali, D.R. Trans Fats: Health, Chemistry, Functionality, and Potential Replacement Solutions. In Trans Fats Replacement Solutions; Kodali, D.R., Ed.; AOCS Press: Urbana, IL, USA, 2014; pp. 1–39. [Google Scholar] [CrossRef]
- Foon, C.S.; Liang, Y.C.; Mat Dian, N.L.H.; May, C.Y.; Hock, C.C.; Ngan, M.A. Crystallisation and Melting Behavior of Methyl Esters of Palm Oil. Am. J. Appl. Sci. 2006, 3, 1859–1863. [Google Scholar] [CrossRef] [Green Version]
- Castro, R.I.; Gallego, J.; García, M.F.; Marican, A.; Forero-Doria, O. Thermal Study and Composition of Edible Oils Combined by TG/DTG Analysis through Predictive Statistical Model. J. Therm. Anal. Calorim. 2020, 1–8. [Google Scholar] [CrossRef]

| HOPO | APO | |
|---|---|---|
| ACYLGLYCEROLS (% W/W) | ||
| MONOACYLGLYCEROL | 0.21 | 0.24 |
| DIACYLGLYCEROL | 3.96 | 4.54 |
| TRIACYLGLYCEROL | 93.73 | 91.44 |
| FATTY ACIDS PROFILE (%) | ||
| OLEIC | 56.34 | 44.81 |
| LINOLEIC | 7.35 | 7.65 |
| LINOLENIC | 2.06 | 1.28 |
| FREE FATTY ACIDS (%, PALMITIC) | 2.1 ± 0.09 | 3.8 ± 0.08 |
| IODINE VALUE (G/100 G) * | 67.7 | 52.3 |
| UNSAPONIFIABLE MATTER (MG/G) | 2.3 ± 0.2 | 1.3 ± 0.3 |
| TOTAL CAROTENE (MG/KG) | 830 ± 6 | 524 ± 4.4 |
| α-CAROTENE | 60 | 51 |
| β-CAROTENE | 504 | 380 |
| TOCOLS (MG/KG) | 883.3 | 728.0 |
| α-TOCOPHEROL | 136.4 | 167.5 |
| β/γ-TOCOPHEROL | 0 | 0 |
| δ-TOCOPHEROL | 0 | 0 |
| α-TOCOTRIENOL | 128.6 | 117.1 |
| β/γ-TOCOTRIENOL | 603.5 | 413.6 |
| δ-TOCOTRIENOL | 15.2 | 29.8 |
| T (°C) | HOPO | APO | ||
|---|---|---|---|---|
| DENSITY (G/CM3) | VISCOSITY (CP) | DENSITY (G/CM3) | VISCOSITY (CP) | |
| 20 | 0.91369 | - | 0.91450 | - |
| 30 | 0.90637 | - | 0.90680 | - |
| 40 | 0.89866 | 40.19 | 0.89739 | 43.79 |
| 50 | 0.88663 | 27.59 | 0.88509 | 25.19 |
| 60 | 0.88396 | 20.4 | 0.88040 | 18.6 |
| 70 | 0.87849 | 15.6 | 0.86810 | 13.8 |
| 80 | 0.87174 | 12.0 | 0.86411 | 10.8 |
| 1° PEAK | AD | 2° PEAK | AD | 3° PEAK | AD | ||
|---|---|---|---|---|---|---|---|
| APO | TM | 5.32 | 0.33 | 18.68 | 0.50 | - | - |
| TRM | −30.2 UP TO 13.5 | - | 13.42 UP TO 38.67 | - | - | - | |
| TC | −49.095 | 0.25 | 1.555 | 0.09 | 15.61 | 0.88 | |
| TRC | −63.32 UP TO −39.55 | - | −39.55 UP TO 5.88 | - | 5.88 UP TO 17.89 | - | |
| HOPO | TM | 2.47 | 0.33 | 11.36 | 0.83 | - | - |
| TRM | −30.0 UP TO 8.5 | - | −0.05 UP TO 33.29 | - | - | - | |
| TC | −47.67 | 0.42 | −4.81 | 0.25 | 9.98 | 0.84 | |
| TRC | −71.88 UP TO 39.01 | - | −39.01 UP TO 1.82 | - | 1.82 UP TO 12.29 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida, E.S.; da Silva Damaceno, D.; Carvalho, L.; Victor, P.A.; dos Passos, R.M.; de Almeida Pontes, P.V.; Cunha-Filho, M.; Sampaio, K.A.; Monteiro, S. Thermal and Physical Properties of Crude Palm Oil with Higher Oleic Content. Appl. Sci. 2021, 11, 7094. https://doi.org/10.3390/app11157094
de Almeida ES, da Silva Damaceno D, Carvalho L, Victor PA, dos Passos RM, de Almeida Pontes PV, Cunha-Filho M, Sampaio KA, Monteiro S. Thermal and Physical Properties of Crude Palm Oil with Higher Oleic Content. Applied Sciences. 2021; 11(15):7094. https://doi.org/10.3390/app11157094
Chicago/Turabian Stylede Almeida, Erislene S., Daniela da Silva Damaceno, Laiane Carvalho, Priscilla Araújo Victor, Rafaela Menezes dos Passos, Paula Virginia de Almeida Pontes, Marcílio Cunha-Filho, Klicia A. Sampaio, and Simone Monteiro. 2021. "Thermal and Physical Properties of Crude Palm Oil with Higher Oleic Content" Applied Sciences 11, no. 15: 7094. https://doi.org/10.3390/app11157094
APA Stylede Almeida, E. S., da Silva Damaceno, D., Carvalho, L., Victor, P. A., dos Passos, R. M., de Almeida Pontes, P. V., Cunha-Filho, M., Sampaio, K. A., & Monteiro, S. (2021). Thermal and Physical Properties of Crude Palm Oil with Higher Oleic Content. Applied Sciences, 11(15), 7094. https://doi.org/10.3390/app11157094






