1. Introduction
The possibility of bonding brackets was an important step in fixed orthodontics, resulting in the shortening of working time for the orthodontist, better hygiene and fewer dental and periodontal pathological conditions after wearing the appliances, better aesthetics and the elimination of the working phase in which the interdental spaces were closed after detaching the bands [
1].
The bond strength between the brackets and the tooth enamel is an extremely important issue in performing the mechanics of orthodontic treatment; the brackets’ detachments cause a series of inconveniences, the reattachment being a difficult, unpleasant maneuver, besides the fact that the detachment of the brackets can cause delays of the treatment results and mechanical injuries of the neighboring soft tissues of the oral cavity [
1,
2].
From a chemical point of view, adhesion is the gluing of two materials that can be different by means of a chemical compound called adhesive or bonding. The difference from physical adhesion is that chemical groups which appear on both surfaces can form an intramolecular or intermolecular chemical bond. Chemical adhesion involves a chemo-absorption process in which the adhesive molecules attached by adsorption to the surface of the material can react with its active groups by forming chemical bonds [
3]. In our case, the purpose of adhesion is to join the enamel surface with another substrate, represented by the materials used for bonding the brackets. The tooth enamel is the hardest tissue of the body due to its hypermineralization. Its hardness varies depending on the dental area in which the tooth is positioned; in areas with intense functional stress, it can reach 5 degrees of hardness on the Mohs scale. The tooth enamel does not retain water, being consequently easy to wash and dry [
4].
Many orthodontists are accustomed to using composite resins for bonding brackets, even if those adhesive systems have high technical sensitivity because they require a completely dry surface throughout the application procedures. The number of clinical steps and the long application time require the patient’s cooperation and the focus of the doctor to eliminate the possible technical errors [
5].
Ekhlassi et al. [
6] showed that Transbond Plus Color Change Adhesive (3M Unitek, Monrovia, CA, USA) is an improved resin that has ionized glass particles in its composition; this adhesive is described by the manufacturer as having excellent adhesion with metal and ceramic brackets. TPCC has been described as having hydrophilic properties.
Because of the chemical composition of ionized particles, Transbond Plus Color Change is an adhesive that slowly releases fluoride, which results in the reduction in enamel demineralization around the brackets’ bases [
7]. Its pink-colored component is activated by light-curing or by exposing it to the natural light, and during the polymerization, the initial pink color changes and turns irreversibly into a shade similar to that of the tooth enamel. The color change restores the aesthetic appearance of the bonding agent during the treatment. The initial pink color helps the doctor to remove excessive bonding material from the tooth surface before light-curing [
6,
8]. Excess adhesive may cause food retention, dental plaque and injuries of the superficial periodontium or the oral mucosa. Bacterial plaque with a large variety of microbial strains and food debris may produce inflammation and infection of the periodontal tissues and demineralization of the tooth enamel, especially in the cervical region of the vestibular surfaces, above the upper edge of the brackets’ bases [
8]. These great advantages of bonding procedures and the entire orthodontic treatment are important reasons why the use of TPCC is preferred by many orthodontists.
Self-etching primers began to be used about 20 years ago because their use was found to result in the formation of a continuum between the adhesive contained and the etched surface by simultaneous acid demineralization and penetration of the treated surface with acidic monomers. Those monomers can be then easily polymerized in situ, so the bonding technique is simplified [
8]. Self-etching primers are combinations of etching acids and bonding resins that are manufactured in order to eliminate the etching step and to reduce the chairside working time and the risk of salivary contamination; using the primer reduces the adhesion process to two steps instead of three.
According to the manufacturer’s (3M Unitek, Monrovia, CA, USA) datasheet, Transbond Plus Color Change adhesive when used together with Transbond Plus Self-Etching Primer (3M) provides a moisture-tolerant bonding system. Transbond Plus Self-Etching Primer (TSEP) contains methacrylate phosphoric acid esters as the main ingredients [
9]. The acidic end group of the resin derivate has hydrophilic properties [
10].
Contamination may occur frequently in clinical activity after application of the primer; in this case, the ability of the primer to create a chemical bond strong enough to allow the proper adhesion of the brackets is to be considered.
Another bonding material used in our study was Fuji Ortho LC, an adhesive made by GC America, Inc.; this dental material has been described by the manufacturer as a light-cured resin-reinforced glass ionomer cement. Resins have been added to ionomeric cements to improve their aesthetic and mechanical characteristics, to increase adhesion and to maintain the fluoride-releasing property [
11].
Fuji Ortho LC is a viscous material that becomes hard by polymerization that occurs upon exposure to ultraviolet or natural light. In orthodontics, it has been frequently used for bonding brackets and bands.
The study described in this work aimed to evaluate the adhesion created by using two adhesive systems with a different chemical composition for bonding brackets, Transbond Plus Color Change with Transbond Plus Self-Etching Primer and pre-etched Fuji Ortho LC in three enamel conditions achievable in regular dental office activity: dry, contaminated with water and contaminated with saliva.
2. Materials and Methods
In this in vitro study, we used a total of 120 mandibular and maxillary premolars extracted for orthodontic purposes. The donor patients approved the use of their extracted teeth in the study by signing a written consent. The criteria for including teeth in the study were:
The crown integrity, namely the absence of any structural or developmental crown defects, decays, restorations or cracks caused by the extraction procedures [
12,
13];
The teeth not having undergone any chemical treatment [
9].
The extracted teeth were washed in water then placed in a 0.1% thymol solution. Later, they were stored for a week in distilled water that was changed once a day. They were removed from distilled water on the day of testing and were gently cleaned with Depural Neo from Spofa Dental, a slightly abrasive fluoride-free paste that is generally used for professional teeth polishing [
14].
We wanted to use only the premolar crowns for easier handling, so we separated the premolar roots and crowns at the anatomical cement–enamel junction using low-speed diamond disks and water cooling [
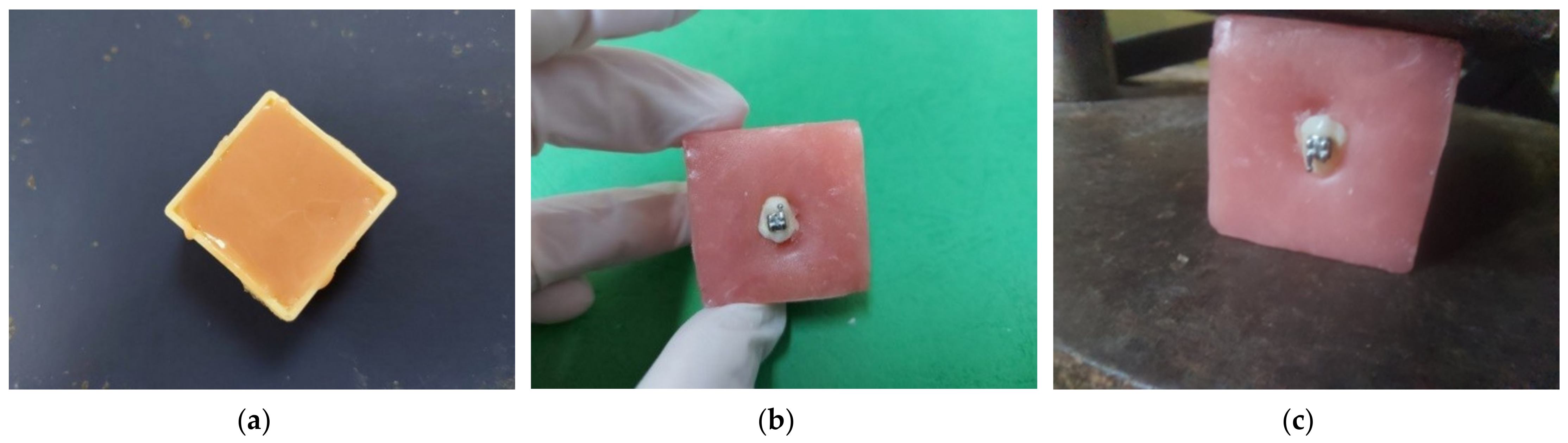
15]. All the premolar crowns were mounted in Duracrol (a self-curing methacrylate resin produced by Spofa Dental, which now is part of Danaher Corporation, California) blocks with only their buccal surfaces exposed (
Figure 1) in order to allow the brackets’ bonding and testing [
15]; the hard self-curing resin kept the crowns of the teeth immobile during the brackets’ debonding. The cubes were prepared by putting the acrylic resin in plastic cubes previously insulated with Vaseline before starting the setting. The size of the cubes was 3.3 cm/3.3 cm/2 cm. Just before the setting, the crowns of the premolars were submerged so that only the vestibular surface remained exposed. We made sure that the vestibular surfaces were not contaminated with Duracrol [
16]. For all the specimens in the study, we used Discovery brackets from DENTAURUM GmbH & Co., Ispringen, Germany.
Thus, prepared teeth were randomly divided into six groups (20 samples for each group) according to the bonding system and enamel preparation:
Group 1: Transbond Plus Color Change together with Transbond Plus Self-Etching Primer (3M). The buccal surfaces of teeth in this group were treated with Transbond Plus Self-Etching Primer; the primer was rubbed onto the buccal surfaces for 10 s using the disposable supplied. Then, the moisture-free spray was used for 10 s in order to deliver air to the primer. We applied Transbond Plus Color Change paste to the brackets’ bases which then were pressed evenly and placed on the teeth, respecting the positioning bonding rules by localizing the center of the buccal surfaces [
17]. Excessive bonding material around the bracket base was then gently removed by using a sealer, and the adhesive was light-cured with a Demetron LC lamp (SDS Kerr, USA) which had a light intensity of 800 mW/cm
2. The specimens were light-cured from occlusal, gingival, mesial and distal aspects for 10 s each, for a total of 40 s [
18,
19].
Group 2: Transbond Plus Color Change together with Transbond Plus Self-Etching Primer (3M) moistened with distilled water. We first prepared the buccal surfaces of the teeth in this group as we did with the specimens from Group 1; the difference was that after the application of the primer and the use of the air spray, we moistened the entire buccal surfaces of the teeth in this group with water. The water was applied by using first a dental syringe and then using a microbrush. Then, we applied the bonding material Transbond Plus Color Change in the same way as we did in Group 1.
Group 3: Transbond Plus Color Change together with Transbond Plus Self-Etching Primer (3M) moistened with saliva. The working steps were the same as in Group 2, except that after applying the primer and using the air spray, a small amount of saliva was applied to the entire buccal surfaces of the group’s specimens. The saliva was donated by one of the authors and applied by using a microbrush. The donor had been asked to clean their teeth well just before collecting the saliva [
5].
Group 4: Fuji Ortho LC in capsules on etched and dry enamel. The specimens were first etched with 37% orthophosphoric acid for 15 s [
11,
16,
18]. Then, the treated surfaces were rinsed thoroughly with the water spray for 15 s and air-dried with the oil-free spray for 20 s [
18].
Fuji Ortho LC was applied to the bracket base. Then, the brackets were pressed evenly on the enamel surfaces, respecting the positioning bonding rules by localizing the center of the buccal surfaces [
17]. In order to achieve the minimum adhesive thickness, the brackets were compressed over the tooth surface by putting a special blade in the slots. Excessive bonding material around the bracket base was then gently removed by using a common sealer. The light-curing was performed with the lamp Demetron LC. The specimens were light-cured from occlusal, gingival, mesial and distal aspects for 10 s each [
18,
19].
Group 5: Fuji Ortho LC in capsules on etched enamel entirely moistened with distilled water before bonding. All the stages of the teeth preparation were similar to those in Group 4. The difference was that after being treated with orthophosphoric acid, washed and dried, the buccal surfaces of the teeth were moistened by using distilled water and a microbrush.
Group 6: Fuji Ortho LC in capsules on etched enamel surfaces entirely moistened with saliva before bonding. All the stages of the teeth preparation were similar to those in Group 4. The only difference was that after being treated with orthophosphoric acid, washed and dried, the buccal surfaces of the teeth were moistened by using saliva donated by one of the authors and a microbrush in the same way as in Group 3 [
5].
We used a universal testing machine (Model DDW-100, DTEC, Deity Testing Equipment Co., Ltd., Beijing, China) (
Figure 2) to determine the force required to detach the brackets. For testing the bond strength, the bracket slot was positioned parallel to the horizontal plane and the surveyor blade was placed perpendicular to the bracket’s base [
18]. We tested the shear bond strength by applying an occlusal–cervical force to the bracket base, with a cross-head speed of 1 mm/min [
5]. The force required to detach the brackets was expressed in megapascal (MPa) by using a common conversion from N/mm
2 to MPa [
12], taking into account the dimension of the bracket base, i.e., 10.3 mm
2 (data obtained from the manufacturer—Dentaurum, Ispringen, Germany).
Data resulting from the experimental investigation were verified to avoid outliers and considered for statistical analysis. The results are expressed as mean ± standard deviation. For testing the normality of data, we used the Kolmogorov–Smirnov test. For the parametric data, we used Student’s t-test. To compare all six groups together, we used the ANOVA test. The statistically significant level was p < 0.05.
4. Discussions
We have chosen to compare these two adhesive systems because they are widely used for bonding brackets in orthodontic clinical practice [
6,
12,
18] and it was very important to determine the difference between their bond strengths in different working conditions; still, they were uniquely studied comparatively in various enamel conditions only in this research by the time of documenting and writing of this report.
In recent decades, primers were manufactured and used for bonding resin composites to the enamel surface. Unfortunately, these coupling agents had poor hydrolytic stability, so they were chemically unstable in the oral environment where the saliva is always present and the risk of enamel contamination is high [
5,
12]. We wanted a good adhesion even in humid conditions, so we had to choose bonding materials with hydrophilic properties.
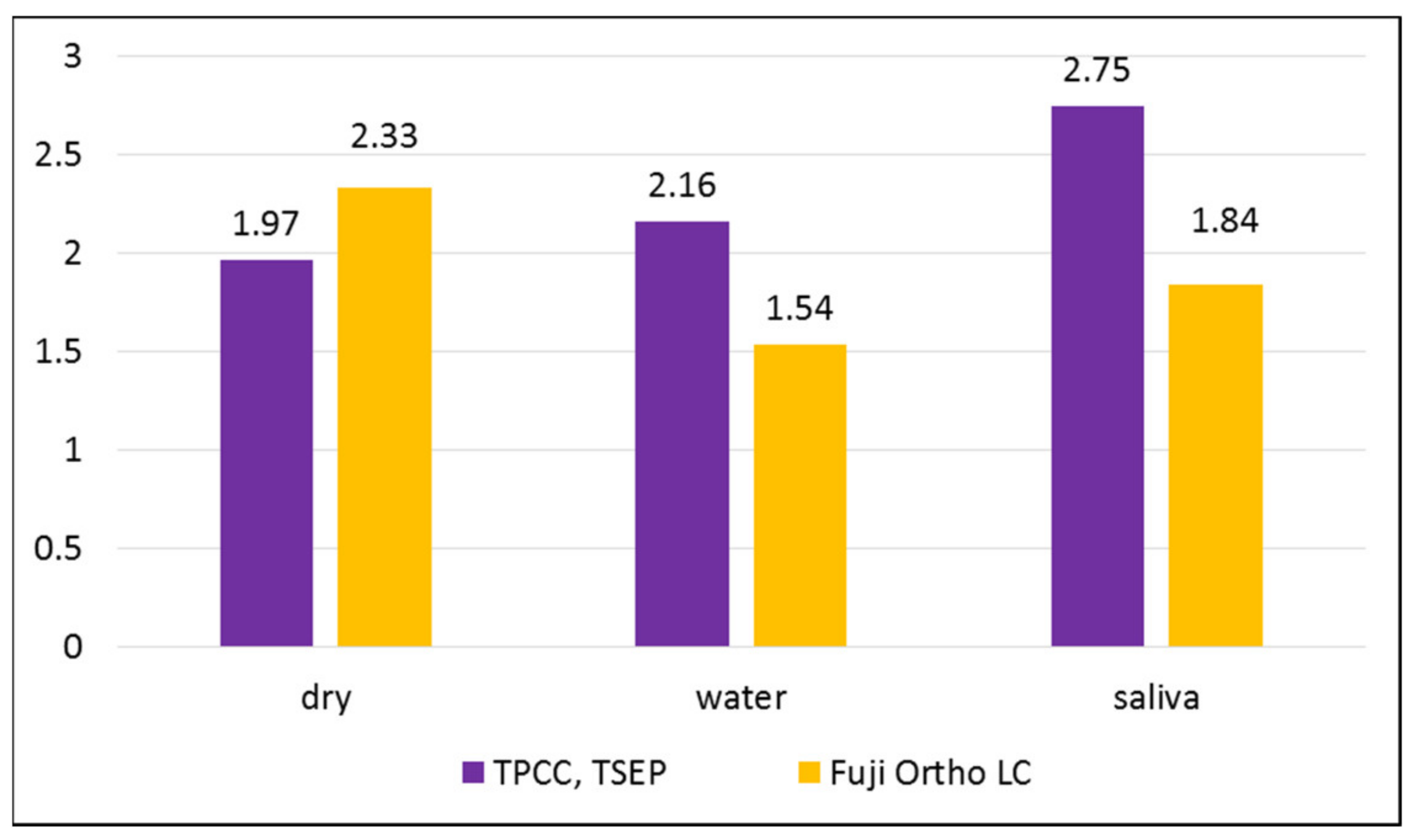
The studied adhesive systems in all described working conditions have demonstrated an adequate adhesion for use as bonding agents for orthodontic brackets. According to Reynolds [
20], the adequate bond strength required for orthodontic needs starts from 5.9–7.8 MPa. This value of adhesion could counteract orthodontic and masticatory forces [
20]. In our study, the highest bond strength was found for the group in which we used TPCC and TSEP on dry enamel, and the lowest bond strength was found for the same adhesive system on the enamel moistened with saliva. A statistically significant difference between water-moistened groups was found: Fuji Ortho LC had a higher bond strength. A very small difference without statistical significance was found between saliva-moistened groups. The ANOVA comparison of the variance of the averages demonstrated a statistically significant difference between groups (
p = 0.000002244).
The results of our study showed that the mean bond strength of the Transbond Plus Color Change used with the primer without contamination was higher than that in the other two groups, with water and saliva contamination. Saliva contamination does not affect the strength of the bond as much as water contamination in the case of using Transbond Plus Color Change with Transbond Plus Self-Etching Primer. The reason why we chose to use the Transbond Plus Self-Etching Primer together with Transbond Plus Color Change is that it has been demonstrated that this combination with a hydrophilic resin gives a superior bond strength compared to that of the same primer used with the conventional Transbond XT, which is a hydrophobic resin, even under conditions of saliva contamination [
12].
Other researchers, such as Ascensión Vicente et al. [
21], found that Transbond Plus with TSEP proves better adhesion under saliva contamination than with water contamination or even without any contamination. In their study, as in ours, the contamination was performed after applying the primer, but the use of Transbond Plus instead of the Transbond Plus Color Change we used could explain the different results [
21].
Mandava Prasad et al. [
5] found that the groups in which they used a self-etch bonding system—TSEP—contaminated with water and saliva had significantly higher bond strength than groups bonded with a conventional bonding system in the same conditions. They found a better tolerance of TSEP in wet conditions compared to Transbond XT (3M Unitek) after acid etching in the same conditions [
5].
In their in vivo study, Mariá D. Campoy et al. [
22] found that saliva contamination before or after application of Transbond Plus Self-Etching primer does not significantly change the bonding strength and does not increase the risk of bond failure [
22].
It has been also demonstrated by Cacciafesta et al. [
23] that Transbond Plus Self-Etching Primer gives higher bonding strength values than two other primers, one conventional and one hydrophilic. It proved to be less affected by water or saliva contamination [
23].
In the molecular structure of TSEP, there is a combination between a phosphoric acid and a methacrylate group. These two groups form a methacrylate phosphoric acid ester. When TSEP is applied on the hard dental structures, the phosphate group dissolves calcium and removes it. The phosphate group and calcium form a complex that is incorporated into the adhesive network during polymerization. By this mechanism, the acid etching of the enamel and the penetration of the monomer into the created microretentions are synchronized. So, the depth at which the enamel is etched coincides with the depth of penetration of the primer [
5].
As a chemical composition, TSEP also has water as a solvent [
9]; this could give the primer tolerance to wet conditions. TSEP’s performance in such conditions could be explained by the self-etching primer’s chemical composition of hydrophilic monomers.
Transbond Plus Color Change may owe its tolerance to humid conditions to the polyethylene glycol dimethacrylate (PEGDMA) and the low concentration of hydrophobic bisphenol A diglycidyl ether dimethacrylate (bis-GMA) in its composition; polyethylene glycol dimethacrylate (PEGDMA) favors the infiltration of bis-GMA adhesives into the wet enamel [
9] and bonding with TSEP even in humid conditions. Saliva is an oral fluid composed of a variety of minerals and electrolytes such as calcium, sodium, magnesium, potassium, bicarbonate and phosphates. The existence of salivary ions probably multiplies the chemical bonds between TSEP and TPCC and increases the bonding strength [
24].
Fuji Ortho LC can be applied on the enamel surface with or without an etching technique [
18]. Because this bonding material is basically a glass ionomer luting cement with a high capacity to slowly release fluoride, it prevents the decalcification of the tooth enamel and the appearance of “white spots” after debonding brackets. Researchers have demonstrated by many studies that the use of phosphoric acid for slight demineralization of the enamel before bonding with glass ionomers improves the bond strength without affecting the mineralization of the tooth because of their ability to gradually release mineral ions [
11,
16].
The superior adhesion of Fuji Ortho LC in the case of acid pretreatment of the enamel with 37% phosphoric acid was even better than that created by using a self-etching adhesive system [
25].
Among the groups in which we used Fuji Ortho LC, we found the highest adhesion strength when the teeth were not contaminated with anything after etching and the lowest adhesion strength when the teeth were moistened with saliva after etching. The difference was statistically significant between the dry and saliva-moistened groups and without statistical significance between the dry and water-moistened groups and water-moistened and saliva-moistened groups. In our opinion, it would be desirable to add chemical components to the composition of this material to increase its adhesion in case of salivary contamination.
Bishara et al. [
26] obtained a result similar to that of our study by finding no statistically significant differences between two experimental groups of teeth, one bonded with Fuji Ortho LC with the enamel etched and moistened with water before bonding and another bonded with Fuji Ortho LC with the enamel etched and moistened with saliva before bonding [
26].
Feizbakhsh et al. [
18] conducted a study partially similar to ours by applying Fuji Ortho LC after etching on dry enamel, enamel moistened with distilled water and enamel moistened with saliva. They found results similar to those of our study. The bonding strength was maximum in the dry enamel group; they found a statistically significant difference between this group and the group of teeth moistened with saliva, which proved to have the lowest bonding strength value among etched groups [
18]. They also found a significant difference between the group of teeth moistened with distilled water and that moistened with saliva. There was no significant difference after etching between the group of teeth moistened with water and the dry enamel group. The very similar results not as absolute values but as differences between the studied groups could be explained by the fact that these authors used acid etching and subsequent contamination with distilled water and saliva in ways similar to our research [
18]. Their study demonstrated that saliva prevents micromechanical bonding between Fuji Ortho LC and tooth enamel to a great extent in the etched groups because of deposition of salivary constituents [
18]; this must be the reason why we also found the lowest adhesion in the group where the contamination after etching was done with saliva.
Other studies, such as that of Cook et al. [
27], demonstrated that “etching the tooth surface with phosphoric acid produced a significantly poorer bond to the enamel” [
27]. Cacciafesta and Toledano have demonstrated that Fuji Ortho LC bonded without any enamel conditioning gives a significantly lower shear bond strength compared with that made by glass ionomer cement on enamel etched with 37% phosphoric acid [
16,
28]. Using acid etching creates a layer of porous enamel that ranges in depth from 5 to 50 μm [
29], which is more suitable to achieve a stronger micromechanical adhesion by increasing the accessible areas for bonding. The acid application eliminates the organic biofilm and increases the enamel surface’s free energy [
30]. It is well known that the adhesion of glass ionomer cements to enamel is also one of a chemical nature [
16,
18].
Cacciafesta et al. [
31] found that in the case of using stainless steel lingual brackets, Fuji Ortho LC had a significantly higher bond strength after application on saliva-moistened enamel, after enamel conditioning with polyacrylic acid, when compared to all other tested enamel conditions: nonetched and dry, nonetched and wet, etched with polyacrylic acid and water-moistened. The hydrophilic monomer HEMA (2-hydroxy ethyl methacrylate) which is a main component of Fuji Ortho LC, may be responsible for infiltration and hydration [
18].
Many studies have been done to compare the bond strengths of glass ionomer cements and composite resins [
32,
33,
34]. Reddy et al. [
32] showed that the bond strength of the composite resin was better than that of glass ionomer, and the adhesion given by both bonding materials decreased after contamination with blood. Yassaei and Rix [
33,
34] also demonstrated that Transbond XT had a better shear bond strength than Fuji Ortho LC.
In our study, we found no significant differences between the two bonding systems on dry enamel and enamel moistened with saliva. The difference between the two bonding systems was statistically significant in their application on water-moistened enamel; Fuji Ortho LC exhibited better shear bond strength.
The laboratory conditions may greatly differ from those in vivo; the debonding forces may act in different directions. Moreover, water and saliva contamination varies in quantity and cannot be controlled as finely as in our study. It would be useful to test other parameters that reflect the adhesion of the brackets, such as the tensile bond strength [
35].