Assessment of Human Pharmaceuticals in Drinking Water Catchments, Tap and Drinking Fountain Waters
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site and Collection
2.2. Standards and Chemicals
2.3. Experimental Procedure
3. Results and Discussion
3.1. Analytical Quality Control
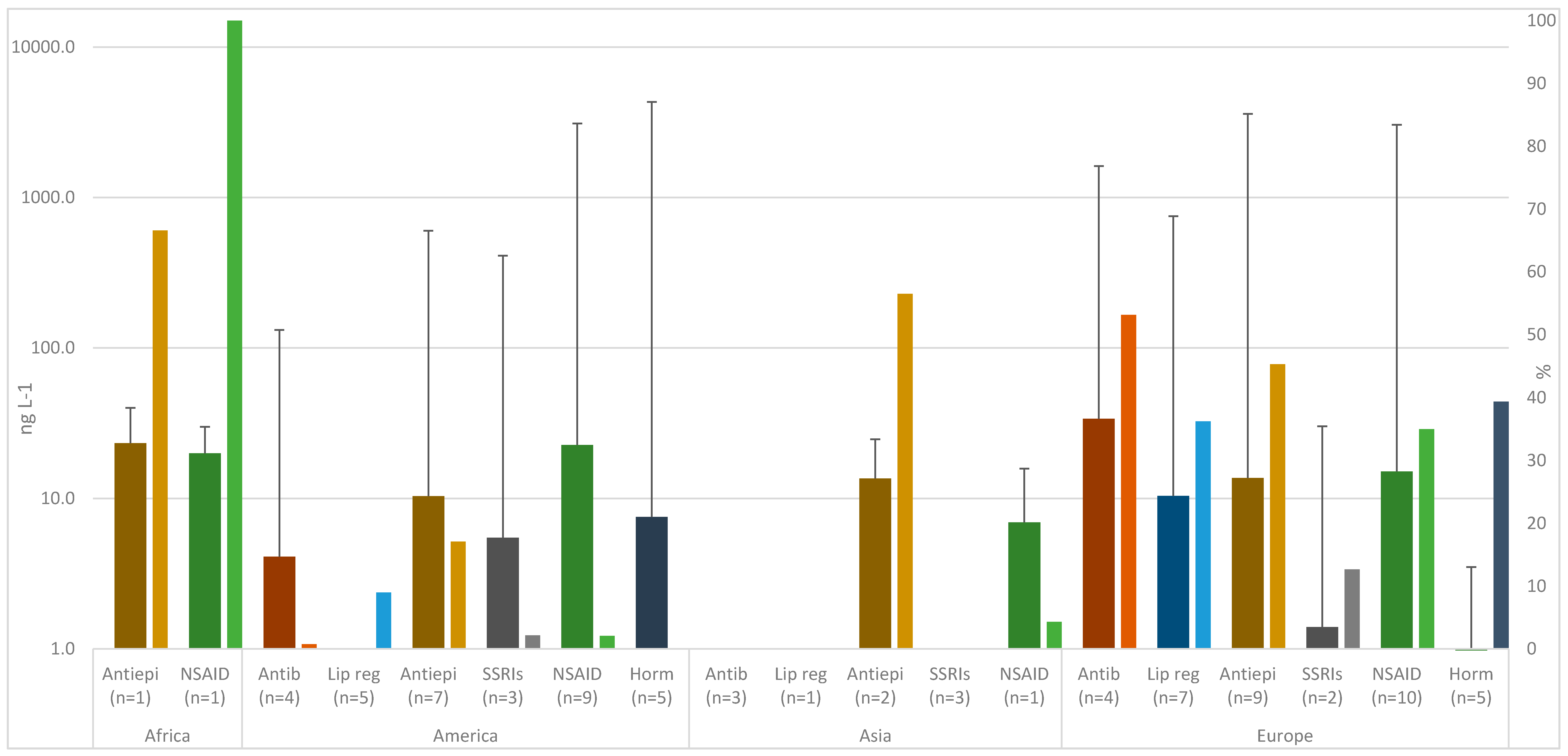
3.2. Occurrence and Comparison with Other Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gros, M.; Petrović, M.; Barceló, D. Development of a multi-residue analytical methodology based on liquid chromatography-tandem mass spectrometry (LC-MS/MS) for screening and trace level determination of pharmaceuticals in surface and wastewaters. Talanta 2006, 70, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaim, F.F.; Abdullah, M.P.; Othman, M.R.; Latip, J.; Zakaria, Z. Multi-residue analytical methodology-based liquid chromatography-time-of-flight-mass spectrometry for the analysis of pharmaceutical residues in surface water and effluents from sewage treatment plants and hospitals. J. Chromatogr. A 2014, 1345, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.P.T.; Silva, L.J.G.; Meisel, L.M.; Lino, C.M.; Pena, A. Environmental impact of pharmaceuticals from Portuguese wastewaters: Geographical and seasonal occurrence, removal and risk assessment. Environ. Res. 2015, 136, 108–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- Bueno, M.J.M.; Gomez, M.J.; Herrera, S.; Hernando, M.D.; Agüera, A.; Fernández-Alba, A.R. Occurrence and persistence of organic emerging contaminants and priority pollutants in five sewage treatment plants of Spain: Two years pilot survey monitoring. Environ. Pollut. 2012, 164, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Narvaez, J.F.V.; Jimenez, C.C. Pharmaceutical Products in the Environment: Sources, Effects and Risks/Productos Farmacéuticos En El Ambiente: Fuentes, Efectos Y Riesgos. Vitae 2012, 19, 93–108. [Google Scholar]
- Pereira, A.; Silva, L.; Laranjeiro, C.; Lino, C.; Pena, A. Selected pharmaceuticals in different aquatic compartments: Part I—Source, fate and occurrence. Molecules 2020, 25, 1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grönwall, J.; Danert, K. Regarding groundwater and drinkingwater access through a human rights lens: Self-Supply as a norm. Water 2020, 12, 419. [Google Scholar] [CrossRef] [Green Version]
- Morris, B.L.; Lawrence, A.R.; Chilton, J.; Adams, B.; Calow, R.C.; Klinck, B.A. Groundwater and Its Susceptibility to Degradation: A Global Assessment of the Problem and Options for Management; No. RS.03; UNEP, United Nations Environment Programme: Nairobi, Kenya, 2003; ISBN 9280722972. [Google Scholar]
- Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. Selective serotonin re-uptake inhibitors (SSRIs) in the aquatic environment: An ecopharmacovigilance approach. Sci. Total Environ. 2012, 437, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Al Aukidy, M.; Verlicchi, P.; Jelic, A.; Petrovic, M.; Barcelò, D. Monitoring release of pharmaceutical compounds: Occurrence and environmental risk assessment of two WWTP effluents and their receiving bodies in the Po Valley, Italy. Sci. Total Environ. 2012, 438, 15–25. [Google Scholar] [CrossRef]
- Rosal, R.; Rodea-Palomares, I.; Boltes, K.; Fernández-Piñas, F.; Leganés, F.; Gonzalo, S.; Petre, A. Ecotoxicity assessment of lipid regulators in water and biologically treated wastewater using three aquatic organisms. Environ. Sci. Pollut. Res. 2010, 17, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Georgia Ștefan, M.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, L.; Snape, J.R.; Verbruggen, B.; Owen, S.F.; Kristiansson, E.; Margiotta-Casaluci, L.; Österlund, T.; Hutchinson, K.; Leverett, D.; Marks, B.; et al. Pharmacology beyond the patient – The environmental risks of human drugs. Environ. Int. 2019, 129, 320–332. [Google Scholar] [CrossRef] [PubMed]
- WHO. Pharmaceuticals in Drinking Water; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef]
- ter Laak, T.L.; Van der Aa, M.; Houtman, C.J.; Stoks, P.G.; Van Wezel, A.P. Relating environmental concentrations of pharmaceuticals to consumption: A mass balance approach for the river Rhine. Environ. Int. 2010, 36, 403–409. [Google Scholar] [CrossRef]
- Bade, R.; Rousis, N.I.; Bijlsma, L.; Gracia-Lor, E.; Castiglioni, S.; Sancho, J.V.; Hernandez, F. Screening of pharmaceuticals and illicit drugs in wastewater and surface waters of Spain and Italy by high resolution mass spectrometry using UHPLC-QTOF MS and LC-LTQ-Orbitrap MS. Anal. Bioanal. Chem. 2015, 407, 8979–8988. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.M.P.T.; Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. Assessing environmental risk of pharmaceuticals in Portugal: An approach for the selection of the Portuguese monitoring stations in line with Directive 2013/39/EU. Chemosphere 2016, 144, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.M.P.T.; Silva, L.J.G.; Laranjeiro, C.S.M.; Meisel, L.M.; Lino, C.M.; Pena, A. Human pharmaceuticals in Portuguese rivers: The impact of water scarcity in the environmental risk. Sci. Total Environ. 2017, 609, 1182–1191. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use; European Medicines Agency: London, UK, 2006.
- Pereira, A.M.P.T.; Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. A critical evaluation of different parameters for estimating pharmaceutical exposure seeking an improved environmental risk assessment. Sci. Total Environ. 2017, 603–604, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.J.G.; Pereira, A.M.P.T.; Meisel, L.M.; Lino, C.M.; Pena, A. A one-year follow-up analysis of antidepressants in Portuguese wastewaters: Occurrence and fate, seasonal influence, and risk assessment. Sci. Total Environ. 2014, 490, 279–287. [Google Scholar] [CrossRef] [PubMed]
- de Gaffney, V.J.; Almeida, C.M.M.; Rodrigues, A.; Ferreira, E.; Benoliel, M.J.; Cardoso, V.V. Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res. 2015, 72, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Henriques, M.; Cardoso, V.V.; Rodrigues, A.M.; Ferreira, E.; Benoliel, M.J.; Almeida, C.M.M. Simultaneous Determination of Ten Endocrine Hormone Disrupters in Water Using SPE/LC-(ESI)MS-MS. J. Water Resour. Prot. 2010, 2, 818–829. [Google Scholar] [CrossRef] [Green Version]
- Paíga, P.; Delerue-Matos, C. Determination of pharmaceuticals in groundwater collected in five cemeteries’ areas (Portugal). Sci. Total Environ. 2016, 569–570, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Stackelberg, P.E.; Gibs, J.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Lippincott, R.L. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci. Total Environ. 2007, 377, 255–272. [Google Scholar] [CrossRef]
- Leung, H.W.; Jin, L.; Wei, S.; Tsui, M.M.P.; Zhou, B.; Jiao, L.; Cheung, P.C.; Chun, Y.K.; Murphy, M.B.; Lam, P.K.S. Pharmaceuticals in Tap Water: Human Health Risk Assessment and Proposed Monitoring Framework in China. Environ. Health Perspect. 2013, 121, 839–846. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Stimulatory drugs of abuse in surface waters and their removal in a conventional drinking water treatment plant. Environ. Sci. Technol. 2008, 42, 6809–6816. [Google Scholar] [CrossRef]
- Snyder, S.A. Occurrence, Treatment, and Toxicological Relevance of EDCs and Pharmaceuticals in Water. Ozone Sci. Eng. 2008, 30, 65–69. [Google Scholar] [CrossRef]
- Simazaki, D.; Kubota, R.; Suzuki, T.; Akiba, M.; Nishimura, T.; Kunikane, S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015, 76, 187–200. [Google Scholar] [CrossRef]
- Vulliet, E.; Cren-Olivé, C.; Grenier-Loustalot, M.-F. Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters. Environ. Chem. Lett. 2009, 9, 103–114. [Google Scholar] [CrossRef]
- Barnes, K.K.; Kolpin, D.W.; Furlong, E.T.; Zaugg, S.D.; Meyer, M.T.; Barber, L.B. A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States — I) Groundwater. Sci. Total Environ. 2008, 402, 192–200. [Google Scholar] [CrossRef]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187C, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Fick, J.; Söderström, H. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef]
- Kleywegt, S.; Pileggi, V.; Yang, P.; Hao, C.; Zhao, X.; Rocks, C.; Thach, S.; Cheung, P.; Whitehead, B. Pharmaceuticals, hormones and bisphenol A in untreated source and finished drinking water in Ontario, Canada—Occurrence and treatment efficiency. Sci. Total Environ. 2011, 409, 1481–1488. [Google Scholar] [CrossRef]
- de Jongh, C.M.; Kooij, P.J.F.; de Voogt, P.; ter Laak, T.L. Screening and human health risk assessment of pharmaceuticals and their transformation products in Dutch surface waters and drinking water. Sci. Total Environ. 2012, 427–428, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Carmona, E.; Andreu, V.; Picó, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water. Sci. Total Environ. 2014, 484, 53–63. [Google Scholar] [CrossRef] [PubMed]
- McEneff, G.; Barron, L.; Kelleher, B.; Paull, B.; Quinn, B. A year-long study of the spatial occurrence and relative distribution of pharmaceutical residues in sewage effluent, receiving marine waters and marine bivalves. Sci. Total Environ. 2014, 476–477, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Magnér, J.; Filipovic, M.; Alsberg, T. Application of a novel solid-phase-extraction sampler and ultra-performance liquid chromatography quadrupole-time-of-flight mass spectrometry for determination of pharmaceutical residues in surface sea water. Chemosphere 2010, 80, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Sacher, F.; Lange, F.T.; Brauch, H.-J.; Blankenhorn, I. Pharmaceuticals in groundwaters. J. Chromatogr. A 2001, 938, 199–210. [Google Scholar] [CrossRef]
- Fram, M.S.; Belitz, K. Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in California. Sci. Total Environ. 2011, 409, 3409–3417. [Google Scholar] [CrossRef] [Green Version]
- Subedi, B.; Codru, N.; Dziewulski, D.M.; Wilson, L.R.; Xue, J.; Yun, S.; Braun-Howland, E.; Minihane, C.; Kannan, K. A pilot study on the assessment of trace organic contaminants including pharmaceuticals and personal care products from on-site wastewater treatment systems along Skaneateles Lake in New York State, USA. Water Res. 2014, 72, 28–39. [Google Scholar] [CrossRef]
- Schriks, M.; Heringa, M.B.; Van der Kooi, M.M.E.; de Voogt, P.; Van Wezel, A.P. Toxicological relevance of emerging contaminants for drinking water quality. Water Res. 2010, 44, 461–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vulliet, E.; Wiest, L.; Baudot, R.; Grenier-Loustalot, M.F. Multi-residue analysis of steroids at sub-ng/L levels in surface and ground-waters using liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2008, 1210, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Zuehlke, S.; Duennbier, U.; Heberer, T. Determination of estrogenic steroids in surface water and wastewater by liquid chromatography-electrospray tandem mass spectrometry. J. Sep. Sci. 2005, 28, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xiang, J.; Que, C.; Chen, F.; Xu, G. Occurrence and fate of psychiatric pharmaceuticals in the urban water system of Shanghai, China. Chemosphere 2015, 138, 486–493. [Google Scholar] [CrossRef]
- Riva, F.; Castiglioni, S.; Fattore, E.; Manenti, A.; Davoli, E.; Zuccato, E. Monitoring emerging contaminants in the drinking water of Milan and assessment of the human risk. Int. J. Hyg. Environ. Health 2018, 221, 451–457. [Google Scholar] [CrossRef]
- K’oreje, K.O.; Vergeynst, L.; Ombaka, D.; De Wispelaere, P.; Okoth, M.; Van Langenhove, H.; Demeestere, K. Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu city, Kenya. Chemosphere 2016, 149, 238–244. [Google Scholar] [CrossRef] [PubMed]
- López-Roldán, R.; de Alda, M.L.; Gros, M.; Petrovic, M.; Martín-Alonso, J.; Barceló, D. Advanced monitoring of pharmaceuticals and estrogens in the Llobregat River basin (Spain) by liquid chromatography-triple quadrupole-tandem mass spectrometry in combination with ultra performance liquid chromatography-time of flight-mass spectrometry. Chemosphere 2010, 80, 1337–1344. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Grujić, S.; Vasiljević, T.; Laušević, M. Determination of multiple pharmaceutical classes in surface and ground waters by liquid chromatography-ion trap-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 4989–5000. [Google Scholar] [CrossRef] [PubMed]
- López-Serna, R.; Jurado, A.; Vázquez-Suñé, E.; Carrera, J.; Petrović, M.; Barceló, D. Occurrence of 95 pharmaceuticals and transformation products in urban groundwaters underlying the metropolis of Barcelona, Spain. Environ. Pollut. 2013, 174, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, G.; Zheng, Q.; Tang, J.; Chen, Y.; Xu, W.; Zou, Y.; Chen, X. Occurrence and risks of antibiotics in the Laizhou Bay, China: Impacts of river discharge. Ecotoxicol. Environ. Saf. 2012, 80, 208–215. [Google Scholar] [CrossRef]
- McEachran, A.D.; Shea, D.; Bodnar, W.; Nichols, E.G. Pharmaceutical occurrence in groundwater and surface waters in forests land-applied with municipal wastewater. Environ. Toxicol. Chem. 2016, 35, 898–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonso-Olivares, C.; Torres-Padrón, M.; Sosa-Ferrera, Z.; Santana-Rodríguez, J. Assessment of the Presence of Pharmaceutical Compounds in Seawater Samples from Coastal Area of Gran Canaria Island (Spain). Antibiotics 2013, 2, 274–287. [Google Scholar] [CrossRef]
| Therapeutic Group/Compound | Matrix-Matched Linearity (r2) | Matrix Effect (%) | MDL (ng L−1) | MQL (ng L−1) |
|---|---|---|---|---|
| Antibiotics | ||||
| Azithromycin (AZI) | 0.9995 | 100 | 3.29 | 9.97 |
| Ciprofloxacin (CIP) | 0.9988 | 100.96 | 5.26 | 15.93 |
| Clarithromycin (CLA) | 0.9999 | 98.77 | 1.51 | 4.58 |
| Erythromycin (ERY) | 0.9991 | 98.84 | 4.49 | 13.60 |
| Lipid regulators | ||||
| Bezafibrate (BEZ) | 0.9998 | 100.00 | 2.26 | 6.84 |
| Gemfibrozil (GEM) | 0.9994 | 94.02 | 3.78 | 11.46 |
| Antiepileptic | ||||
| Carbamazepine (CAR) | 0.9992 | 101.27 | 4.22 | 12.80 |
| Selective serotonin reuptake inhibitors (SSRIs) | ||||
| Citalopram (CIT) | 1.0000 | 100.00 | 1.13 | 3.41 |
| Fluoxetine (FLU) | 0.9999 | 100.61 | 1.84 | 5.59 |
| Sertraline (SER) | 0.9996 | 99.44 | 3.04 | 9.22 |
| Non-steroidal anti-inflammatory drugs (NSAIDs) | ||||
| Diclofenac (DIC) | 0.9991 | 101.96 | 4.61 | 13.97 |
| 4-hydroxydiclofenac (4-OH-DIC) (metabolite) | 0.9990 | 99.33 | 4.92 | 14.92 |
| Ibuprofen (IBU) | 0.9994 | 93.55 | 3.66 | 11.09 |
| Naproxen (NAP) | 0.9992 | 101.27 | 4.38 | 13.27 |
| Paracetamol (PARA) | 0.9995 | 99.20 | 3.57 | 10.82 |
| Hormones | ||||
| Estrone (E1) (natural hormone/metabolite) | 0.9987 | 100.68 | 5.45 | 16.53 |
| 17β-estradiol (E2) | 0.9991 | 102.68 | 4.56 | 13.82 |
| 17α-ethinylestradiol (EE2) | 0.9990 | 99.44 | 4.94 | 14.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.; Silva, L.; Laranjeiro, C.; Pena, A. Assessment of Human Pharmaceuticals in Drinking Water Catchments, Tap and Drinking Fountain Waters. Appl. Sci. 2021, 11, 7062. https://doi.org/10.3390/app11157062
Pereira A, Silva L, Laranjeiro C, Pena A. Assessment of Human Pharmaceuticals in Drinking Water Catchments, Tap and Drinking Fountain Waters. Applied Sciences. 2021; 11(15):7062. https://doi.org/10.3390/app11157062
Chicago/Turabian StylePereira, André, Liliana Silva, Célia Laranjeiro, and Angelina Pena. 2021. "Assessment of Human Pharmaceuticals in Drinking Water Catchments, Tap and Drinking Fountain Waters" Applied Sciences 11, no. 15: 7062. https://doi.org/10.3390/app11157062
APA StylePereira, A., Silva, L., Laranjeiro, C., & Pena, A. (2021). Assessment of Human Pharmaceuticals in Drinking Water Catchments, Tap and Drinking Fountain Waters. Applied Sciences, 11(15), 7062. https://doi.org/10.3390/app11157062







