Human Milk Fat Substitutes from Lard and Hemp Seed Oil Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Human Milk Substitutes—Enzymatic Interesterification
2.3. Analysis
2.3.1. Determination of Fatty Acid Composition
2.3.2. Determination of Generated sn-2 Monoacylglycerols
2.3.3. Determination of Free Fatty Acid Content
2.3.4. Determination of the Peroxide Value
2.3.5. DSC Measurements
2.4. Statistical Analysis
3. Results
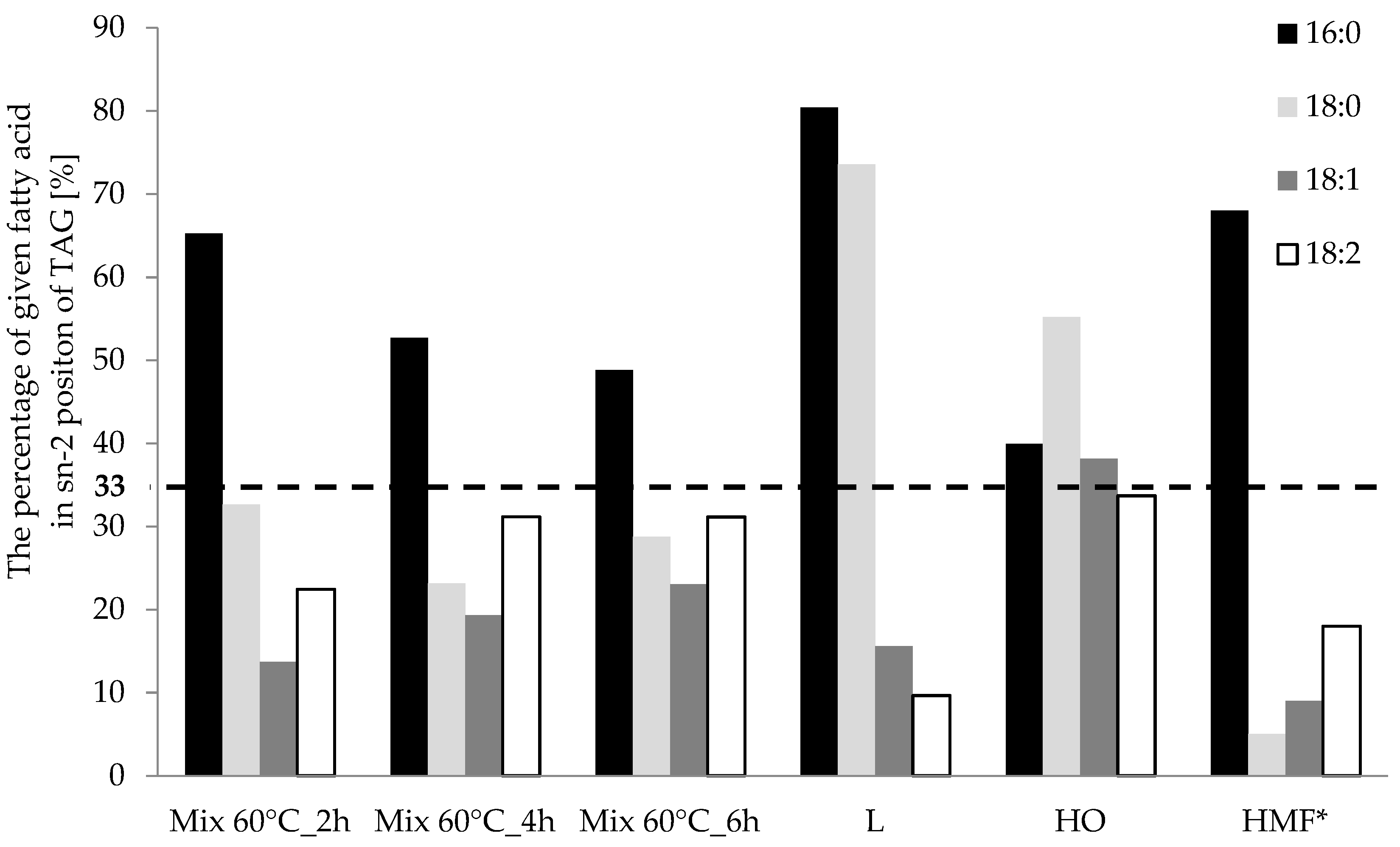
3.1. Fatty Acid Profile and Their Distribution in Triacylglycerols (TAGs)
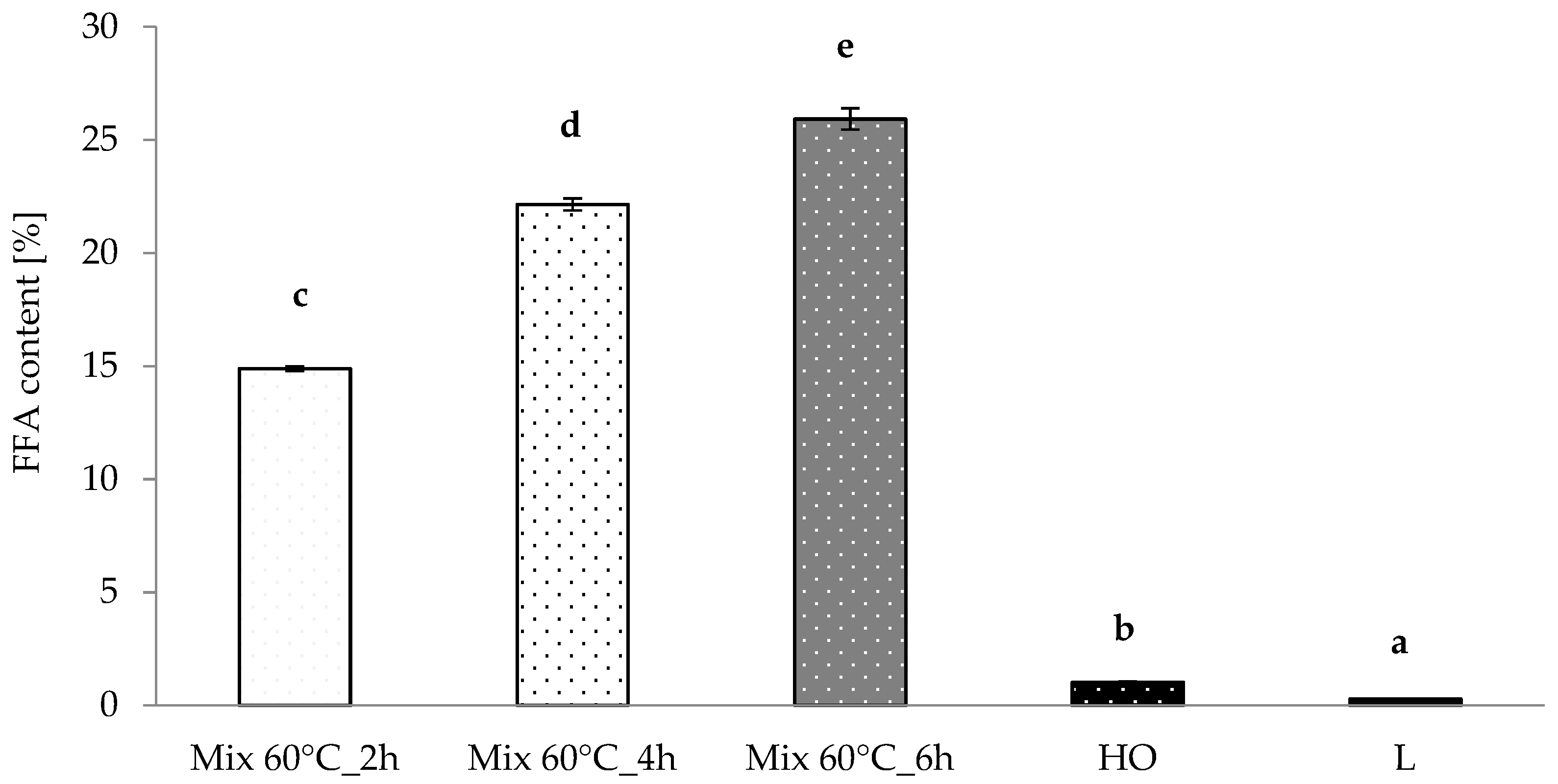
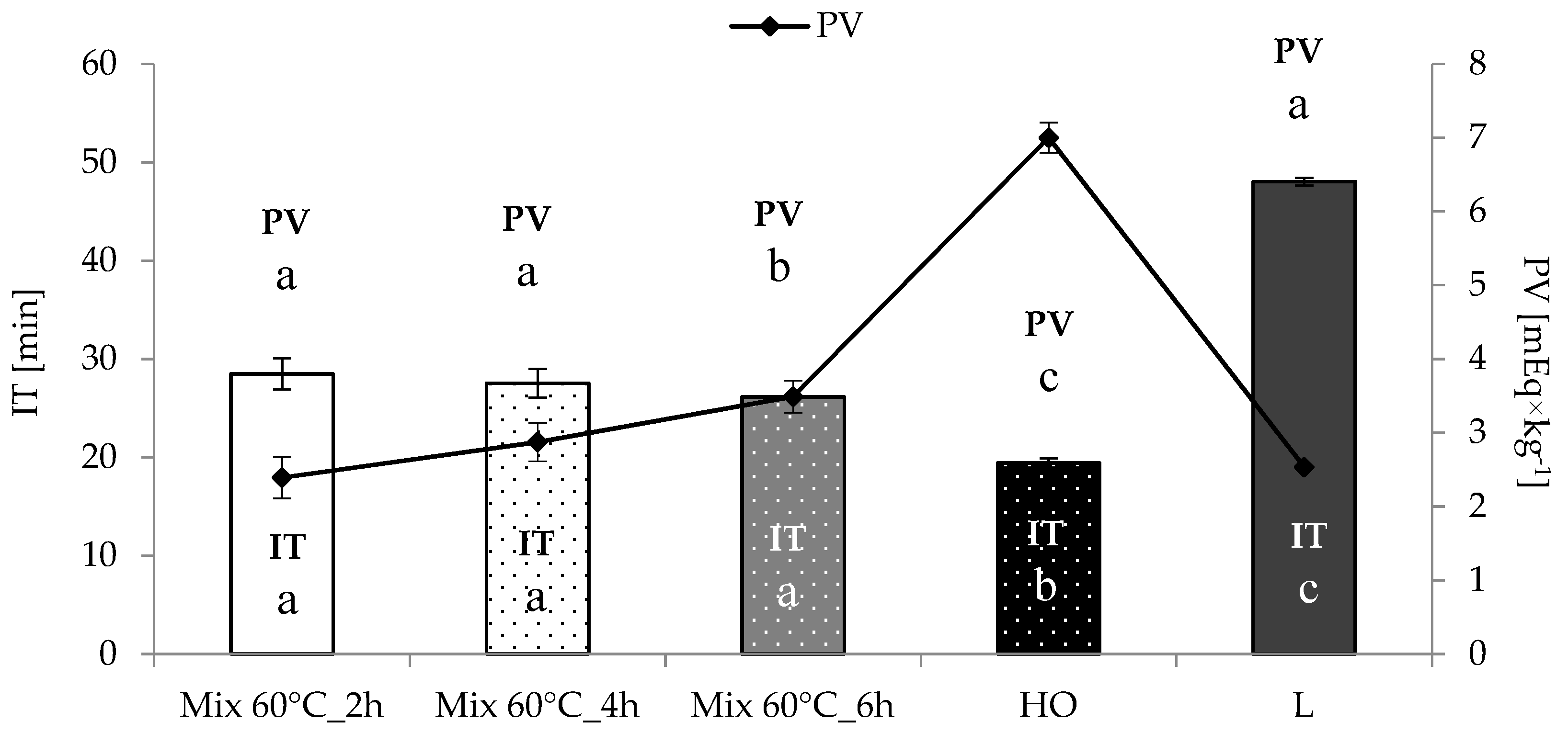
3.2. Free Fatty Acid Content and Oxidative Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, J.N.; Gioielli, L.A. Chemical interesterification of milkfat and milkfat-corn oil blends. Food Res. Int. 2003, 36, 149–159. [Google Scholar] [CrossRef]
- Karabulut, I.; Turan, S.; Ergin, G. Effects of chemical interesterification on solid fat content and slip melting point of fat/oil blends. Eur. Food Res. Technol. 2004, 218, 224–229. [Google Scholar] [CrossRef]
- Idris, N.A.; Dian, N.L.H.M. Interesterified palm products as alternatives to hydrogenation. Asia Pac. J. Clin. Nutr. 2005, 14, 396–401. [Google Scholar] [PubMed]
- Farfán, M.; Villalón, M.J.; Ortíz, M.E.; Nieto, S.; Bouchon, P. The effect of interesterification on the bioavailability of fatty acids in structured lipids. Food Chem. 2013, 139, 571–577. [Google Scholar] [CrossRef]
- De Paula, A.V.; Nunes, G.F.M.; de Castro, H.F.; dos Santos, J.C. Performance of packed bed reactor on the enzymatic interesterification of milk fat with soybean oil to yield structure lipids. Int. Dairy J. 2018, 86, 1–8. [Google Scholar] [CrossRef]
- Santoro, V.; Dal Bello, F.; Aigotti, R.; Gastaldi, D.; Romaniello, F.; Forte, E.; Magni, M.; Baiocchi, C.; Medana, C. Characterization and determination of interesterification markers (triacylglycerol regioisomers) in confectionery oils by liquid chromatography-mass spectrometry. Foods 2018, 7, 23. [Google Scholar] [CrossRef]
- Norizzah, A.R.; Nur Azimah, K.; Zaliha, O. Influence of enzymatic and chemical interesterification on crystallisation properties of refined, bleached and deodourised (RBD) palm oil and RBD palm kernel oil blends. Food Res. Int. 2018, 106, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, A.G.; Rousseau, D. Engineering triacylglyerols: The role of interesterification. Trends Food Sci. Technol. 1995, 6, 329–335. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, X.; Mu, H.; Nilsson, J.; Adler-Nissen, J.; Høy, C.E. Lipozyme IM-catalyzed interesterification for the production of margarine fats in a 1 kg scale stirred tank reactor. Eur. J. Lipid Sci. Technol. 2000, 102, 411–418. [Google Scholar] [CrossRef]
- Michalskia, M.-C.; Briarda, V.; Juanedab, P. CLA profile in native fat globules of different sizes selected from raw milk. Int. Dairy J. 2005, 15, 1089–1094. [Google Scholar] [CrossRef]
- Meng, F.; Uniacke-Lowe, T.; Ryan, A.C.; Kelly, A.L. The composition and physico-chemical properties of human milk: A review. Trends Food Sci. Technol. 2021, 112, 608–621. [Google Scholar] [CrossRef]
- Şahin-Yeşilçubuk, N.; Akoh, C.C. Biotechnological and Novel Approaches for Designing Structured Lipids Intended for Infant Nutrition. J. Am. Oil Chem. Soc. 2017, 94, 1005–1034. [Google Scholar] [CrossRef]
- Jensen, R.G. The lipids in human milk. Prog. Lipid Res. 1996, 35, 53–92. [Google Scholar] [CrossRef]
- Alles, M.S.; Scholtens, P.A.M.J.; Bindels, J.G. Current trends in the composition of infant milk formulas, Curr. Paediatr. 2004, 14, 51–63. [Google Scholar]
- López-López, A.; Castellote-Bargalló, A.I.; Campoy-Folgoso, C.; Rivero-Urgel, M.; Tormo-Carnicé, R.; Infante-Pinad, D.; López-Sabatera, M.C. The influence of dietary palmitic acid triacylglyceride position on the fatty acid, calcium and magnesium contents of at term newborn faeces. Early Hum. Dev. 2001, 65 (Suppl. S2), 83–94. [Google Scholar] [CrossRef]
- Koletzko, B.; Rodriguez-Palmero, M.; Demmelmair, H. Physiological aspects of human milk lipids. Early Hum. Dev. 2001, 65, 3–18. [Google Scholar] [CrossRef]
- Martysiak-Żurowska, D. Content of odd-numbered carbon fatty acids in the milk of lactating women and in infant formula and follow-on formula. Acta Sci. Pol. Technol. Aliment. 2008, 7, 75–82. [Google Scholar]
- Maduko, C.O.; Park, Y.W.; Akoh, C.C. Characterization and oxidative stability of structured lipids: Infant milk fat analog. J. Am. Oil Chem. Soc. 2008, 85, 197–204. [Google Scholar] [CrossRef]
- Bryś, J.; Wirkowska, M.; Górska, A.; Ostrowska-Ligęza, E.; Bryś, A. Application of the calorimetric and spectroscopic methods in analytical evaluation of the human milk fat substitutes. J. Therm. Anal. Calorim. 2014, 118, 841–848. [Google Scholar] [CrossRef]
- Yang, T.; Xu, X.; He, C.; Li, L. Lipase-catalyzed modification of lard to produce human milk fat substitutes. Food Chem. 2003, 80, 473–481. [Google Scholar] [CrossRef]
- Ilyasoglu, H. Production of human fat milk analogue containing α-linolenic acid by solvent-free enzymatic interesterification. LWY-Food Sci. Technol. 2013, 54, 179–185. [Google Scholar] [CrossRef]
- Lu, J.; Wang, P.; Ke, Z.; Liu, X.; Kang, Q.; Hao, L. Effect of metal ions on the enzymatic hydrolysis of hemp seed oil by lipase Candida sp.99-125. Int. J. Biol. Macromol. 2018, 114, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Siudem, P.; Wawer, I.; Paradowska, K. Rapid evaluation of edible hemp oil quality using NMR and FT-IR spectroscopy. J. Mol. Struct. 2019, 1177, 204–208. [Google Scholar] [CrossRef]
- PN EN ISO 5509:2001. Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids; Polish Committee for Standardization: Warsaw, Poland, 2001. [Google Scholar]
- Bryś, J.; Vaz Flores, I.F.; Górska, A.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Bryś, A. Use of GC and PDSC methods to characterize human milk fat substitutes obtained from lard and milk thistle oil mixtures. J. Therm. Anal. Calorim. 2017, 130, 319–327. [Google Scholar] [CrossRef]
- ISO 660:2009. Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- ISO 3960:2007. Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- Lien, E.L. The role of fatty acid composition and positional distribution in fat absorption in infants. J. Pediatr. 1994, 125 Pt 2, 562–568. [Google Scholar] [CrossRef]
- López-López, A.; López-Sabater, M.C.; Campoy-Folgoso, C.; Rivero-Urgell, M.; Castellote-Bargalló, A.I. Fatty acid and sn-2 fatty acid composition in human milk from Granada (Spain) and infant formulas. Eur. J. Clin. Nutr. 2002, 56, 1242–1254. [Google Scholar] [CrossRef]
- Kowalski, B.; Tarnowska, K.; Gruczyńska, E.; Bekas, W. Chemical and enzymatic interesterification of beef tallow and rapeseed oil blend with low content of tallow. J. Oleo Sci. 2004, 53, 479–488. [Google Scholar] [CrossRef][Green Version]
- Nielsen, N.S.; Yang, T.; Xu, X.; Jacobsen, C. Production and oxidative stability of a human milk fat substitute produced from lard by enzyme technology in a pilot packed-bed reactor. Food Chem. 2006, 94, 53–60. [Google Scholar] [CrossRef]
- Small, D.M. The effects of glyceride structure on adsorption and metabolism. Annu. Rev. Nutr. 1991, 11, 413–434. [Google Scholar] [CrossRef]
- Forsyth, J.S. Lipids and infant formulas. Nutr. Res. Rev. 1998, 1, 255–278. [Google Scholar] [CrossRef]
- Innis, S.M.; King, D.J. Trans Fatty acids in human milk are inversely associated with concentrations of essential all-cis n-6 and n-3 fatty acids and determine trans, but not n-6 and n-3, fatty acids in plasma lipids of breast-fed infants. Am. J. Clin. Nutr. 1999, 70, 383–390. [Google Scholar] [CrossRef]
- Xu, X. Production of specific-structured triacylglycerols by lipase-catalyzed reactions: A review. Eur. J. Lipid Sci. Technol. 2000, 102, 287–303. [Google Scholar] [CrossRef]
- Bryś, J.; Wirkowska, M.; Górska, A.; Ostrowska-Ligęza, E.; Bryś, A.; Koczoń, P. The use of DSC and FT-IR spectroscopy for evaluation of oxidative stability of interesterified fats. J. Therm. Anal. Calorim. 2013, 112, 481–487. [Google Scholar] [CrossRef]
- He, Y.; Wu, T.; Sun, H.; Sun, P.; Liu, B.; Luo, M.; Chen, F. Comparison of fatty acid composition and positional distribution of microalgae triacylglycerols for human milk fat substitutes. Algal Res. 2019, 37, 40–50. [Google Scholar] [CrossRef]
- Stolarczyk, A.; Socha, P. Fat in nutrition of infants. Nowa Pediatria 2002, 3, 200–203. [Google Scholar]
- Ledóchowska, E.; Datta, I. Enzymatyczne i chemiczne przeestryfikowanie mieszaniny oleju rzepakowego i stearyny palmowej. Tłuszcze Jadalne 1995, 30, 169–183. [Google Scholar]
- Martin, D.; Reglero, G.; Señoráns, F.J. Oxidative stability of structured lipids. Eur. Food Res. Technol. 2010, 231, 635–653. [Google Scholar] [CrossRef]
- Thurgood, J.; Ward, R.; Martini, S. Oxidation kinetics of soybean oil/anhydrous milk fat blends: A differential scanning calorimetry study. Food Res. Int. 2007, 40, 1030–1037. [Google Scholar] [CrossRef]
- Yankah, V.V.; Akoh, C.C. Batch enzymatic synthesis, characterization and oxidative stability of DHA-containing structured lipids. J. Food Lipids 2007, 7, 247–261. [Google Scholar] [CrossRef]
- Özdemir, I.S.; Karaoğlu, Ö.; Dağ, Ç.; Bekiroğlu, S. Assessment of sesame oil fatty acid and sterol composition with FT-NIR spectroscopy and chemometrics. Turk. J. Agric. For. 2018, 42, 444–452. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Jakubczyk, E.; Górska, A.; Wirkowska, M.; Bryś, J. The use of moisture sorption isotherms and glass transition temperature to assess the stability of powdered baby formulas. J. Therm. Anal. Calorim. 2014, 118, 911–918. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Bryś, J.; Sujka, K.; Koczoń, P. Assessment of the hazelnuts roasting process by pressure differential scanning calorimetry and MID-FT-IR spectroscopy. Food Anal. Methods 2015, 8, 2465–2473. [Google Scholar] [CrossRef]
- Sørensen, A.-D.M.; Xu, X.; Zhang, L.; Kristensen, J.B.; Jacobsen, C. Human milk fat substitute from butterfat: Production by enzymatic interesterification and evaluation of oxidative stability. J. Therm. Anal. Calorim. 2010, 87, 185–194. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Adamczak, M.; Soumanou, M.M. Lipase-catalyzed synthesis of modified lipids. In Lipids for Functional Foods and Nutraceuticals, 1st ed.; Gunstone, F.D., Ed.; The Oily Press: Bridgwater, UK, 2003; pp. 149–182. [Google Scholar]

| Type of Sample | Fatty Acids (wt%) | ||||
|---|---|---|---|---|---|
| 16:0 | 18:0 | 18:1 | 18:2 | ||
| sn-2 | Mix 60 °C_2 h | 51.8 | 13.1 | 12.5 | 11.4 |
| Mix 60 °C_4 h | 47.1 | 8.5 | 16.5 | 15.1 | |
| Mix 60 °C_6 h | 41.3 | 11.7 | 19.6 | 16.1 | |
| sn-1,3 | Mix 60 °C_2 h | 13.8 | 13.5 | 39.5 | 19.8 |
| Mix 60 °C_4 h | 21.1 | 14.1 | 34.4 | 16.6 | |
| Mix 60 °C_6 h | 21.7 | 14.5 | 32.8 | 17.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryś, J.; Górska, A.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M.; Bryś, A.; Brzezińska, R.; Dolatowska-Żebrowska, K.; Małajowicz, J.; Ziarno, M.; Obranović, M.; et al. Human Milk Fat Substitutes from Lard and Hemp Seed Oil Mixtures. Appl. Sci. 2021, 11, 7014. https://doi.org/10.3390/app11157014
Bryś J, Górska A, Ostrowska-Ligęza E, Wirkowska-Wojdyła M, Bryś A, Brzezińska R, Dolatowska-Żebrowska K, Małajowicz J, Ziarno M, Obranović M, et al. Human Milk Fat Substitutes from Lard and Hemp Seed Oil Mixtures. Applied Sciences. 2021; 11(15):7014. https://doi.org/10.3390/app11157014
Chicago/Turabian StyleBryś, Joanna, Agata Górska, Ewa Ostrowska-Ligęza, Magdalena Wirkowska-Wojdyła, Andrzej Bryś, Rita Brzezińska, Karolina Dolatowska-Żebrowska, Jolanta Małajowicz, Małgorzata Ziarno, Marko Obranović, and et al. 2021. "Human Milk Fat Substitutes from Lard and Hemp Seed Oil Mixtures" Applied Sciences 11, no. 15: 7014. https://doi.org/10.3390/app11157014
APA StyleBryś, J., Górska, A., Ostrowska-Ligęza, E., Wirkowska-Wojdyła, M., Bryś, A., Brzezińska, R., Dolatowska-Żebrowska, K., Małajowicz, J., Ziarno, M., Obranović, M., & Škevin, D. (2021). Human Milk Fat Substitutes from Lard and Hemp Seed Oil Mixtures. Applied Sciences, 11(15), 7014. https://doi.org/10.3390/app11157014













