Abstract
Background and Objectives: The endodontic system and the periodontium are closely interrelated and the infection of both leads to the appearance of endodontic-periodontal lesions. Along with the endodontic and periodontal classic treatment, in most cases, there is a need for regenerative periodontal therapy for the repair of the damaged tissue. One material that stimulates bone healing is represented by platelet-rich fibrin (PRF). The aim of this study was to determine if the inclusion of PRF in the treatment protocol of endodontic-periodontal lesions is effective. Materials and Methods: This review was conducted according to the PRISMA guidelines. Four databases, MEDLINE (through PubMed), Scopus, Web of Science, and Google Scholar, were used in order to find all significant articles on the topic. Relevant keywords were used in different combinations. Results: The inclusion criteria were met by six studies, published between 2014 and 2020 and they were selected for the review. The use of PRF for the regenerative therapy of endodontic-periodontal lesions showed favorable outcomes in all of the studies included, with significant reductions in the probing depths. Conclusion: While platelet-rich fibrin may be beneficial, further research is needed.
1. Introduction
Endodontic-periodontal lesions involve the infection at the same time of endodontic and periodontal structures, that are closely interrelated. Both have mesodermal origins and as the tooth develops, three main connective paths are created between them: the apical foramen, dentinal tubules, lateral, and accessory canal [1]. The lesions can be caused by the progression of an endodontic or a periodontal infection or they can have different origins [2]. The first classification of endodontic-periodontal lesions was performed in 1972, by Simon and Glick [2] and included the following types: primary endodontic lesions, primary periodontal lesions, primary endodontic lesions with secondary periodontal involvement, primary periodontal lesions with secondary endodontic involvement and true combined lesions. A more recent classification published by Carranza [3] divides the endodontic-periodontal lesions into four types: primary pulpal infection which can result in periradicular periodontitis, primary periodontal infection which can result in endodontic infection, both primary pulpal and periodontal infections that occur either simultaneously (‘independent’ endodontic-periodontal lesions) or extensively (‘combined’ endodontic-periodontal lesions). Making a correct diagnosis contributes to an adequate treatment plan and may offer a better prognosis of the affected tooth [1,2,3].
Usually, the primary infection is the one to be treated first when the endodontic or periodontal lesion is present and the infection of the other structure has just started [1,3,4,5], but when the second infection is chronic, both endodontic and periodontal treatments need to be conducted [6]. Frequently, the root canal treatment is performed first [5,7], then the treatment of the residual periodontal pocket, which can be more anticipable after a successful endodontic treatment [3]. Scaling and root planning are included in the periodontal treatment, as well as regenerative procedures, which are essential to be taken into consideration for the repair of the damaged tissue (Figure 1) [8]. In a combined endodontic and periodontal lesion, the configuration of the bone defect and the degree of destruction of the periodontal attachment have a considerable influence on the long-term prognosis of the tooth [3]. In case of an advanced periodontal lesion, the success of the therapy revolves around the possibility of filling the defect using regenerative procedures [3].
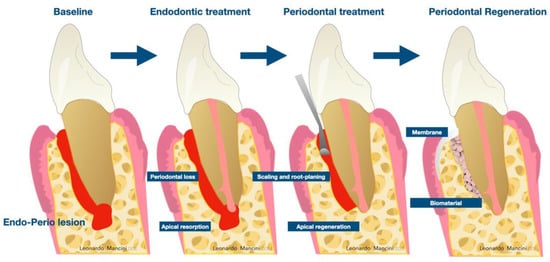
Figure 1.
Workflow for the treatment of endodontic-periodontal lesions consist of a first phase of endodontic treatment, a second phase of non-surgical periodontal therapy and a third phase of regeneration of the residual bony defect.
For regenerative periodontal therapy, a material or a combination of materials that can stimulate healing in bone and soft tissue are used. One of the materials that possesses these properties is represented by platelet-rich fibrin (PRF) [9,10]. It is an autogenous material that is formed of a fibrin matrix and has platelets, leukocytes, cytokines, circulating stem cells [11], macrophages [12], and growth factors [12,13,14] such as transforming growth factor-1 (TGF-β1), vascular endothelial growth factor (VEGF), platelet derived growth factors (PDGFs) and insulin-like growth factors (IGFs). In the literature several protocols aimed at improving the quality of the fibrin cloth which include variations in the spin time and speed. Included, in the PRF family are: leucocyte-platelet rich fibrin (L-PRF), titanium-platelet rich fibrin (T-PRF), advanced-PRF (A-PRF), and BioPRF. They can differ not only for the spin time and speed but also for the inclination and the surface treatment of the tubes used (Table 1). Time and speed are crucial determinants of the most effective autologous blood derivate. Marchetti and coworkers [9] have stated that the fibrin scaffold and the concentration of growth factor differs between protocols; thus, the regenerative potential may differ. The inclination of the tubes is another factor that needs to be taken into consideration. Miron et al. have reported that inclined spin might reduce the concentration of growth factor and the reduced production of the fibrin scaffold [10]. The PRF family stimulates bone growth and maturation, hemostasis [12,13,14] and when used in a combination with a bone graft, it improves the stabilization of the graft material and also improves its handling characteristics [8,15,16]. Due to its properties, PRF can bring benefits in treating periodontal defects that persisted after the endodontic treatment and scaling and root planning. Recent research has noticed the potential benefits of PRF material used for the regeneration of infrabony defects [17]. The purpose of this review was to determine if PRF alone has favorable outcomes when included in the regenerative periodontal therapy of the periodontal defects caused by endodontic-periodontal lesions.

Table 1.
Characteristics of several PRF isolation protocols, RCF, relative centrifugal force.
2. Material and Methods
This study was conducted following the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) [18]. The research question was: “Is the use of PRF in the treatment of endodontic-periodontal lesions effective?”
- P: Patients with endodontic-periodontal lesions
- I: The use of PRF in adjunct to endodontic and periodontal treatment
- C: Open flap debridement or only endodontic treatment
- O: Regenerative potential of PRF in endodontic-periodontal lesions (in terms of probing depth, clinical attachment loss, bleeding on probing, size of periapical lesion)
2.1. Eligibility Criteria
The inclusion criteria were clinical trials, case reports or case studies, published in English, where the treatment of endodontic-periodontal lesions included PRF. The exclusion criteria were the followings: studies on animals, letters to the editor, incomplete data, probing depths (PD) not mentioned and patients under 18 years.
2.2. Literature Search
MEDLINE (through PubMed), Scopus, Web of Science and Google Scholar databases were searched to find all relevant studies published in English from date of inception up to April 2021. The following combination of keywords was used: (“endodontic-periodontal lesion” OR “endo-perio lesion” OR “endo-periodontal lesion” OR “endodontic periodontal” OR “endoperio”) AND (“platelet-rich fibrin” OR “PRF”). Titles and abstracts of retrieved articles were assessed for eligibility and those who were on the topic of this review were excluded. Full texts of articles previously obtained were read and assessed for inclusion. For further studies, the reference lists of the included articles were searched.
2.3. Data Extraction
The following data were extracted using a standard data collection form: first author, year of study, number of patients, the age and the gender of the patients, diagnosis, tooth treated, the treatment conducted, the parameters before and after the treatment endodontic-periodontal lesions with PRF (probing depth, clinical attachment loss, bleeding on probing, size of periapical lesion). Extracted data was assessed by two researchers (M.I.A. and L.E.N). in case of disagreement a third author (A.M.) was decisive.
3. Results
3.1. Search Results
The search provided a total of 388 studies from PubMed, Scopus, Web of Science and Google Scholar databases. After removing the duplicates, a total of 366 articles were screened. The articles were reviewed by title and abstract and 14 articles were identified for full-text assessment. At the end of the analysis, 6 articles met the inclusion criteria [19,20,21,22,23,24]. Reasons for the exclusion of reviewed full text articles [7,8,15,25,26,27,28,29] are shown in Figure 2.
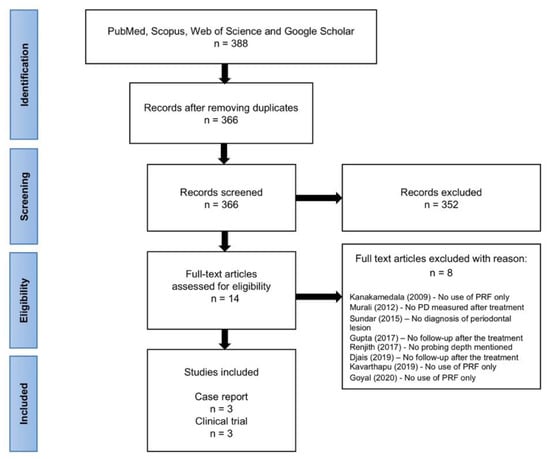
Figure 2.
Flow chart of methodology used to identify relevant literature according to PRISMA.
3.2. General Characteristics
From the retrieved articles, the 6 studies meeting the inclusion criteria were published between 2014 and 2020, involving 174 patients treated with PRF. Of these, three of the studies were case reports (Karunakar et al., 2014 [19]; Shashikumar et Nisha, 2016 [21]; Betancourt et al., 2017 [22]) and three were clinical trials (Dhiman et al., 2015 [20]; Razi et al., 2020 [24]; Ustaoğlu et al., 2020 [23]). The sample size varied from 1 to 140 patients. All studies reported the age of the participants, which ranged from 18 to 59 years. The gender of the patients was reported in all of the studies included, as shown in Table 2.

Table 2.
General characteristics of the included studies.
3.3. Clinical Assessments for the Diagnosis of Endodontic-Periodontal Lesions
For the diagnosis of the endodontic-periodontal lesions, a detailed medical history was taken, and a clinical examination was performed. One patient reported that the affected tooth had previously suffered trauma [21]. Inspection for cavities, incorrect restorations, discoloration, cracks, fractures, erosion, and abrasion was assessed. In one case, the affected tooth presented a discoloration and an abfraction on the buccal side [22]. Also, the mucosa and gingiva were examined for ulcerations, inflammation or for sinus tracts, which are often associated with necrosis [1]. Gingival inflammation was observed in two cases [19,21] and a sinus tract opening on the labial aspect of the tooth was reported in one case [21]. In order to detect the presence of a periradicular inflammatory process, palpation and percussion were assessed, however this could not predict if the lesion was of endodontic or periodontal origin [1]. Three patients [19,21] presented pain on percussion and one of them [21] had the tooth tender on palpation also.
For a certain diagnosis, a pulp vitality test was performed, and the probing depths were measured [30]. A negative response to the vitality tests was obtained, meaning the teeth were non-vital [19,20,21,22,23]. Significant values of the probing depth were reported after periodontal examination, from 8 mm to 11 mm in the case reports [19,21,22]. The inclusion criteria for the clinical trial conducted by Ustaoğlu and coworkers [23] were probing depths equal to or more than 5 mm and infrabony defects with 2 or 3 walls with a depth over 3 mm, after endodontic and non-surgical periodontal treatment. In the clinical trial conducted by Dhiman et al. [20], patients with probing depth greater than 6 mm were included. Also, tooth mobility was tested. A mandatory assessment for the diagnosis of the endodontic-periodontal cases is represented by radiographic examination, which was performed in all the cases included [19,20,21,22,23,24]. It showed the bone loss around the affected tooth and it could be useful in detecting the cause of the lesions, as for example a deep cavity or a failed root canal treatment. One study did not report the procedure of diagnosis of the endodontic-periodontal lesion [24].
3.4. Treatment of Endodontic-Periodontal Lesions
In the studies included, the treatment was divided into two phases. In the first phase, an endodontic and a periodontal treatment were carried out. The periodontal treatment included scaling and root planning. An evaluation of the situation was conducted after a period of time. In two studies [19,21], the period of time was of three months, which is the recommended period of time [1]. In two studies, the evaluation was performed earlier, after 2 weeks [22] or after 6 weeks [23] and in the rest of the studies the period of time was not mentioned [20,24]. For the reason that periodontal parameters did not improve, regenerative periodontal therapy was planned for phase two. PRF was used alone for the regenerative periodontal procedures. More types of platelet concentrates were used: pure platelet-rich fibrin, leukocyte- and platelet-rich fibrin (L-PRF), and titanium-prepared platelet-rich fibrin (T-PRF). The patients were recalled after 10 days for the removal of the sutures, then they were scheduled for regular check-ups at 3, 6, 9, 12 months after the surgery.
Encouraging results were obtained after the periodontal regenerative therapy. The efficiency of pure PRF for regenerative therapy was observed in two report cases [19,21] which showed improvement in the clinical parameters. At the nine-month follow up, the probing depths of tooth 4.6 and tooth 4.4 were reduced from 10 mm to 4 mm, respectively from 8 mm to 3 mm [19]. In the other report case, at the 12-month follow up, the probing depth of tooth 3.1 was reduced from 8 mm to 4 mm [21]. Furthermore, in a clinical study [20] conducted on 15 patients diagnosed with primary endodontic lesions with secondary periodontal involvement, after endodontic microsurgery, the PRF was applied in the periodontal defects and the success rate of the treatment was of 83.33%.
The performance of L-PRF was emphasized in the study conducted by Betancourt et al. [22], where it showed great improvement in the periodontal parameters, with a decrease of the probing depth from 11 mm to 3 mm on mesio-vestibular side and 14 mm to 5 mm on mesio-palatal side of tooth 2.4. In two comparative clinical trials [23,24] T-PRF was used for the periodontal regenerative therapy. In the first one [23] where the comparison was between T-PRF, guided tissue regeneration (GTR) and then open flap debridement, it was concluded that both T-PRF and GTR are more efficient than the open flap debridement. Moreover, regarding T-PRF and GTR, the decrease of the probing depth and the clinical attachment loss were similar, the only difference was noticed in the reduction of the infrabony defect, which was greater when GTR was used. Comparing PRF and T-PRF, Razi et al. [24] discovered that the outcomes were similar and there was no statistically significant difference between them, both reducing the probing depths and being beneficial in the management of difficult endodontic-periodontal lesions.
4. Discussion
Dental practitioners may face many challenges when it comes to the diagnosis of endodontic-periodontal lesions. On this account, taking a proper patient history, doing a thoroughly clinical examination, that includes inspection for cavities, palpation, percussion, the vitality test and the probing depth measurement and having a periapical radiography of the suspected tooth are of utmost importance in order to diagnose correctly the lesion [8]. Bacteria, dental trauma, teeth preparation under no irrigation, incorrect dental restorations, irritative materials, and malocclusion may lead to the inflammation of the pulp [31]. Untreated it can lead to pulp necrosis and to the inflammatory response of the periodontal ligament at the apical foramen or opening of accessory canals [1,31]. Through the apical foramen, dentinal tubules, lateral and accessory canals that communicate to the periodontium, inflammatory byproducts of pulpal origin may reach to the periodontal tissue and provoke an inflammatory response [1].
As for periodontal disease leading to the infection of the endodontic system, it occurs indirectly, when gingival recession is present [1]. If exposed cementum does not protect the accessory canals, they become a way for pathogens from the oral cavity to penetrate the pulp, resulting in chronic pulpitis and necrosis [1]. Usually, when pulp or periodontium is affected and the second infection of the other one has just begun, the primary disease is the first to be treated [1,4,5]. In the case that the secondary infections is established and chronic, both endodontic and periodontal treatment is mandatory [7]. Routinely, the root canal treatment is performed first, then the periodontal therapy. [5,7]. The periodontium is likely to heal completely after a successful root canal treatment [7]. An unfavorable outcome was noticed in a study where endodontic-periodontal lesions were treated without using an accompanying regenerative procedure, with a score of success ranging from 27% to 37% [32]. In addition to the endodontic and periodontal treatment, regenerative procedures are also needed for the regeneration of the damaged tissue [8].
Platelet-rich fibrin is an innovative material that can be used in regenerative therapy to stimulate healing in bone and soft tissue [9,10]. It is a fibrin matrix which contains platelets, leukocytes, cytokines, circulating stem cells [11], and macrophages [12]. The most important growth factors found in PRF [12,13,14] are: platelet derived growth factors (PDGFs), transforming growth factor-1 (TGF-β1), Insulin-like growth factors (IGFs), vascular endothelial growth factor (VEGF). As the resorption of the fibrin matrix goes, growth factors are released progressively over a period of time, leading to favorable healing course [33]. The beneficial impact of PRF on bone healing can be explained by its components action. Macrophages stimulate the osteogenesis and support the bone formation process [34,35,36,37]. After the activation of platelets and leukocytes, cytokines are released which stimulate bone healing [13]. TGF-β1 stimulates the synthesis of collagen, one of the components of the bone and fibronectin, which is responsible for the adhesion and migration of the cells and also promotes the osteogenic differentiation [38,39]. VEGF has an angiogenic effect [40]. PDGFs and IGF-I have a proliferative and differentiating effect on the osteoblasts [41,42].
There are a series of patients related factors that may affect PRF’s characteristics: age, systemic diseases (diabetes, thrombocytopenia), diet, environmental changes, ethnic differences, the immune system and the genetics [43]. In one study conducted by Yajamanya and coworkers [44], it was concluded that the fibrous proteins found in PRF modified with age, with a reduction in the number of platelets and leukocytes. In the studies included, all the patients were reported as systemically healthy, and this factor might not interfere with the results. The use of PRF as a material for the regenerative periodontal therapy of endodontic-periodontal lesions showed good results in all the cases which lead to reductions in the probing depths. So far, there is not a gold standard procedure in the treatment of endodontic-periodontal lesions. Other therapies that may be taken into account are open flap debridement (OFD) alone, OFD in combination with enamel matrix derivate, OFD in combination with enamel matrix derivate, synthetic bone, OFD in combination with synthetic bone or OFD in combination with PRF and synthetic bone. Oktawati and collaborators have mentioned in their systematic review that root canal treatment in combination with synthetic bone was a widely used approach [45]. The use of PRF in other regenerative techniques showed promising and valid results as reported by Mancini and Tarallo [36,37]. Mancini et al. showed a statistically significant result comparing coronally advanced flap alone (CAF) with CAF + PRF, in terms of recession reduction and gingival thickness. Tarallo et al. investigate the use of PRF in furcation defects showing statistically significant data for PD and CAL.
Our review had several limitations. One of them would be the limited number patients with endodontic-periodontal lesions treated using PRF in the protocol scheme. Also, there was a short follow-up of one year after the periodontal regenerative therapy. Another drawback was that the preparation of PRF procedure that was not the same in the studies included. Moreover, other limitations are the heterogeneity of the data collected, poor quality of the studies included (e.g., case reports) and the missing of a recognized guidelines and clinical protocols for the treatment of endodontic-periodontal lesions with PRF. Another aspect that needs to be considered is the anatomical variable that in this review was assessed. The anatomical structure of molars and premolars is different to the single rooted teeth and thus the regenerative procedure might be less reliable [46].
The systematic search through four databases and precise data extraction represents strengths of our paper. Another strength of our research is represented by the diagnosis of endodontic-periodontal lesions and the benefits and the limitations of using PRF. Moreover, it is the first study that collects and describes data regarding the use of PRF in endodontic and periodontal lesions. Nevertheless, the absence of randomized clinical trials in which PRF was used, makes these data a preliminary result from the literature.
5. Conclusion
Platelet-rich fibrin, used alone, showed improvements of endodontic-periodontal lesions in a low number of patients. While platelet-rich fibrin may be beneficial, further research is needed.
Author Contributions
Conceptualization, M.I.O. and A.M.; Methodology, M.I.O. and L.E.N.; Validation, A.M.; Formal analysis, M.I.O. and L.E.N.; Investigation, M.I.O., L.E.N., and A.M.; Data curation, M.I.O. and A.M.; Writing—original draft preparation, M.I.O., A.M., and F.O.; Writing—review and editing, A.M., F.O., and L.M.; Supervision, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rotstein, I.; Simon, J.H.S. Diagnosis, prognosis and decision-making in the treatment of combined periodontal-endodontic lesions. Periodontology 2000 2004, 34, 165–203. [Google Scholar] [CrossRef]
- Simon, J.H.S.; Glick, D.H.; Frank, A.L. The Relationship of Endodontic-Periodontic Lesions. J. Periodontol. 1972, 43, 202–208. [Google Scholar] [CrossRef]
- Newman, M.G.; Takei, H.; Klokkevold, P.R.; Carranza, F.A. Carranza’s Clinical Periodontology, 12th ed.; Elsevier: Philadelphia, PA, USA, 2015. [Google Scholar]
- Walton, R.E.; Torabinejad, M. Principles and Practice of Endodontics, 4th ed.; Elsevier: St. Louis, MO, USA, 2010. [Google Scholar]
- Harrington, G.W.; Steiner, D.R.; Ammons, W.F. The periodontal-endodontic controversy. Periodontol. 2000 2002, 30, 123–130. [Google Scholar] [CrossRef]
- Thorat, M.; Pradeep, A.R.; Pallavi, B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: A controlled clinical trial. J. Clin. Periodontol. 2011, 38, 925–932. [Google Scholar] [CrossRef]
- Murali, K.V.; Shahabe, S.A.; Patil, S.G.; Ahmed, M.N.; Bhandi, S. Periodontal Management of Non Healing Endodontic Lesion—A Case Report. Int. J. Clin. Dent. Sci. 2012, 3, 57931988. [Google Scholar]
- Goyal, L.; Gupta, N.; Gupta, N.D. Autologous platelet-rich derivatives along with alloplastic bone substitute in the management of complex perio-endo cases. J. Indian Soc. Periodontol. 2020, 24, 182–185. [Google Scholar] [CrossRef]
- Marchetti, E.; Mancini, L.; Bernardi, S.; Bianchi, S.; Cristiano, L.; Torge, D.; Marzo, G.; Macchiarelli, G. Evaluation of Different Autologous Platelet Concentrate Biomaterials: Morphological and Biological Comparisons and Considerations. Materials 2020, 13, 2282. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Chai, J.; Zheng, S.; Feng, M.; Sculean, A.; Zhang, Y. A novel method for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation. J. Biomed. Mater. Res. Part A 2019, 107, 2257–2271. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e56–e60. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, N.; Mici, E.; Calvo, A.; Runci, M.; Crimi, S.; Lauritano, F.; Belli, E. Preliminary Results of Bone Regeneration in Oromaxillomandibular Surgery Using Synthetic Granular Graft. BioMed Res. Int. 2018, 2018, 8503427. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Cortellini, S.; Temmerman, A.; Li, X.; Pinto, N.; Teughels, W.; Quirynen, M. Characterization of the Leukocyte- and Platelet-Rich Fibrin Block: Release of Growth Factors, Cellular Content, and Structure. Int. J. Oral Maxillofac. Implants 2019, 34, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Kanakamedala, A.; Ari, G.; Sudhakar, U.; Vijayalakshmi, R.; Ramakrishnan, T.; Emmadi, P. Treatment of a Furcation Defect with a Combination of Platelet-Rich Fibrin and Bone Graft-a Case Report. Endod. Pract. Today 2009, 3, 127–135. [Google Scholar]
- Raja, V.S.; Naidu, E.M. Platelet-rich fibrin: Evolution of a second-generation platelet concentrate. Indian J. Dent. Res. 2008, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, X.; Yu, J.; Wang, J.; Zhai, P.; Chen, S.; Liu, M.; Zhou, Y. Platelet-Rich Fibrin as a Bone Graft Material in Oral and Maxillofacial Bone Regeneration: Classification and Summary for Better Application. BioMed Res. Int. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Jayadev, M.; Shravani, G.S.; Karunakar, P.; Prasanna, J.S. Platelet-rich fibrin, “a faster healing aid” in the treatment of combined lesions: A report of two cases. J. Indian Soc. Periodontol. 2014, 18, 651–655. [Google Scholar] [CrossRef]
- Dhiman, M.; Kumar, S.; Duhan, J.; Sangwan, P.; Tewari, S. Effect of Platelet-rich Fibrin on Healing of Apicomarginal Defects: A Randomized Controlled Trial. J. Endod. 2015, 41, 985–991. [Google Scholar] [CrossRef]
- Nisha, S.; Shashikumar, P. Autologous platelet concentrate as a potential regenerative biomaterial in the treatment of endo-perio lesion. Indian J. Oral Health Res. 2016, 2, 106. [Google Scholar] [CrossRef]
- Betancourt, P.; Elgueta, R.; Fuentes, R. Treatment of endo-periodontal lesion using leukocyte-platelet-rich fibrin. A case report. Colomb. Med. 2017, 48, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Ustaoğlu, G.; Aydin, Z.U.; Özelçi, F. Comparison of GTR, T-PRF and open-flap debridement in the treatment of intrabony defects with endo-perio lesions: A randomized controlled trial. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e117–e123. [Google Scholar] [CrossRef]
- Razi, M.A.; Mahajan, A.; Qamar, S.; Mehra, S.; Roy, T.R.; Kumari, P. A Comparative Study of Platelet-rich Fibrin (PRF) and Titanium-prepared Platelet-rich Fibrin (T-PRF) in Management of Endo-perio Lesions. J. Contemp. Dent. Pract. 2020, 21, 997–1001. [Google Scholar] [CrossRef]
- Sundar, J.S.; Varma, K.M.; Satish, R.K.; Sajjan, G.S.; Tanikonda, R. A Biological Approach in Repair of Damaged Dental Pulp and Periapical Tissues Using Platelet Rich Fibrin, Mineral Trioxide Aggregate and Laser Biostimulation. IJSS Case Rep. Rev. 2015, 1, 44–50. [Google Scholar]
- Renjith, K.P.; Harish Kumar, V.V.; Santhosh, V.C.; Sreekanth, P.; Shabeer, M. PRF and Bonegraft-Magical Tools in Periodontics-A Case Report. Int. J. Periodontol. Implantol. 2017, 2, 23–26. [Google Scholar]
- Gupta, P.; Mittal, S.; Pall, S.; Deswal, H. A Potential Opportunity for Treating Hopeless Tooth with Hemisection and Platelet Rich Fibrin as a Regenerative Tool: A Case Report. IP Int. J. Periodontol. Implantol. 2017, 2, 95–97. [Google Scholar]
- Djais, A.I.; Akbar, F.H.; Adam, M.; Oktawati, S.; Tahir, H.; Gani, A.; Supiaty; Rizki, S.S. Application of Platelet Rich Fibrin (PRF) on Endodontic-Periodontic Lesion in Periodontal Tissue Regereneration: Case Report. J. Int. Dent. Med. Res. 2019, 12, 1189–1195. [Google Scholar]
- Kavarthapu, A.; Malaiappan, S. Management of periodontic-endodontic lesion in aggressive periodontitis-9 months follow-up: Report of a case. Indian J. Dent. Res. 2019, 30, 149–153. [Google Scholar] [PubMed]
- Tsesis, I.; Nemcovsky, C.E.; Nissan, J.; Rosen, E. Endodontic-Periodontal Lesions, 1st ed.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Tanzer, J.M.; Livingston, J.; Thompson, A.M. The Microbiology of Primary Dental Caries in Humans. J. Dent. Educ. 2001, 65, 1028–1037. [Google Scholar] [CrossRef]
- Hirsch, J.-M.; Ahlström, U.; Henrikson, P.-Å.; Heyden, G.; Peterson, L.-E. Periapical surgery. Int. J. Oral Surg. 1979, 8, 173–185. [Google Scholar] [CrossRef]
- Corso, M.D.; Toffler, M.; Ehrenfest, D.M. Use of an Autologous Leukocyte and Plate Let Rich Fibrin (L-PRF) Membrane in Post–Avulsion Sites: An Overview of Choukroun’s PRF. J. Implant Adv. Clin. Dent. 2010, 9, 27–35. [Google Scholar]
- Mise-Omata, S.; Alles, N.; Fukazawa, T.; Aoki, K.; Ohya, K.; Jimi, E.; Obata, Y.; Doi, T. NF-κB RELA-deficient bone marrow macrophages fail to support bone formation and to maintain the hematopoietic niche after lethal irradiation and stem cell transplantation. Int. Immunol. 2014, 26, 607–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalski, M.N.; Koh, A.J.; Weidner, S.; Roca, H.; McCauley, L.K. Modulation of Osteoblastic Cell Efferocytosis by Bone Marrow Macrophages. J. Cell. Biochem. 2016, 117, 2697–2706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, L.; Tarallo, F.; Quinzi, V.; Fratini, A.; Mummolo, S.; Marchetti, E. Platelet-Rich Fibrin in Single and Multiple Coronally Advanced Flap for Type 1 Recession: An Updated Systematic Review and Meta-Analysis. Medicina 2021, 57, 144. [Google Scholar] [CrossRef]
- Tarallo, F.; Mancini, L.; Pitzurra, L.; Bizzarro, S.; Tepedino, M.; Marchetti, E. Use of Platelet-Rich Fibrin in the Treatment of Grade 2 Furcation Defects: Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2104. [Google Scholar] [CrossRef] [PubMed]
- Bachman, H.; Nicosia, J.; Dysart, M.M.; Barker, T.H. Utilizing Fibronectin Integrin-Binding Specificity to Control Cellular Responses. Adv. Wound Care 2015, 4, 501–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marie, P.J.; Hay, E.; Saidak, Z. Integrin and cadherin signaling in bone: Role and potential therapeutic targets. Trends Endocrinol. Metab. 2014, 25, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, E.; McLean, W.; Ng, Y.-S.; Fukai, N.; Reginato, A.M.; Lovejoy, S.; D’Amore, P.A.; Olsen, B.R. Skeletal defects in VEGF120/120 mice reveal multiple roles for VEGF in skeletogenesis. Development 2002, 129, 1893–1904. [Google Scholar] [CrossRef]
- Piché, J.; Graves, D. Study of the growth factor requirements of human bone-derived cells: A comparison with human fibroblasts. Bone 1989, 10, 131–138. [Google Scholar] [CrossRef]
- Hock, J.M.; Centrella, M.; Canalis, E. Insulin-Like Growth Factor I Has Independent Effects on Bone Matrix Formation and Cell Replication. Endocrinology 1988, 122, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, M.; Pulikkotil, S.J.; Sonia, N. Platelet Rich Fibrin in Periodontal Regeneration. Open Dent. J. 2016, 10, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Yajamanya, S.; Chatterjee, A.; Babu, C.; Karunanithi, D. Fibrin network pattern changes of platelet-rich fibrin in young versus old age group of individuals: A cell block cytology study. J. Indian Soc. Periodontol. 2016, 20, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Oktawati, S.; Siswanto, H.; Mardiana, A.; Supiaty; Neormansyah, I.; Basir, I. Endodontic–periodontic lesion management: A systematic review. Med. Clin. Pract. 2020, 3, 100098. [Google Scholar] [CrossRef]
- Mancini, L.; Romandini, M.; Fratini, A.; Americo, L.; Panda, S.; Marchetti, E. Biomaterials for Periodontal and Peri-Implant Regeneration. Materials 2021, 14, 3319. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).