Biomass-Based Chemical Looping Gasification: Overview and Recent Developments
Abstract
1. Introduction
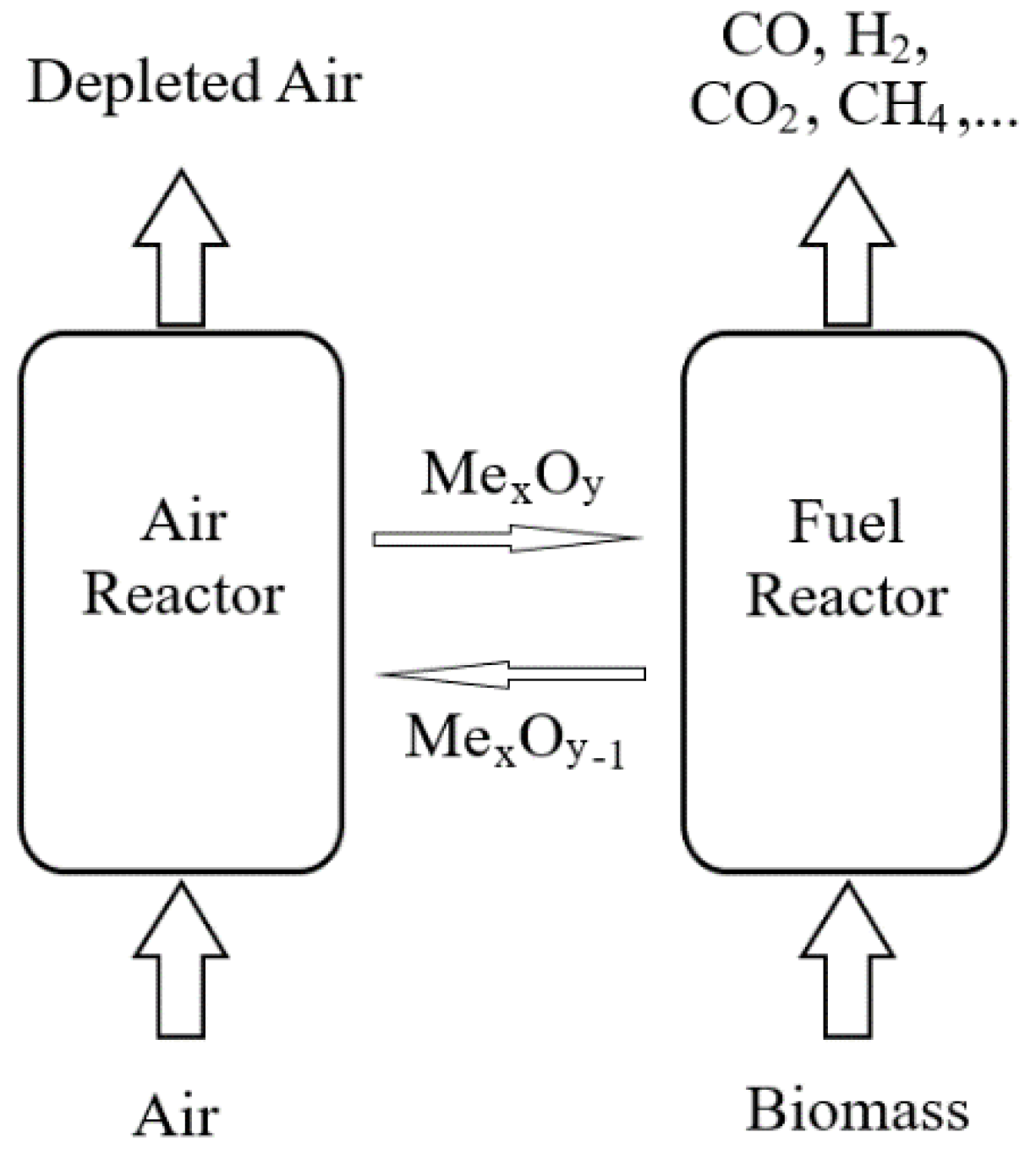
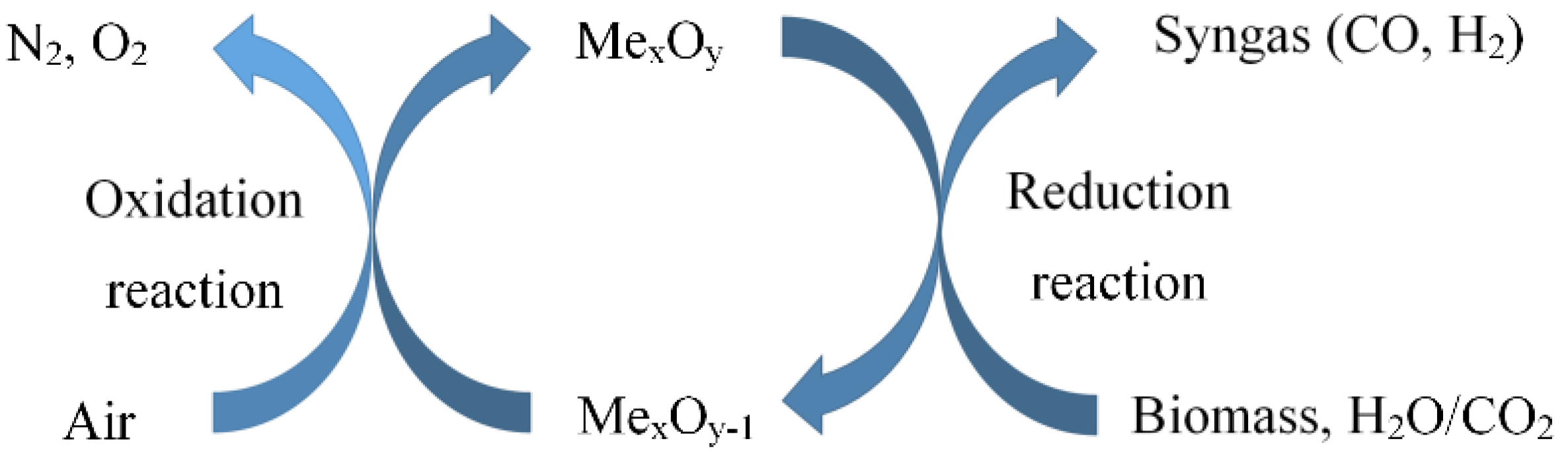
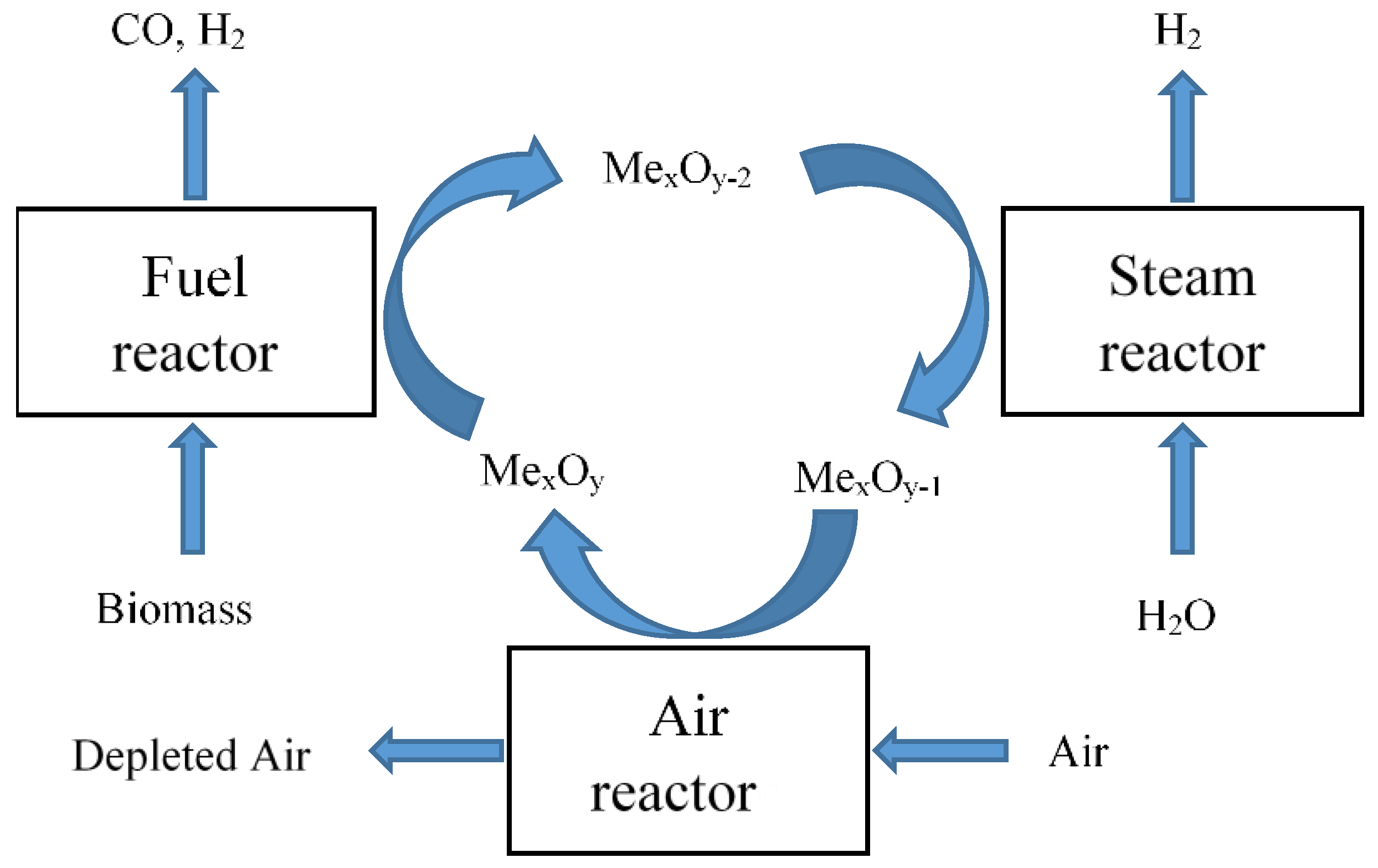
2. Process of Chemical Looping Gasification of Biomass
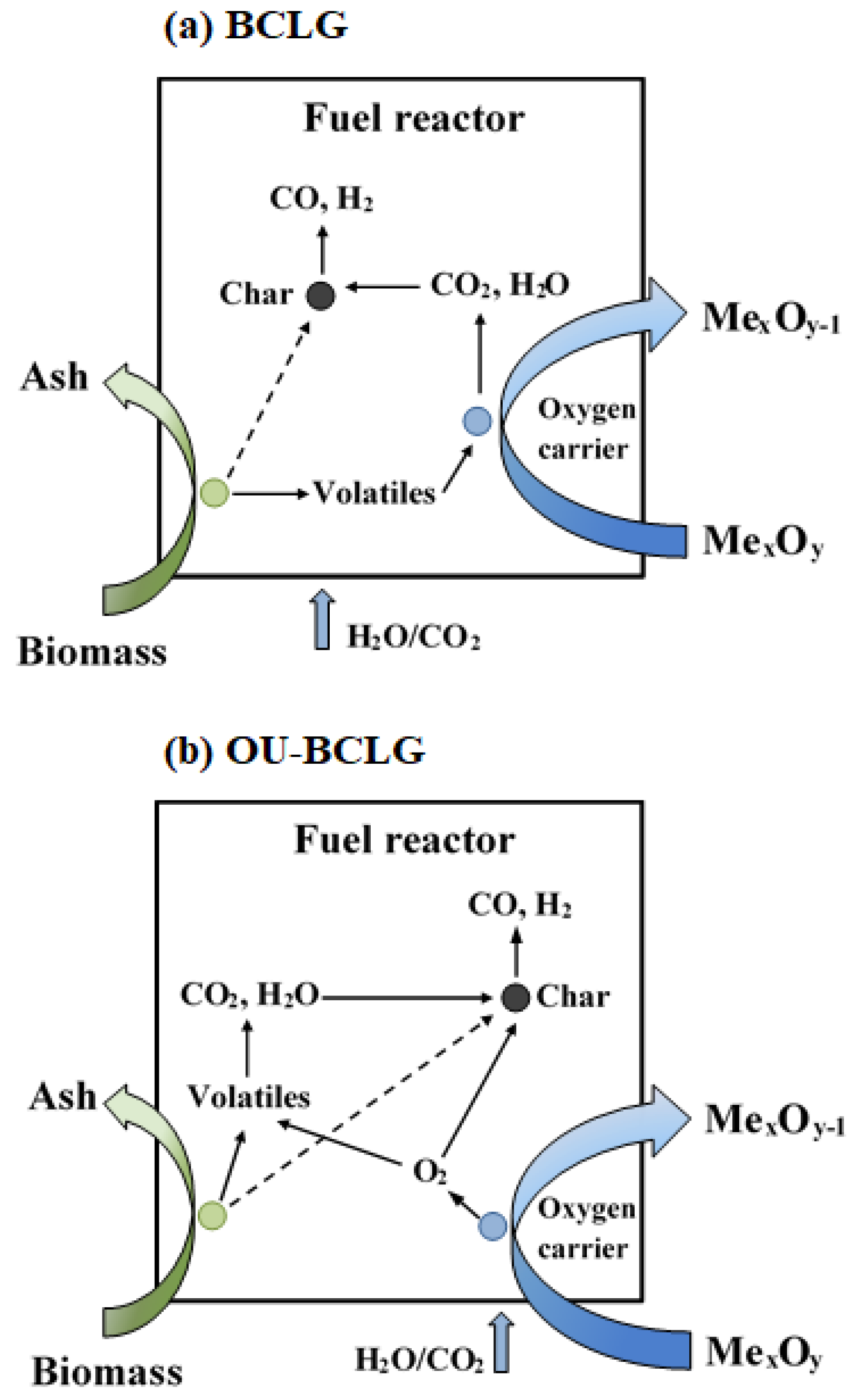
2.1. Process Description
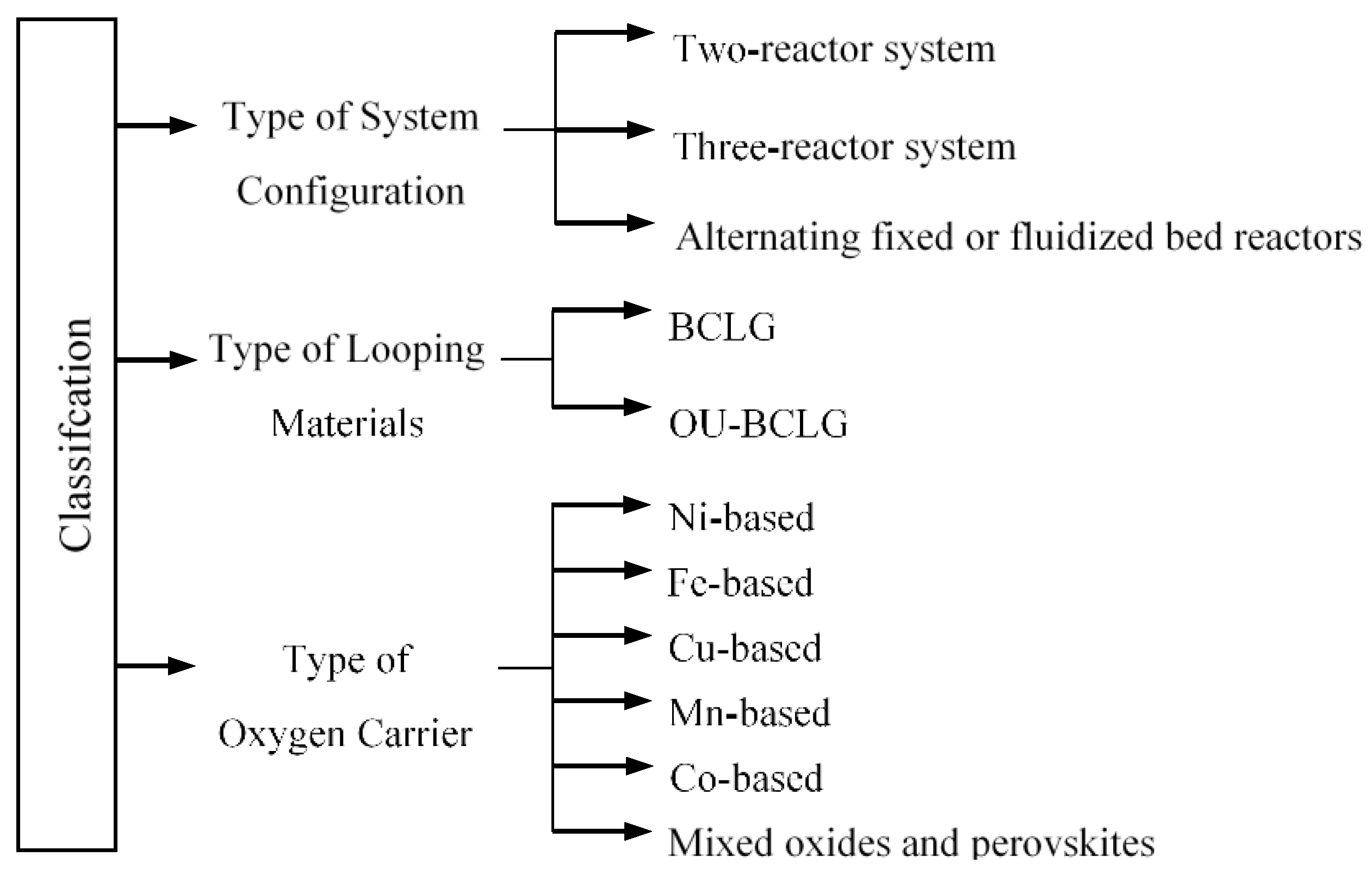
2.2. Process Classification
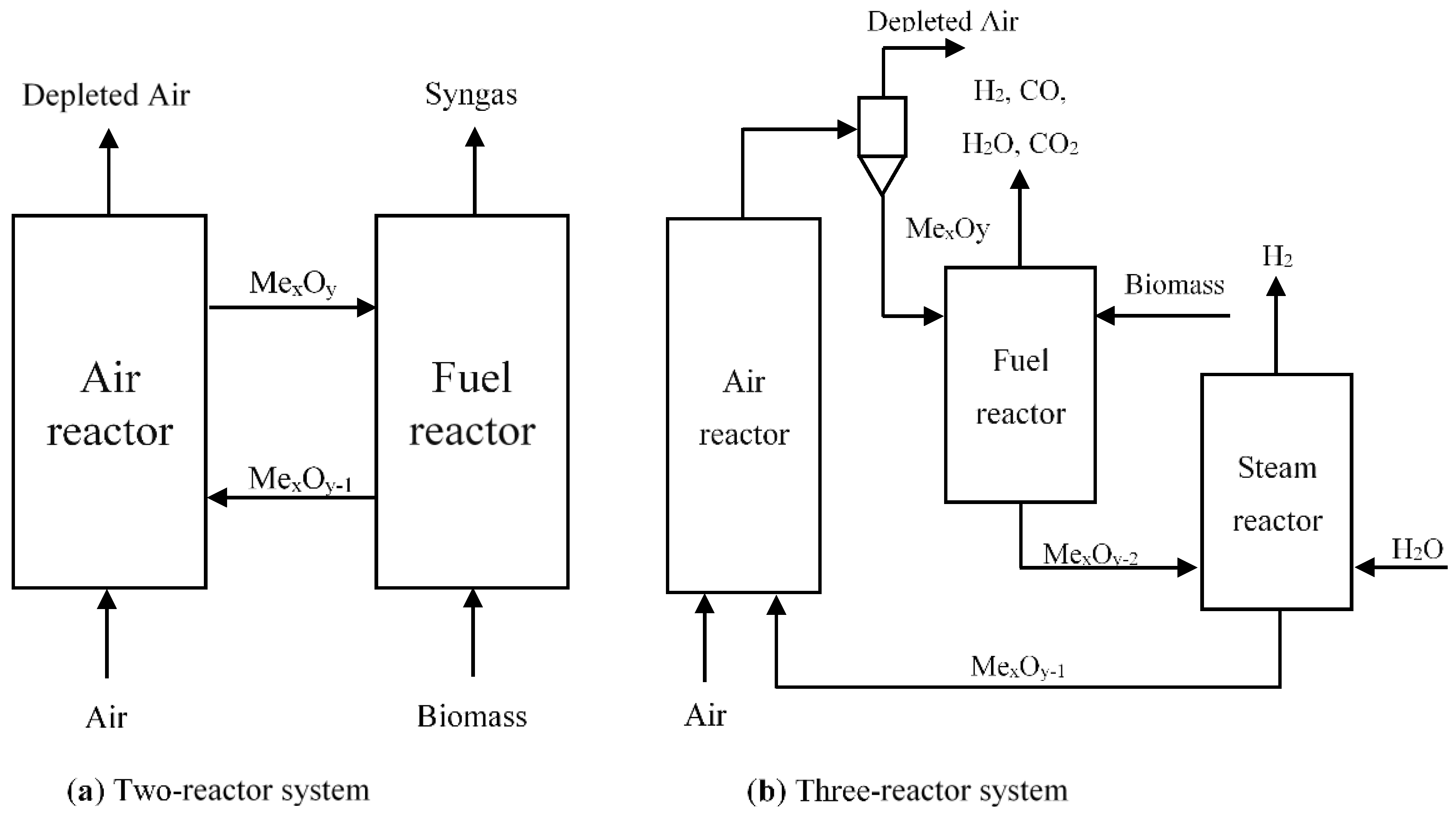
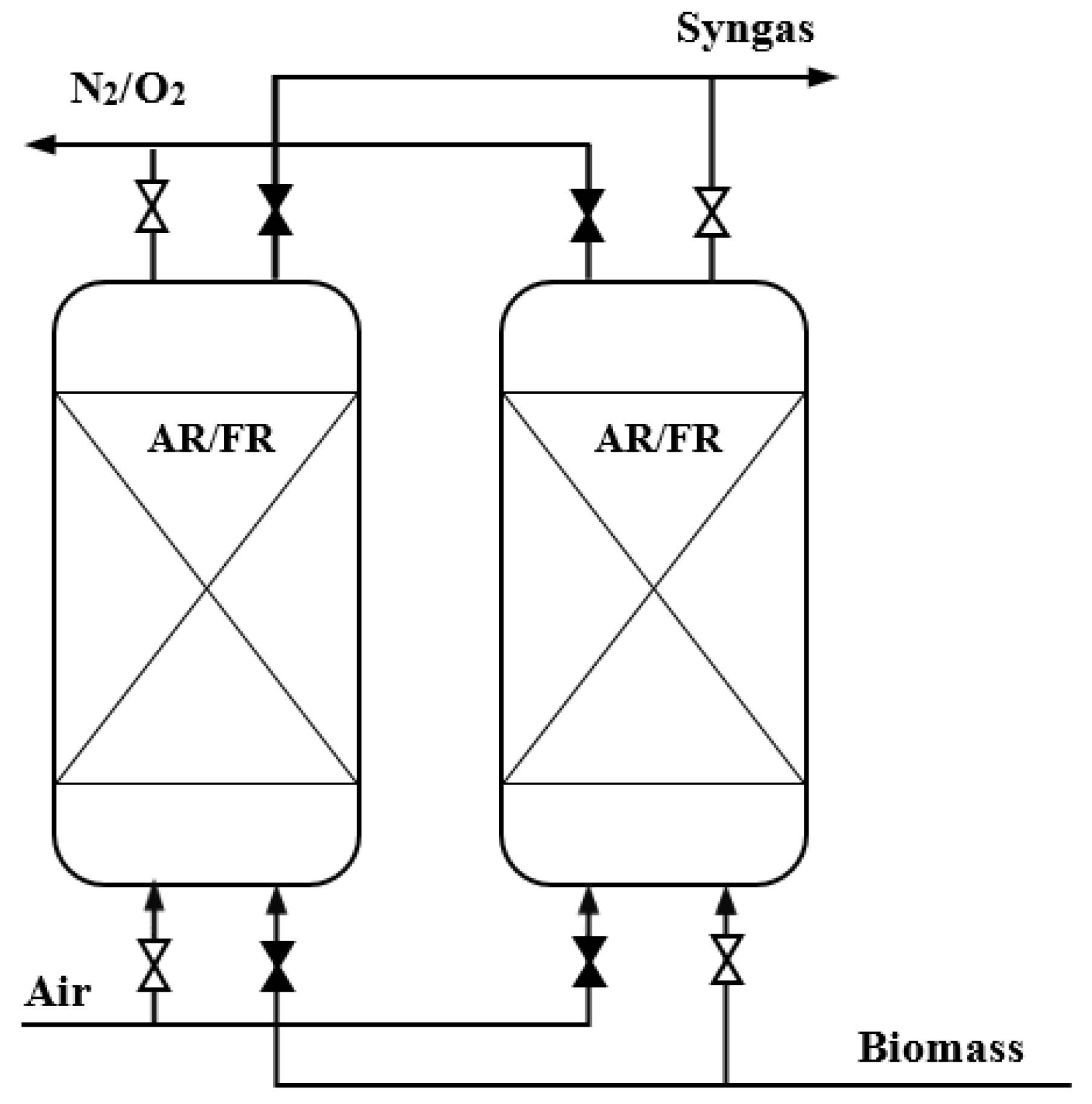
2.2.1. System Configuration
- (i)
- There should be sufficient particle circulation between the FR and the AR.
- (ii)
- There should be sufficient contact between the fuel/air and the solid oxygen carriers to achieve maximum conversion.
- (iii)
- High temperature and high-pressure operations must be carried out to achieve higher overall efficiency.
- (iv)
- There should be limited gas leakage between the FR and the AR.
Two-Reactor System
Three-Reactor System
Packed and Fluidized-Bed
2.2.2. System Complexity and Challenges
System Complexity
Fouling and Corrosion
3. Looping Materials in Chemical Looping Gasification of Biomass
3.1. Looping Materials
3.2. Type of Oxygen Carrier
- (i)
- Sufficient oxygen transport capacity.
- (ii)
- Favorable thermodynamics and reactivity regarding the solid fuel for the reduction reactions.
- (iii)
- High reactivity in the oxidation reactions.
- (iv)
- Selectivity towards CO and H2.
- (v)
- Resistance to attrition to minimize losses of elutriated solid.
- (vi)
- Minimal carbon deposition.
- (vii)
- Good fluidization characteristics (no presence of agglomeration) and high melting points.
- (viii)
- Reasonable cyclability/circulation for using over several redox reactions.
- (ix)
- Low cost and long lifetime.
- (x)
- Environmentally friendly properties.
- (xi)
- High mechanical strength and resistance to frictional stresses.
- (xii)
- Capability of converting biomass to gaseous products.
- (xiii)
- Propensity to convert methane
- (i)
- Ni-based oxygen carriers.
- (ii)
- Cu-based oxygen carriers.
- (iii)
- Fe-based oxygen carriers.
- (iv)
- Mn-based oxygen carriers.
- (v)
- Co-based oxygen carriers.
- (vi)
- Perovskite-type complex metal oxides
- (vii)
- Other oxygen carriers.
- (i)
- Increase the reactivity and/or stability of particles.
- (ii)
- Improve the conversion of the fuel gas.
- (iii)
- Improve the mechanical strength and resistance to the attrition of particles.
- (iv)
- Improve tar decomposition.
- (v)
- Improve carbon dioxide adsorption.
- (vi)
- Decrease the carbon deposition.
- (vii)
- Decrease the preparation cost of the oxygen carrier.
- (viii)
- Decrease the use of toxic metals.
3.3. Performance of Looping Materials
4. Effect of Process Parameters on Biomass Chemical Looping Gasification
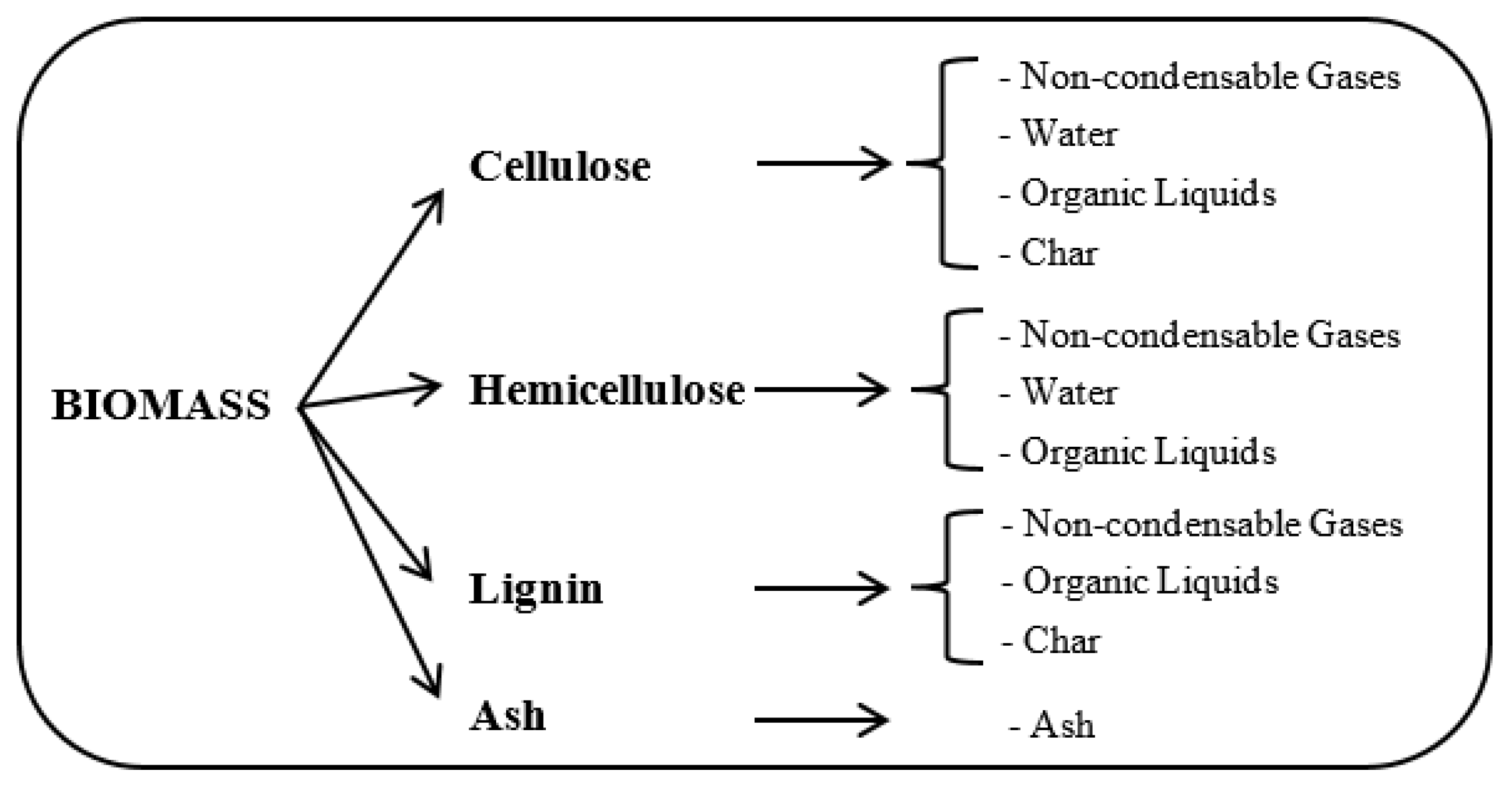
4.1. Biomass Characteristics
- (i)
- The chemical constituents: cellulose, hemicellulose, and lignin.
- (ii)
- Elemental composition.
- (iii)
- Inherent mineral content.
- (iv)
- Proximate analysis: moisture, volatile, fixed carbon, and ash contents.
- (v)
- Physical properties: particle size, shape, and density.
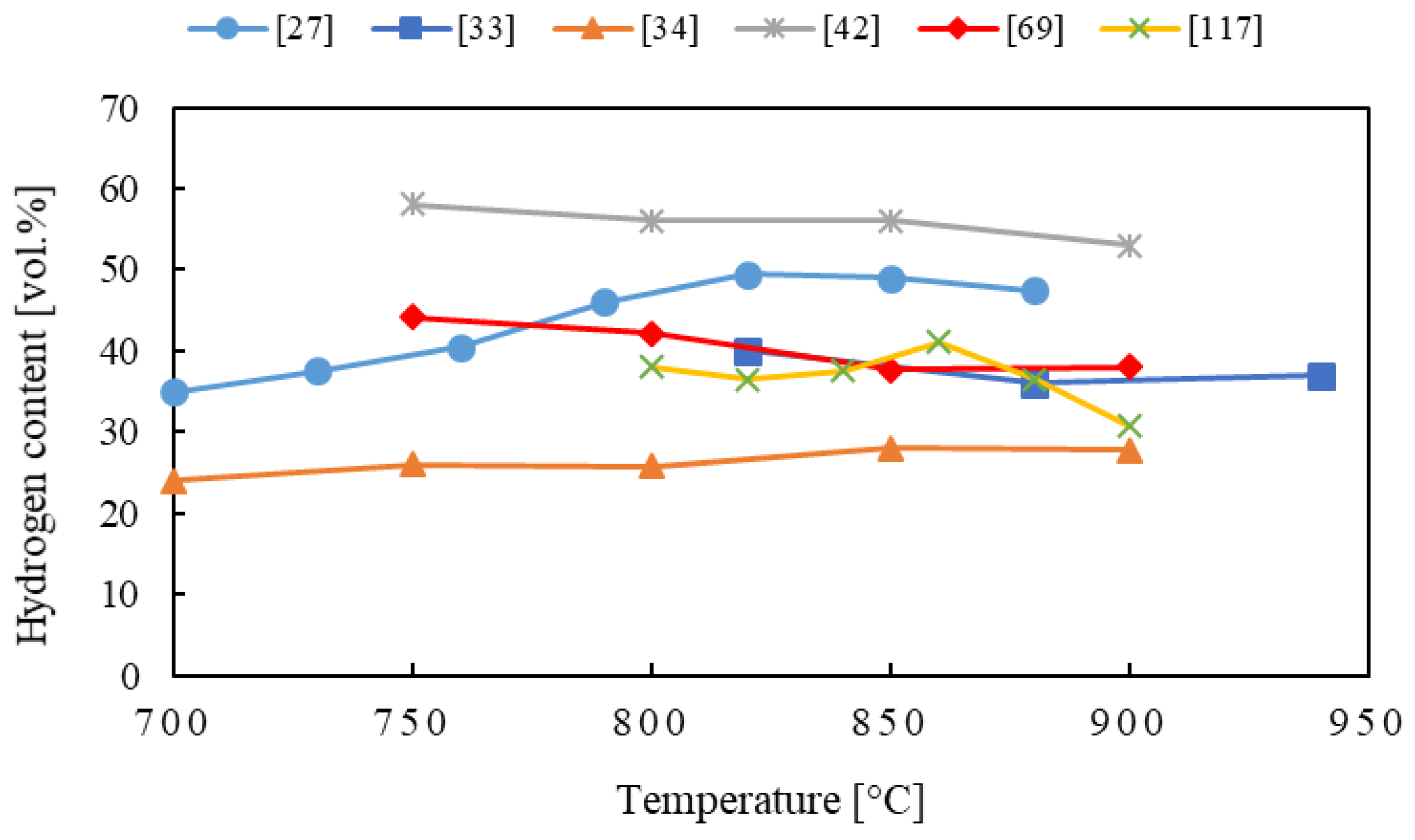
4.2. Gasification Temperature
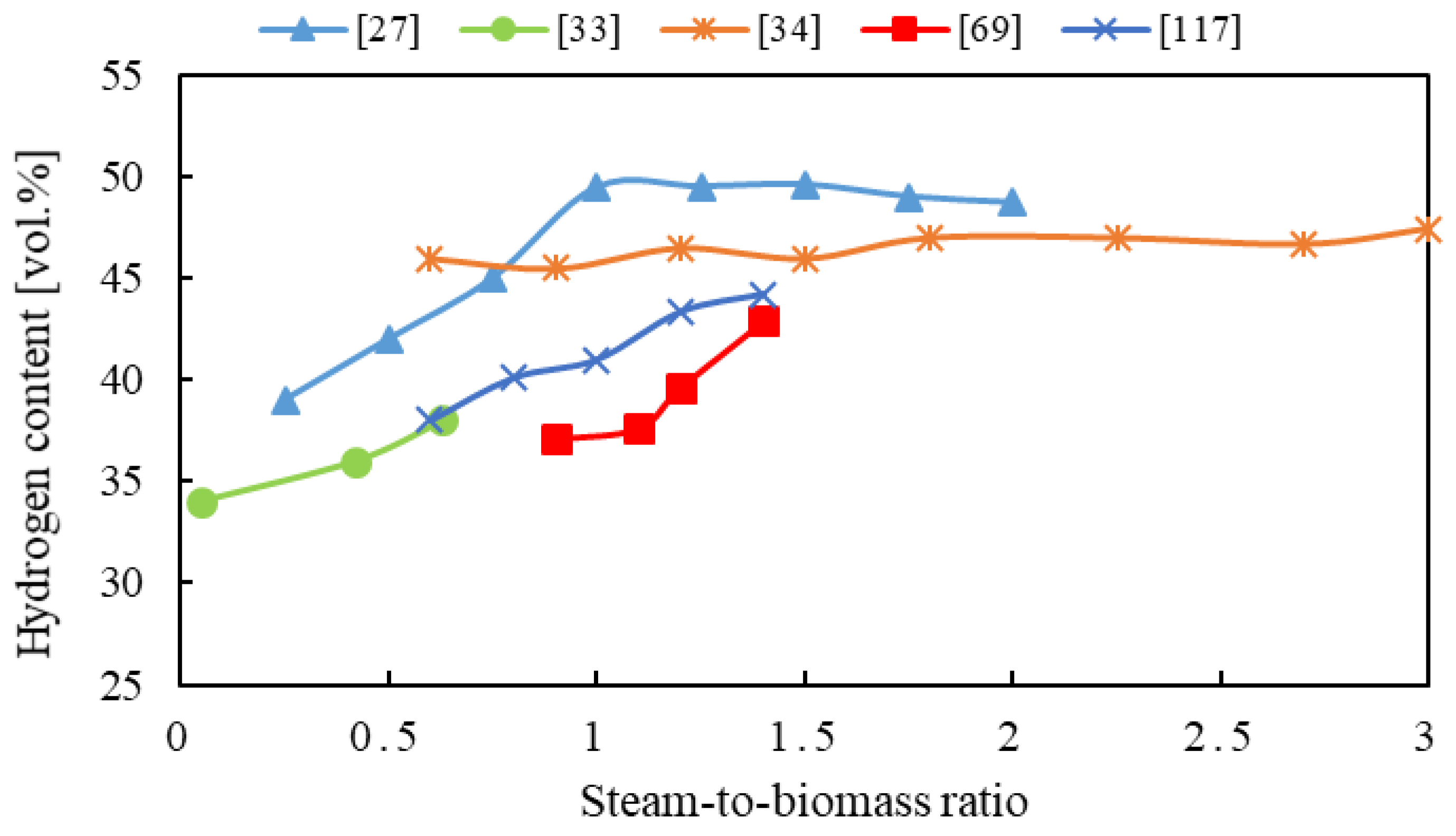
4.3. Steam-to-Biomass Ratio
4.4. Oxygen Carrier-to-Biomass Ratio (OBR)
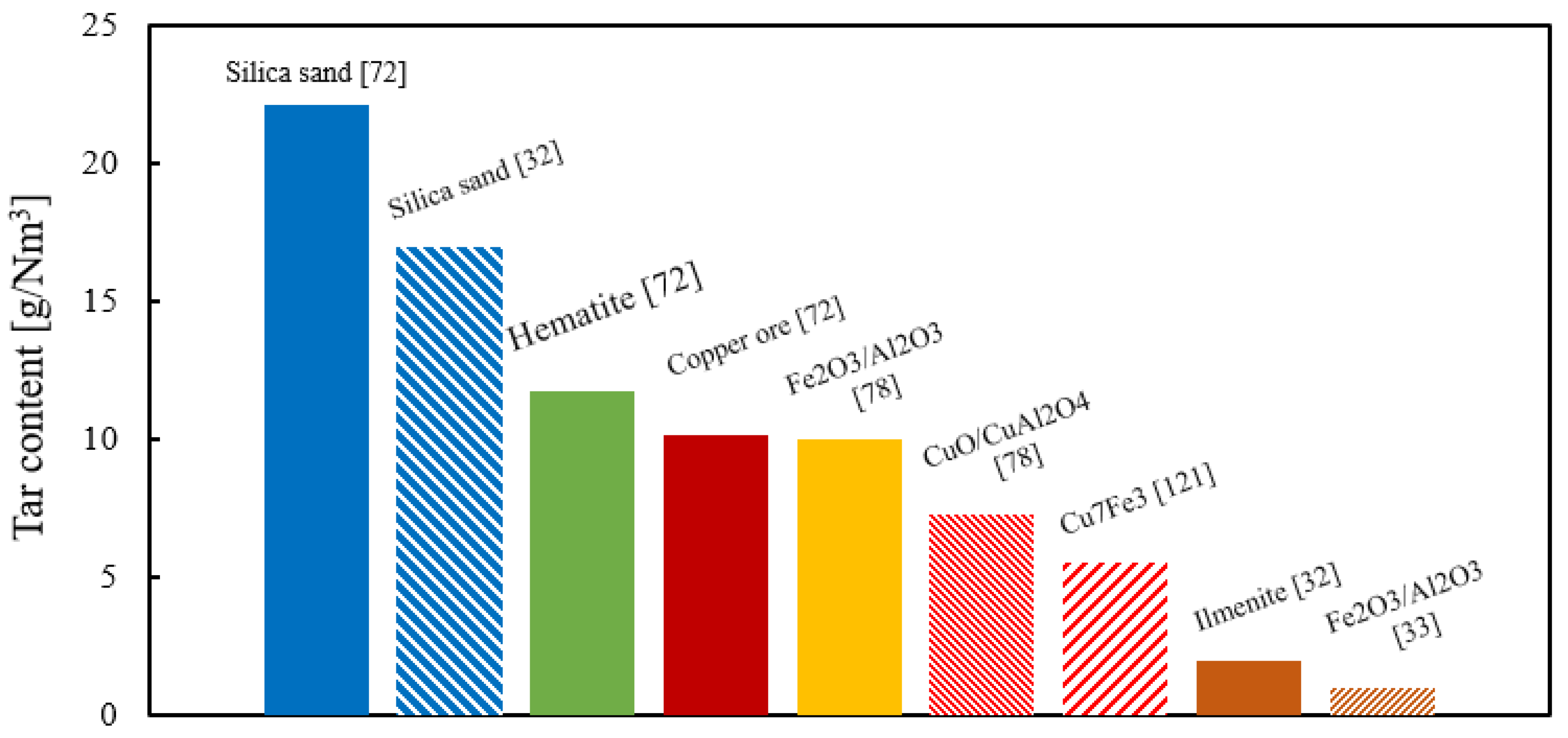
4.5. Effect of Operating Conditions on Tar Formation
5. Progress in Biomass Chemical Looping Gasification
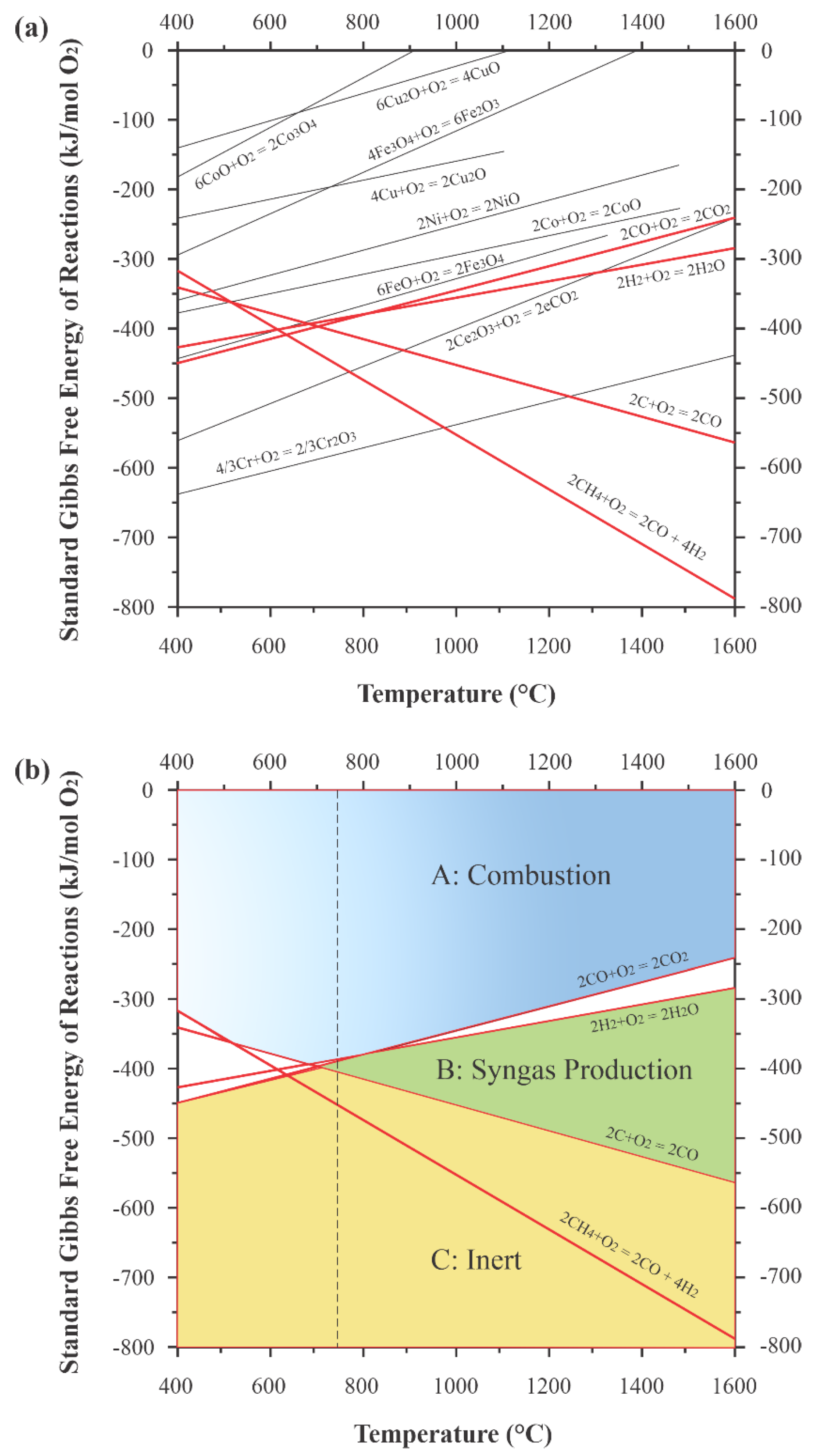
5.1. Thermodynamic Analysis and Kinetic Studies
5.2. Pilot-Scale Investigations
5.3. Simulation/Modeling Studies
- Thermodynamic equilibrium
- Restricted thermodynamic equilibrium
- Kinetic mechanism
- Experimental data
6. Conclusions and Future Prospects
- Description of principles and developments of biomass-based chemical looping gasification technology.
- Recent research strategies and achievements in biomass-based chemical looping gasification.
- Prospects and challenges of biomass-based chemical looping gasification towards commercialization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Air reactor |
| BCLG | Biomass chemical looping gasification |
| CFD | Computational fluid dynamics |
| CLC | Chemical looping combustion |
| CLG | Chemical looping gasification |
| CLR | Chemical looping reforming |
| CGC | Cold gas cleanup |
| CV | Calorific value |
| FR | Fuel reactor |
| GHG | Greenhouse gas |
| HHV | Higher heating value |
| LHV | Lower heating value |
| IEO | International energy outlook |
| LM | Looping material |
| HGC | Hot gas cleanup |
| OC | Oxygen carrier |
| WGC | Warm gas cleanup |
| TGA | Thermogravimetric analysis |
References
- United Nations. Paris Climate Agreement. 2015. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 10 May 2021).
- Administration USEI. International Energy Outlook 2016 with Projections to 2040; U.S. Energy Information Administration: Washington, DC, USA, 2016; p. 290.
- Federal Ministry for the Environment NC, Building and Nuclear Safety. Climate Action Plan 2050. Principles and Goals of the German Government’s Climate Policy; Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety: Berlin, Germany, 2016; p. 92.
- Lamers, P.; Hoefnagels, R.; Junginger, M.; Hamelinck, C.; Faaij, A. Global solid biomass trade for energy by 2020: An assessment of potential import streams and supply costs to North-West Europe under different sustainability constraints. GCB Bioenergy 2015, 7. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Mendiara, T.; García-Labiano, F.; Abad, A.; Gayán, P.; de Diego, L.F.; Izquierdo, M.; Adánez, J. Negative CO2 emissions through the use of biofuels in chemical looping technology: A review. Appl. Energy 2018, 232, 657–684. [Google Scholar] [CrossRef]
- Mattison, T.; Hildor, F.; Li, Y.; Linderholm, C. Negative emissions of carbon dioxide through chemical-looping combustion (CLC) and gasification (CLG) using oxygen carriers based on manganese and iron. Mitig. Adapt. Strat. Glob. Chang. 2020, 25, 497–517. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.-H.A.; Fennell, P.S.; Shen, L.; Fan, L.-S. Biomass-based chemical looping technologies: The good, the bad and the future. Energy Environ. Sci. 2017, 10, 1885–1910. [Google Scholar] [CrossRef]
- Ishida, M.; Zheng, D.; Akehata, T. Evaluation of a chemical-looping-combustion power-generation system by graphic exergy analysis. Energy 1987, 12, 147–154. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; García-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Nandya, A.; Lohaa, C.; Gub, S.; Sarkarc, P.; Karmakara, M.K.; Chatterjeea, P.K. Present status and overview of Chemical Looping Combustion technology. Renew. Sustain. Energy Rev. 2016, 59, 597–619. [Google Scholar] [CrossRef]
- Pujara, M.; Sheth, M.; Rachchh, N.; Bhoraniya, R.; Harichandan, A.B. Chemical looping reforming (CLR) system for H2 production—A review. In Blockchain Technology and Innovations in Business Processes; Springer: Berlin, Germany, 2020; pp. 267–276. [Google Scholar]
- Luo, M.; Yi, Y.; Wang, S.; Wang, Z.; Du, M.; Pan, J.; Wang, Q. Review of hydrogen production using chemical-looping technology. Renew. Sustain. Energy Rev. 2018, 81, 3186–3214. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Wang, Y.; Huo, R.; Huang, Z.; Liu, M.; Wei, G.; Zhao, Z.; Li, H.; Fang, Y. Review of Biomass Chemical Looping Gasification in China. Energy Fuels 2020, 34, 7847–7862. [Google Scholar] [CrossRef]
- Cuadrat, A.; Abad, A.; García-Labiano, F.; Gayán, P.; de Diego, L.; Adánez, J. Relevance of the coal rank on the performance of the in situ gasification chemical-looping combustion. Chem. Eng. J. 2012, 195, 91–102. [Google Scholar] [CrossRef]
- Dieringer, P.; Marx, F.; Alobaid, F.; Ströhle, J.; Epple, B. Process Control Strategies in Chemical Looping Gasification—A Novel Process for the Production of Biofuels Allowing for Net Negative CO2 Emissions. Appl. Sci. 2020, 10, 4271. [Google Scholar] [CrossRef]
- Protasova, L.; Snijkers, F. Recent developments in oxygen carrier materials for hydrogen production via chemical looping processes. Fuel 2016, 181, 75–93. [Google Scholar] [CrossRef]
- Mendiara, T.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Gayan, P.; Adanez, J. Biomass combustion in a CLC system using an iron ore as an oxygen carrier. Int. J. Greenh. Gas Control. 2013, 19, 322–330. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, A.; Deng, Z.; Wei, G.; Zhao, K.; Chen, D.; He, F.; Zhao, Z.; Li, H.; Li, F. In-situ removal of toluene as a biomass tar model compound using NiFe2O4 for application in chemical looping gasification oxygen carrier. Energy 2020, 190, 116360. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield—A review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Wolf, J. CO2 Mitigation in Advanced Power Cycles. Ph.D. Thesis, KTH-Royal Institute of Technology, Kemiteknik, Stockholm, Sweden, 2004. [Google Scholar]
- Noorman, S.; Annaland, M.V.S.; Kuipers, H. Packed Bed Reactor Technology for Chemical-Looping Combustion. Ind. Eng. Chem. Res. 2007, 46, 4212–4220. [Google Scholar] [CrossRef]
- Huijun, G.; Laihong, S.; Fei, F.; Shouxi, J. Experiments on biomass gasification using chemical looping with nickel-based oxygen carrier in a 25 kWth reactor. Appl. Therm. Eng. 2015, 85, 52–60. [Google Scholar] [CrossRef]
- Huseyin, S.; Wei, G.-Q.; Li, H.-B.; He, F.; Huang, Z. Chemical-looping gasification of biomass in a 10 kWth interconnected fluidized bed reactor using Fe2O3/Al2O3 oxygen carrier. J. Fuel Chem. Technol. 2014, 42, 922–931. [Google Scholar] [CrossRef]
- Zeng, J.; Xiao, R.; Zhang, H.; Wang, Y.; Zeng, D.; Ma, Z. Chemical looping pyrolysis-gasification of biomass for high H2/CO syngas production. Fuel Process. Technol. 2017, 168, 116–122. [Google Scholar] [CrossRef]
- Hedayati, A.; Soleimanisalim, A.H.; Linderholm, C.J.; Mattisson, T.; Lyngfelt, A. Experimental evaluation of manganese ores for chemical looping conversion of synthetic biomass volatiles in a 300 W reactor system. J. Environ. Chem. Eng. 2021, 9, 105112. [Google Scholar] [CrossRef]
- Marx, F.; Dieringer, P.; Ströhle, J.; Epple, B. Design of a 1 MWth pilot plant for Chemical looping gasification of Biogenic Residues. Energies 2021, 14, 2581. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Basu, P. Chemical-Looping Gasification of Biomass for Hydrogen-Enriched Gas Production with In-Process Carbon Dioxide Capture. Energy Fuels 2009, 23, 5077–5083. [Google Scholar] [CrossRef]
- Wei, G.; He, F.; Zhao, Z.; Huang, Z.; Zheng, A.; Zhao, K.; Li, H. Performance of Fe–Ni bimetallic oxygen carriers for chemical looping gasification of biomass in a 10 kWth interconnected circulating fluidized bed reactor. Int. J. Hydrogen Energy 2015, 40, 16021–16032. [Google Scholar] [CrossRef]
- Condori, O.; García-Labiano, F.; de Diego, L.F.; Izquierdo, M.T.; Abad, A.; Adánez, J. Biomass chemical looping gasification for syngas production using ilmenite as oxygen carrier in a 1.5 kWth unit. Chem. Eng. J. 2021, 405, 126679. [Google Scholar] [CrossRef]
- Samprón, I.; de Diego, L.F.; García-Labiano, F.; Izquierdo, M.T.; Abad, A.; Adánez, J. Biomass Chemical Looping Gasification of pine wood using a synthetic Fe2O3/Al2O3 oxygen carrier in a continuous unit. Bioresour. Technol. 2020, 316, 123908. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Huang, Z.; Wei, G.; Zhao, K.; Wang, G.; Kong, X.; Feng, Y.; Tan, H.; Hou, S.; Lv, Y.; et al. Biomass chemical-looping gasification coupled with water/CO2-splitting using NiFe2O4 as an oxygen carrier. Energy Convers. Manag. 2019, 201, 112157. [Google Scholar] [CrossRef]
- Wolf, J.; Yan, J. Parametric study of chemical looping combustion for tri-generation of hydrogen, heat, and electrical power with CO2 capture. Int. J. Energy Res. 2005, 29, 739–753. [Google Scholar] [CrossRef]
- Cormos, C.-C. Hydrogen production from fossil fuels with carbon capture and storage based on chemical looping systems. Int. J. Hydrogen Energy 2011, 36, 5960–5971. [Google Scholar] [CrossRef]
- Chiesa, P.; Lozza, G.; Malandrino, A.; Romano, M.C.; Piccolo, V. Three-reactors chemical looping process for hydrogen production. Int. J. Hydrogen Energy 2008, 33, 2233–2245. [Google Scholar] [CrossRef]
- Xiang, W.; Chen, S.; Xue, Z.; Sun, X. Investigation of coal gasification hydrogen and electricity co-production plant with three-reactors chemical looping process. Int. J. Hydrogen Energy 2010, 35, 8580–8591. [Google Scholar] [CrossRef]
- Fan, L.-S.; Li, F. Chemical Looping Technology and Its Fossil Energy Conversion Applications. Ind. Eng. Chem. Res. 2010, 49, 10200–10211. [Google Scholar] [CrossRef]
- Gupta, P.; Velazquez-Vargas, L.G.; Fan, L.-S. Syngas Redox (SGR) Process to Produce Hydrogen from Coal Derived Syngas. Energy Fuels 2007, 21, 2900–2908. [Google Scholar] [CrossRef]
- Li, F.; Kim, H.R.; Sridhar, D.; Wang, F.; Zeng, L.; Chen, J.; Fan, L.-S. Syngas Chemical Looping Gasification Process: Oxygen Carrier Particle Selection and Performance. Energy Fuels 2009, 23, 4182–4189. [Google Scholar] [CrossRef]
- Yan, J.; Sun, R.; Shen, L.; Bai, H.; Jiang, S.; Xiao, Y.; Song, T. Hydrogen-rich syngas production with tar elimination via biomass chemical looping gasification (BCLG) using BaFe2O4/Al2O3 as oxygen carrier. Chem. Eng. J. 2020, 387, 124107. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, C.; Peng, B.; Liu, C.; Li, Z.; Wu, K.; Zhang, H.; Xiao, R. High H2/CO ratio syngas production from chemical looping co-gasification of biomass and polyethylene with CaO/Fe2O3 oxygen carrier. Energy Convers. Manag. 2019, 199, 111951. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Feng, Y.; Zhao, K.; Zheng, A.; Chang, S.; Wei, G.; Zhao, Z.; Li, H. Biomass Char Direct Chemical Looping Gasification Using NiO-Modified Iron Ore as an Oxygen Carrier. Energy Fuels 2014, 28, 183–191. [Google Scholar] [CrossRef]
- Liu, G.; Liao, Y.; Wu, Y.; Ma, X. Enhancement of Ca2Fe2O5 oxygen carrier through Mg/Al/Zn oxide support for biomass chemical looping gasification. Energy Convers. Manag. 2019, 195, 262–273. [Google Scholar] [CrossRef]
- Wang, S.; Song, T.; Yin, S.; Hartge, E.-U.; Dymala, T.; Shen, L.; Heinrich, S.; Werther, J. Syngas, tar and char behavior in chemical looping gasification of sawdust pellet in fluidized bed. Fuel 2020, 270, 117464. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Woolcock, P.J.; Brown, R.C. A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 2013, 52, 54–84. [Google Scholar] [CrossRef]
- Sharma, S.; Dolan, M.; Park, D.; Morpeth, L.; Ilyushechkin, A.; McLennan, K.; Harris, D.; Thambimuthu, K. A critical review of syngas cleaning technologies—Fundamental limitations and practical problems. Powder Technol. 2008, 180, 115–121. [Google Scholar] [CrossRef]
- Asadullah, M. Barriers of commercial power generation using biomass gasification gas: A review. Renew. Sustain. Energy Rev. 2014, 29, 201–215. [Google Scholar] [CrossRef]
- Milne, T.A.; Evans, R.J.; Abatzaglou, N. Biomass Gasifier “Tars”: Their Nature, Formation, and Conversion; National Renewable Energy Laboratory: Golden, CO, USA, 1998.
- Nunes, L.; Matias, J.; Catalão, J.P.S. Biomass combustion systems: A review on the physical and chemical properties of the ashes. Renew. Sustain. Energy Rev. 2016, 53, 235–242. [Google Scholar] [CrossRef]
- Heakal, F.E.-T.; Fouda, A.; Radwan, M. Inhibitive effect of some thiadiazole derivatives on C-steel corrosion in neutral sodium chloride solution. Mater. Chem. Phys. 2011, 125, 26–36. [Google Scholar] [CrossRef]
- Roos, C.J. Clean Heat and Power Using Biomass Gasification for Industrial and Agricultural Project; U.S. Department of Energy, Northwest CHP Application Center: Washington, DC, USA, 2010; p. 64.
- Schmid, J.C.; Wolfesberger, U.; Koppatz, S.; Pfeifer, C.; Hofbauer, H. Variation of feedstock in a dual fluidized bed steam gasifier-influence on product gas, tar content, and composition. Environ. Prog. Sustain. Energy 2012, 31, 205–215. [Google Scholar] [CrossRef]
- Ran, J.; Fu, F.; Qin, C.; Zhang, P.; Yang, L.; Wang, W. Evaluation of CuO/MgAl2O4 in Biomass Chemical Looping Gasification with Oxygen Uncoupling. Bioresources 2016, 11, 2109–2123. [Google Scholar] [CrossRef][Green Version]
- Zhang, B.; Wu, Z.; Zhang, J.; Guo, W.; Yang, B. Chemical Looping with Oxygen Uncoupling of the Lignocellulosic Biomass Main Model Compound: Product Distribution and Kinetic Analysis on Lignin. Energy Fuels 2020, 34, 10968–10979. [Google Scholar] [CrossRef]
- Wang, M.; Li, T.; Xiao, Y.; Wang, W. Study of chemical looping co-gasification of lignite and rice husk with Cu-Ni oxygen carrier. Int. J. Low-Carbon Technol. 2021. [Google Scholar] [CrossRef]
- Mendiara, T.; Johansen, J.M.; Utrilla, R.; Jensen, A.D.; Glarborg, P. Evaluation of different oxygen carriers for biomass tar reforming (II): Carbon deposition in experiments with methane and other gases. Fuel 2011, 90, 1370–1382. [Google Scholar] [CrossRef]
- Park, H.J.; Park, S.H.; Sohn, J.M.; Park, J.; Jeon, J.-K.; Kim, S.-S.; Park, Y.-K. Steam reforming of biomass gasification tar using benzene as a model compound over various Ni supported metal oxide catalysts. Bioresour. Technol. 2010, 101, S101–S103. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, N.; Nobuta, K.; Kimura, T.; Hosokai, S.; Hayashi, J.-I.; Tago, T.; Masuda, T. Production of chemicals by cracking pyrolytic tar from Loy Yang coal over iron oxide catalysts in a steam atmosphere. Fuel Process. Technol. 2011, 92, 771–775. [Google Scholar] [CrossRef]
- Wang, P.; Means, N.; Shekhawat, D.; Berry, D.; Massoudi, M. Chemical-Looping Combustion and Gasification of Coals and Oxygen Carrier Development: A Brief Review. Energies 2015, 8, 10605–10635. [Google Scholar] [CrossRef]
- Guo, Q.; Cheng, Y.; Liu, Y.; Jia, W.; Ryu, H.-J. Coal Chemical Looping Gasification for Syngas Generation Using an Iron-Based Oxygen Carrier. Ind. Eng. Chem. Res. 2013, 53, 78–86. [Google Scholar] [CrossRef]
- Voitic, G.; Hacker, V. Recent advancements in chemical looping water splitting for the production of hydrogen. RSC Adv. 2016, 6, 98267–98296. [Google Scholar] [CrossRef]
- Zhu, X.; Li, K.; Neal, L.M.; Li, F. Perovskites as Geo-inspired Oxygen Storage Materials for Chemical Looping and Three-Way Catalysis: A Perspective. ACS Catal. 2018, 8, 8213–8236. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Fu, J.; Yu, L.; Chen, M.; Liu, S.; He, F.; Chen, D.; Wei, G.; Zhao, K.; et al. Chemical looping gasification of biomass char using iron ore as an oxygen carrier. Int. J. Hydrogen Energy 2016, 41, 17871–17883. [Google Scholar] [CrossRef]
- Zeng, J.; Xiao, R.; Zhang, H.; Chen, X.; Zeng, D.; Ma, Z. Syngas production via biomass self-moisture chemical looping gasification. Biomass-Bioenergy 2017, 104, 1–7. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Alobaid, F.; Epple, B. Chemical looping gasification of torrefied woodchips in a bubbling fluidized bed test rig using iron-based oxygen carriers. Renew. Energy 2021, 172, 34–45. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, G.; Deng, Z.; Zhao, K.; He, F.; Chen, D.; Wei, G.; Zheng, A.; Zhao, Z.; Li, H. Investigation on gasification performance of sewage sludge using chemical looping gasification with iron ore oxygen carrier. Int. J. Hydrogen Energy 2017, 42, 25474–25491. [Google Scholar] [CrossRef]
- Tian, X.; Niu, P.; Ma, Y.; Zhao, H. Chemical-looping gasification of biomass: Part II. Tar yields and distributions. Biomass-Bioenergy 2018, 108, 178–189. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, H.; Zheng, C. Synthesis Gas Generation by Chemical-Looping Reforming of Biomass with Natural Copper Ore as Oxygen Carrier. Waste Biomass-Valorization 2015, 6, 81–89. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Solar chemical looping gasification of biomass with the ZnO/Zn redox system for syngas and zinc production in a continuously-fed solar reactor. Fuel 2018, 215, 66–79. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; He, F.; Chen, D.; Wei, G.; Zhao, K.; Zheng, A.; Zhao, Z.; Li, H. Reactivity investigation on chemical looping gasification of biomass char using nickel ferrite oxygen carrier. Int. J. Hydrogen Energy 2017, 42, 14458–14470. [Google Scholar] [CrossRef]
- Shen, T.; Ge, H.; Shen, L. Characterization of combined Fe-Cu oxides as oxygen carrier in chemical looping gasification of biomass. Int. J. Greenh. Gas Control. 2018, 75, 63–73. [Google Scholar] [CrossRef]
- Adanez, J.; de Diego, L.F.; García-Labiano, F.; Gayan, P.; Abad, A.; Palacios, J.M. Selection of Oxygen Carriers for Chemical-Looping Combustion. Energy Fuels 2004, 18, 371–377. [Google Scholar] [CrossRef]
- Shen, L.; Wu, J.; Gao, Z.; Xiao, J. Reactivity deterioration of NiO/Al2O3 oxygen carrier for chemical looping combustion of coal in a 10kWth reactor. Combust. Flame 2009, 156, 1377–1385. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, L.; Zou, X. Chemical-looping auto-thermal reforming of biomass using Cu-based oxygen carrier. Appl. Energy 2015, 157, 408–415. [Google Scholar] [CrossRef]
- Yu, Z.; Li, C.; Fang, Y.; Huang, J.; Wang, Z. Reduction Rate Enhancements for Coal Direct Chemical Looping Combustion with an Iron Oxide Oxygen Carrier. Energy Fuels 2012, 26, 2505–2511. [Google Scholar] [CrossRef]
- Gu, H.; Shen, L.; Xiao, J.; Zhang, S.; Song, T.; Chen, D. Iron ore as oxygen carrier improved with potassium for chemical looping combustion of anthracite coal. Combust. Flame 2012, 159, 2480–2490. [Google Scholar] [CrossRef]
- Andrus, H.E.; Chiu, J.H.; Thibeault, P.R.; Brautsch, A. Alstom’s Calcium Oxide Chemical Looping Combustion Coal Power Technology Development. In Proceedings of the 34th International Technical Conference on Clean Coal and Fuel Systems, Clearwater Beach, FL, USA, 31 May–4 June 2009; pp. 1179–1900. [Google Scholar]
- Yu, Z.; Yang, Y.; Yang, S.; Zhang, Q.; Zhao, J.; Fang, Y.; Hao, X.; Guan, G. Iron-based oxygen carriers in chemical looping conversions: A review. Carbon Resour. Convers. 2019, 2, 23–34. [Google Scholar] [CrossRef]
- Chen, Y.; Galinsky, N.; Wang, Z.; Li, F. Investigation of perovskite supported composite oxides for chemical looping conversion of syngas. Fuel 2014, 134, 521–530. [Google Scholar] [CrossRef]
- Chen, S.; Shi, Q.; Xue, Z.; Sun, X.; Xiang, W. Experimental investigation of chemical-looping hydrogen generation using Al2O3 or TiO2 -supported iron oxides in a batch fluidized bed. Int. J. Hydrogen Energy 2011, 36, 8915–8926. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, Y.; Liu, G.; Ma, X. Syngas production by chemical looping gasification of biomass with steam and CaO additive. Int. J. Hydrogen Energy 2018, 43, 19375–19383. [Google Scholar] [CrossRef]
- Hu, Q.; Shen, Y.; Chew, J.W.; Ge, T.; Wang, C.-H. Chemical looping gasification of biomass with Fe2O3/CaO as the oxygen carrier for hydrogen-enriched syngas production. Chem. Eng. J. 2020, 379, 122346. [Google Scholar] [CrossRef]
- Kang, K.-S.; Kim, C.-H.; Bae, K.-K.; Cho, W.-C.; Kim, S.-H.; Park, C.-S. Oxygen-carrier selection and thermal analysis of the chemical-looping process for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 12246–12254. [Google Scholar] [CrossRef]
- Zheng, Z.; Luo, L.; Feng, A.; Iqbal, T.; Li, Z.; Qin, W.; Dong, C.; Zhang, S.; Xiao, X. CaO-Assisted Alkaline Liquid Waste Drives Corn Stalk Chemical Looping Gasification for Hydrogen Production. ACS Omega 2020, 5, 24403–24411. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Basu, P. An investigation into steam gasification of biomass for hydrogen enriched gas production in presence of CaO. Int. J. Hydrogen Energy 2010, 35, 1582–1589. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, X.; Russell, C.K.; Dyar, M.D.; Sklute, E.C.; Toan, S.; Fan, M.; Duan, L.; Xiang, W. Synergistic enhancement of chemical looping-based CO2 splitting with biomass cascade utilization using cyclic stabilized Ca2Fe2O5 aerogel. J. Mater. Chem. A 2019, 7, 1216–1226. [Google Scholar] [CrossRef]
- Udomsirichakorn, J.; Salam, P.A. Review of hydrogen-enriched gas production from steam gasification of biomass: The prospect of CaO-based chemical looping gasification. Renew. Sustain. Energy Rev. 2014, 30, 565–579. [Google Scholar] [CrossRef]
- Han, L.; Wang, Q.; Yang, Y.; Yu, C.; Fang, M.; Luo, Z. Hydrogen production via CaO sorption enhanced anaerobic gasification of sawdust in a bubbling fluidized bed. Int. J. Hydrogen Energy 2011, 36, 4820–4829. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, S.; Russell, C.K.; Hu, J.; Rony, A.H.; Tan, G.; Chen, A.; Duan, L.; Boman, J.; Tang, J.; et al. Improvement of H2-rich gas production with tar abatement from pine wood conversion over bi-functional Ca2Fe2O5 catalyst: Investigation of inner-looping redox reaction and promoting mechanisms. Appl. Energy 2018, 212, 931–943. [Google Scholar] [CrossRef]
- Wu, J.; Bai, L.; Tian, H.; Riley, J.; Siriwardane, R.; Wang, Z.; He, T.; Li, J.; Zhang, J.; Wu, J. Chemical looping gasification of lignin with bimetallic oxygen carriers. Int. J. Greenh. Gas Control 2020, 93, 102897. [Google Scholar] [CrossRef]
- He, F.; Galinsky, N.; Li, F. Chemical looping gasification of solid fuels using bimetallic oxygen carrier particles—Feasibility assessment and process simulations. Int. J. Hydrogen Energy 2013, 38, 7839–7854. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Zhu, H.; Chen, D.; Zhao, K.; Wei, G.; Feng, Y.; Zheng, A.; Zhao, Z.; Li, H. Thermodynamic analysis and thermogravimetric investigation on chemical looping gasification of biomass char under different atmospheres with Fe2O3 oxygen carrier. Appl. Energy 2015, 157, 546–553. [Google Scholar] [CrossRef]
- Dueso, C.; Thompson, C.; Metcalfe, I. High-stability, high-capacity oxygen carriers: Iron oxide-perovskite composite materials for hydrogen production by chemical looping. Appl. Energy 2015, 157, 382–390. [Google Scholar] [CrossRef]
- de Diego, L.F.; García-Labiano, F.; Adánez, J.; Gayán, P.; Abad, A.; Corbella, B.M.; Palacios, J.M. Development of Cu-based oxygen carriers for chemical-looping combustion. Fuel 2004, 83, 1749–1757. [Google Scholar] [CrossRef]
- Wang, B.; Yan, R.; Lee, D.H.; Liang, D.T.; Zheng, Y.; Zhao, H.; Zheng, C. Thermodynamic Investigation of Carbon Deposition and Sulfur Evolution in Chemical Looping Combustion with Syngas. Energy Fuels 2008, 22, 1012–1020. [Google Scholar] [CrossRef]
- de Diego, L.F.; Gayán, P.; García-Labiano, F.; Celaya, J.; Abad, A.; Adánez, J. Impregnated CuO/Al2O3 Oxygen Carriers for Chemical-Looping Combustion: Avoiding Fluidized Bed Agglomeration. Energy Fuels 2005, 19, 1850–1856. [Google Scholar] [CrossRef]
- Gu, H.; Shen, L.; Zhong, Z.; Zhou, Y.; Liu, W.; Niu, X.; Ge, H.; Jiang, S.; Wang, L. Interaction between biomass ash and iron ore oxygen carrier during chemical looping combustion. Chem. Eng. J. 2015, 277, 70–78. [Google Scholar] [CrossRef]
- Niu, X.; Shen, L.; Gu, H.; Jiang, S.; Xiao, J. Characteristics of hematite and fly ash during chemical looping combustion of sewage sludge. Chem. Eng. J. 2015, 268, 236–244. [Google Scholar] [CrossRef]
- Niu, X.; Shen, L.; Gu, H.; Song, T.; Xiao, J. Sewage sludge combustion in a CLC process using nickel-based oxygen carrier. Chem. Eng. J. 2015, 260, 631–641. [Google Scholar] [CrossRef]
- Florin, N.; Harris, A.T. Enhanced hydrogen production from biomass with in situ carbon dioxide capture using calcium oxide sorbents. Chem. Eng. Sci. 2008, 63, 287–316. [Google Scholar] [CrossRef]
- de Lasa, H.; Salaices, E.; Mazumder, J.; Lucky, R. Catalytic Steam Gasification of Biomass: Catalysts, Thermodynamics and Kinetics. Chem. Rev. 2011, 111, 5404–5433. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Schüth, F. Design of solid catalysts for the conversion of biomass. Energy Environ. Sci. 2009, 2, 610–626. [Google Scholar] [CrossRef]
- Brown, R. Biochar production technology. In Biochar for Environmental Management; Routledge: London, UK, 2009; p. 448. [Google Scholar]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.-P. The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]
- Mok, W.S.-L.; Antal, M.J. Effects of pressure on biomass pyrolysis. II. Heats of reaction of cellulose pyrolysis. Thermochim. Acta 1983, 68, 165–186. [Google Scholar] [CrossRef]
- Volpe, R.; Zabaniotou, A.A.; Skoulou, V.K. Synergistic Effects between Lignin and Cellulose during Pyrolysis of Agricultural Waste. Energy Fuels 2018, 32, 8420–8430. [Google Scholar] [CrossRef]
- Pasangulapati, V.; Ramachandriya, K.D.; Kumar, A.; Wilkins, M.; Jones, C.L.; Huhnke, R.L. Effects of cellulose, hemicellulose and lignin on thermochemical conversion characteristics of the selected biomass. Bioresour. Technol. 2012, 114, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.C.-C.; Chang, H.-F.; Lin, F.-J.; Lin, K.-H.; Chen, C.-H. Biomass gasification for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 14252–14260. [Google Scholar] [CrossRef]
- Raut, M.K.; Basu, P.; Acharya, B. The Effect of Torrefaction Pre-Treatment on the Gasification of Biomass. Int. J. Renew. Energy Biofuels 2016, 2016, 1–14. [Google Scholar] [CrossRef][Green Version]
- Lv, P.; Xiong, Z.; Chang, J.; Wu, C.; Chen, Y.; Zhu, J. An experimental study on biomass air–steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda-Almansa, G.; Bula, A. Gasification of biomass wastes in an entrained flow gasifier: Effect of the particle size and the residence time. Fuel Process. Technol. 2010, 91, 681–692. [Google Scholar] [CrossRef]
- Di Blasi, C. Kinetic and Heat Transfer Control in the Slow and Flash Pyrolysis of Solids. Ind. Eng. Chem. Res. 1996, 35, 37–46. [Google Scholar] [CrossRef]
- Ge, H.; Guo, W.; Shen, L.; Song, T.; Xiao, J. Biomass gasification using chemical looping in a 25kWth reactor with natural hematite as oxygen carrier. Chem. Eng. J. 2016, 286, 174–183. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Alobaid, F.; May, J.; Peters, J.; Epple, B. Experimental study on steam gasification of torrefied woodchips in a bubbling fluidized bed reactor. Energy 2020, 202, 117744. [Google Scholar] [CrossRef]
- Reed, T.B. Biomass Gasification: Principle and Technology. Energy Technol. Rev. 1981, 67, 123. [Google Scholar]
- Huang, Z.; He, F.; Zhao, K.; Feng, Y.; Zheng, A.; Chang, S.; Zhao, Z.; Li, H. Natural iron ore as an oxygen carrier for biomass chemical looping gasification in a fluidized bed reactor. J. Therm. Anal. Calorim. 2014, 116, 1315–1324. [Google Scholar] [CrossRef]
- Niu, P.; Ma, Y.; Tian, X.; Ma, J.; Zhao, H. Chemical looping gasification of biomass: Part I. screening Cu-Fe metal oxides as oxygen carrier and optimizing experimental conditions. Biomass-Bioenergy 2018, 108, 146–156. [Google Scholar] [CrossRef]
- Yan, L.; Yue, G.; He, B. Thermodynamic analyses of a biomass–coal co-gasification power generation system. Bioresour. Technol. 2016, 205, 133–141. [Google Scholar] [CrossRef]
- Sarafraz, M.; Jafarian, M.; Arjomandi, M.; Nathan, G. Potential use of liquid metal oxides for chemical looping gasification: A thermodynamic assessment. Appl. Energy 2017, 195, 702–712. [Google Scholar] [CrossRef]
- Riley, J.; Siriwardane, R.; Tian, H.; Benincosa, W.; Poston, J. Kinetic analysis of the interactions between calcium ferrite and coal char for chemical looping gasification applications: Identifying reduction routes and modes of oxygen transfer. Appl. Energy 2017, 201, 94–110. [Google Scholar] [CrossRef]
- Wang, K.; Yu, Q.; Qin, Q.; Hou, L.; Duan, W. Thermodynamic analysis of syngas generation from biomass using chemical looping gasification method. Int. J. Hydrogen Energy 2016, 41, 10346–10353. [Google Scholar] [CrossRef]
- Chein, R.-Y.; Hsu, W.-H. Thermodynamic equilibrium analysis of H2-rich syngas production via sorption-enhanced chemical looping biomass gasification. Renew. Energy 2020, 153, 117–129. [Google Scholar] [CrossRef]
- Yang, J.; Ma, L.; Yang, J.; Liu, H.; Liu, S.; Yang, Y.; Mu, L.; Wei, Y.; Ao, R.; Guo, Z.; et al. Thermodynamic and kinetic analysis of CuO-CaSO4 oxygen carrier in chemical looping gasification. Energy 2019, 188, 116109. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; Chen, D.; He, F.; Liu, S.; Zhao, K.; Wei, G.; Zheng, A.; Zhao, Z.; Li, H. Thermodynamic analysis and kinetic investigations on biomass char chemical looping gasification using Fe-Ni bimetallic oxygen carrier. Energy 2017, 141, 1836–1844. [Google Scholar] [CrossRef]
- Chen, L.; Yang, L.; Liu, F.; Nikolic, H.S.; Fan, Z.; Liu, K. Evaluation of multi-functional iron-based carrier from bauxite residual for H2-rich syngas production via chemical-looping gasification. Fuel Process. Technol. 2017, 156, 185–194. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Zhang, B.; Khajeh, A.; Shahbazi, A. Iron oxide supported on silicalite-1 as a multifunctional material for biomass chemical looping gasification and syngas upgrading. Chem. Eng. J. 2020, 401, 125943. [Google Scholar] [CrossRef]
- Hildor, F.; Leion, H.; Linderholm, C.J.; Mattisson, T. Steel converter slag as an oxygen carrier for chemical-looping gasification. Fuel Process. Technol. 2020, 210, 106576. [Google Scholar] [CrossRef]
- Myöhänen, K.; Palonen, J.; Hyppänen, T. Modelling of indirect steam gasification in circulating fluidized bed reactors. Fuel Process. Technol. 2018, 171, 10–19. [Google Scholar] [CrossRef]
- Liu, H.; Cattolica, R.J.; Seiser, R. CFD studies on biomass gasification in a pilot-scale dual fluidized-bed system. Int. J. Hydrogen Energy 2016, 41, 11974–11989. [Google Scholar] [CrossRef]
- Gopaul, S.G.; Dutta, A.; Clemmer, R. Chemical looping gasification for hydrogen production: A comparison of two unique processes simulated using ASPEN Plus. Int. J. Hydrogen Energy 2014, 39, 5804–5817. [Google Scholar] [CrossRef]
- Detchusananard, T.; Ponpesh, P.; Saebea, D.; Authayanun, S.; Arpornwichanop, A. Modeling and Analysis of Sorption Enhanced Chemical Looping Biomass Gasification. Chem. Eng. Trans. 2017, 57, 6. [Google Scholar]
- Cormos, C.-C. Biomass direct chemical looping for hydrogen and power co-production: Process configuration, simulation, thermal integration and techno-economic assessment. Fuel Process. Technol. 2015, 137, 16–23. [Google Scholar] [CrossRef]
- Aghabararnejad, M.; Patience, G.S.; Chaouki, J. Techno-Economic Comparison of a 7-MWthBiomass Chemical Looping Gasification Unit with Conventional Systems. Chem. Eng. Technol. 2015, 38, 867–878. [Google Scholar] [CrossRef]
- Li, F.; Zeng, L.; Fan, L.-S. Biomass direct chemical looping process: Process simulation. Fuel 2010, 89, 3773–3784. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, H.; Guo, W.; Song, T.; Shen, L. System simulation and experimental verification: Biomass-based integrated gasification combined cycle (BIGCC) coupling with chemical looping gasification (CLG) for power generation. Fuel 2019, 241, 118–128. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Chen, J.-R.; Wu, W.; Chang, J.-S. Hydrogen production from biomass using iron-based chemical looping technology: Validation, optimization, and efficiency. Chem. Eng. J. 2018, 337, 405–415. [Google Scholar] [CrossRef]
- Li, Z.; Xu, H.; Yang, W.; Zhou, A.; Xu, M. CFD simulation of a fluidized bed reactor for biomass chemical looping gasification with continuous feedstock. Energy Convers. Manag. 2019, 201, 112143. [Google Scholar] [CrossRef]
- Li, Z.; Xu, H.; Yang, W.; Xu, M.; Zhao, F. Numerical investigation and thermodynamic analysis of syngas production through chemical looping gasification using biomass as fuel. Fuel 2019, 246, 466–475. [Google Scholar] [CrossRef]
| No. | Name of Reaction | Chemical Reaction |
|---|---|---|
| 1 | Boudouard reaction | C + CO2 2CO |
| 2 | Char reforming/water gas | C + H2O CO + H2 |
| 3 | Methanation | C + 2H2 CH4 |
| 4 | Water gas shift reaction | CO + H2O CO2 + H2 |
| 5 | Steam reforming of methane | CH4 + H2O CO + 3H2 |
| 6 | Dry reforming | CH4 + CO2 2CO + 2H2 |
| 7 | Oxygen carrier reduction | CO + MexOy → CO2 + MexOy−1 |
| 8 | Oxygen carrier reduction | H2 + MexOy → H2O + MexOy−1 |
| 9 | Oxygen carrier reduction | CH4 + MexOy → 2H2 + CO + MexOy−1 |
| 10 | Oxygen carrier reduction | C + MexOy → CO + MexOy−1 |
| 11 | Oxygen carrier reduction | C + 2MexOy → CO2 + 2MexOy−1 |
| 12 | Tars’ reforming | Tars + H2O CO + H2 + CO2 + hydrocabons + … |
| 13 | Hydrocarbon reforming | Hydrocabons + H2O CO + H2 +CO2+ … |
| Redox Couples | CaSO4/CaS | Co3O4/Co | CuO/Cu | CuO/Cu2O | NiO/Ni |
| Ro | 0.47 | 0.27 | 0.20 | 0.1 | 0.21 |
| Redox couples | Mn2O3/MnO | Mn2O3/Mn3O4 | Fe2O3/FeO | Fe2O3/Fe3O4 | Fe2TiO5/FeTiO3 |
| Ro | 0.1 | 0.034 | 0.1 | 0.034 | 0.05 |
| Redox couples | CuAl2O4/Cu.Al2O3 | CuAlO2/Cu.Al2O3 | CuAl2O4/Cu.AlO2 | Fe2O3/Al2O3/ FeAl2O4 | FeAl2O4/Ni.Al2O3 |
| Ro | 0.089 | 0.066 | 0.044 | 0.045 | 0.091 |
| Type of OC | Operating Temp. [°C] | Support Materials | Advantage | Disadvantage |
|---|---|---|---|---|
| Ni-based | 900–1100 | Al2O3, MgAl2O3, ZrO2, Bentonite, TiO2, MgO, SiO2 | Very high reactivity and selectivity, strong catalytic properties for hydrocarbon conversion, high oxygen transport capacity, high stability, low agglomeration | Sulfur deactivation, high cost, health, and safety issues |
| Fe-based | Al2O3, MgAl2O4, TiO2, SiO2, YSZ, CeO2, ZrO2 | Environmentally friendly and non-toxicity, low cost, high mechanical strength, high chemical stability | Relatively low reactivity, low oxygen transport capacity, agglomeration issue, low solid circulation rate | |
| Cu-based | <800 | Al2O3, CuAl2O4, TiO2, SiO2, CeO2, ZrO2, Bentonite, MgO, MgAl2O4 | High reactivity and oxygen transport capacity, low cost and toxicity, high chemical and mechanical stability, environmentally friendly. No demand for external heat | Agglomeration and de-fluidization due to the low melting point of Cu |
| Mn-based | Al2O3, MgAl2O4, TiO2, SiO2, ZrO2, bentonite | Low cost and non-toxicity, environmentally friendly | Sulfur deactivation, relatively low oxygen transport capacity, agglomeration, low reactivity with fuels | |
| Co-based | YSZ, Al2O3, CoAl2O4, TiO2, SiO2, ZrO2, bentonite | High oxygen transport capacity, high reactivity with CH4 and CO | High cost and environmental impact and health issue, low reactivity with fuels |
| Biomass Type | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|
| Hardwood | 42–50 | 20–38 | 16–25 |
| Softwood | 35–50 | 24–35 | 16–33 |
| Straws | 33–40 | 20–45 | 15–20 |
| Corn stover | 33–35 | 21–24 | 17–22 |
| Switchgrass | 30–50 | 10–40 | 5–20 |
| Biomass | Moisture a | VM | FC | Ash | LHV (MJ/kg) |
|---|---|---|---|---|---|
| Wood | 20 | 82 | 17 | 1 | 18.6 |
| Wheat straw | 16 | 59 | 21 | 4 | 17.3 |
| Barley straw | 30 | 46 | 18 | 6 | 16.1 |
| Torrefied woodchips | 5.28 | 70.75 | 22.82 | 1.15 | 19.3 |
| Pine sawdust | - | 84.1 | 15.6 | 0.4 | 19.3 |
| Pine wood | 5.6 | 78.5 | 15.3 | 0.6 | 17.4 |
| References | Biomass | Type of OC | Fuel Reactor | Air Reactor | Remarks |
|---|---|---|---|---|---|
| Huijun et al. [25] Interconnected FB (25 kWth) | Rice straw | NiO/A3 | Bubbling fluidized bed Operating temperature: 650–850 °C | High-velocity fluidized bed |
|
| Huseyin et al. [26] Interconnected FB (10 kWth) | Sawdust of Pine | Fe2O3/Al2O3 | Bubbling fluidized bed Operating temperature: 750–900 °C | Fast fluidized bed |
|
| He et al. [34] | Biomass | NiFe2O4 | Fixed bed Operating temperature 700–900 °C | Fixed bed |
|
| Yan et al. [42] | Sawdust | BaFe2O4/Al2O3 | Fixed bed reactor Operating temperature: 750–900 °C | - |
|
| Liu et al. [43] | Pine wood + polyethylene | CaO/Fe2O3 | Fixed bed reactor Operating temperature: 750–850 °C | Fixed bed reactor |
|
| Liu et al. [45] | Pine sawdust | CaFe2O5/ MgO, ZnO, Al2O3 | Fixed bed reactor Operating temperature: 850 °C | Fixed bed reactor |
|
| Zeng et al. [68] | Pine sawdust | Natural hematite (Fe2O3) | Fixed bed reactor Operating temperature: 800 °C | - |
|
| Huang et al. [67] | Biomass char | Hematite (Fe2O3) | Fixed bed reactor Operating temperature: 850 °C | Fixed bed reactor |
|
| Hedayati et al. [28] | Woody based biomass | Mn ores | 300 W fluidized bed reactor 850–900 °C | Fluidized bed |
|
| Hildor et al. [131] | Wood pellet | Steel converter slag (LD slag) | Batch fluidized bed 820–970 °C |
|
| References | Biomass | Type of OC | Type of Model | Remarks |
|---|---|---|---|---|
| Gopaul et al. [134] | Poultry litter | CaO and Fe3O4 | ASPEN Plus (process modeling) |
|
| Detchusananard et al. [135] | Wood residue | NiO/CaO | ASPEN Plus (process modeling) |
|
| Cormos et al. [136] | Sawdust Coal/sawdust | Ilmenite | Computational methods |
|
| Aghabararnejad et al. [137] | Biomass | Co3O4/Al2O3 | ASPEN Plus (process modeling) |
|
| Li et al. [138] | Dried poplar | Iron-based (Fe2O3) | ASPEN Plus (process modeling) |
|
| Ge et al. [139] | Rice straw | Hematite (Fe2O3) | ASPEN Plus (process modeling) |
|
| Kuo et al. [140] | Raw wood and torrefied wood | Iron-based | Matlab and ASPEN Plus |
|
| Li et al. [141] | Pine sawdust | Iron-based (Fe2O3) | Numerical method (CFD) |
|
| Li et al. [142] | Microalgae | Fe2O3 | Numerical method |
|
| Dieringer et al. [17] | Wood pellet | Ilmenite | Matlab and ASPEN Plus (equilibrium process modeling) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.M.; Alobaid, F.; Dieringer, P.; Epple, B. Biomass-Based Chemical Looping Gasification: Overview and Recent Developments. Appl. Sci. 2021, 11, 7069. https://doi.org/10.3390/app11157069
Nguyen NM, Alobaid F, Dieringer P, Epple B. Biomass-Based Chemical Looping Gasification: Overview and Recent Developments. Applied Sciences. 2021; 11(15):7069. https://doi.org/10.3390/app11157069
Chicago/Turabian StyleNguyen, Nhut Minh, Falah Alobaid, Paul Dieringer, and Bernd Epple. 2021. "Biomass-Based Chemical Looping Gasification: Overview and Recent Developments" Applied Sciences 11, no. 15: 7069. https://doi.org/10.3390/app11157069
APA StyleNguyen, N. M., Alobaid, F., Dieringer, P., & Epple, B. (2021). Biomass-Based Chemical Looping Gasification: Overview and Recent Developments. Applied Sciences, 11(15), 7069. https://doi.org/10.3390/app11157069







