Biohydrogel Based on Dynamic Covalent Bonds for Wound Healing Applications
Abstract
:1. Introduction
2. Materials and Methods
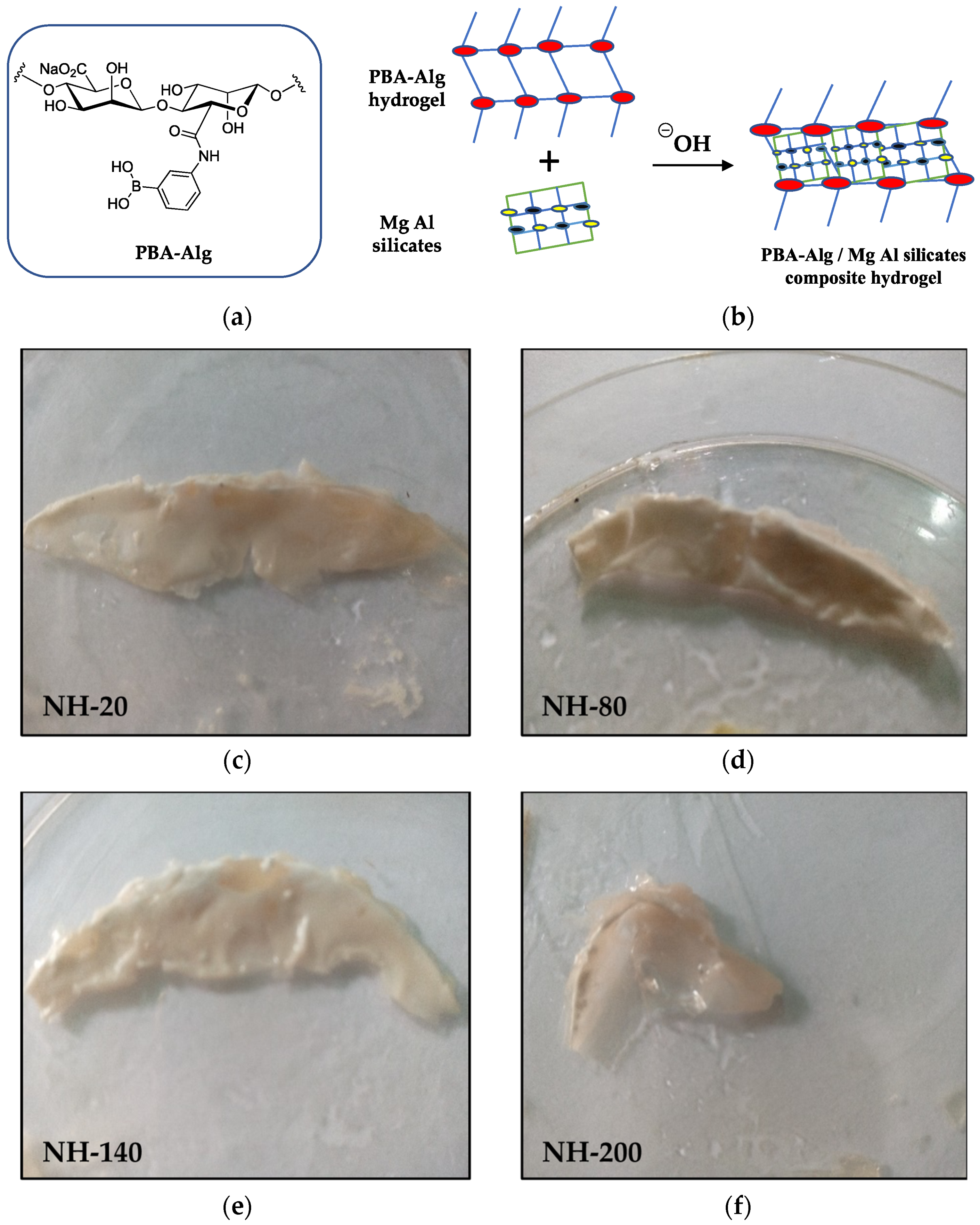
2.1. Synthesis of Phenyl Boronic Acid–Alginate Complex
2.2. Preparation of PBA-Alg Hydrogel
2.3. Preparation of Nanocomposite Hydrogels of PBA-Alg and Magnesium Aluminosilicate (Neusilin® FH2)
2.4. Determination of Interfacial Tension of the Hydrogel with Phosphate-Buffered Saline and Water
2.5. Moisture Uptake and Loss of Hydrogel Films
2.6. Extraction and Purification of Fresh Acid-Soluble Collagen
2.7. Fourier Transform Infrared Spectroscopy
2.8. Effect of Phenylboronic Acid–Alginate Conjugate and Nanocomposites on Blood Clotting Time
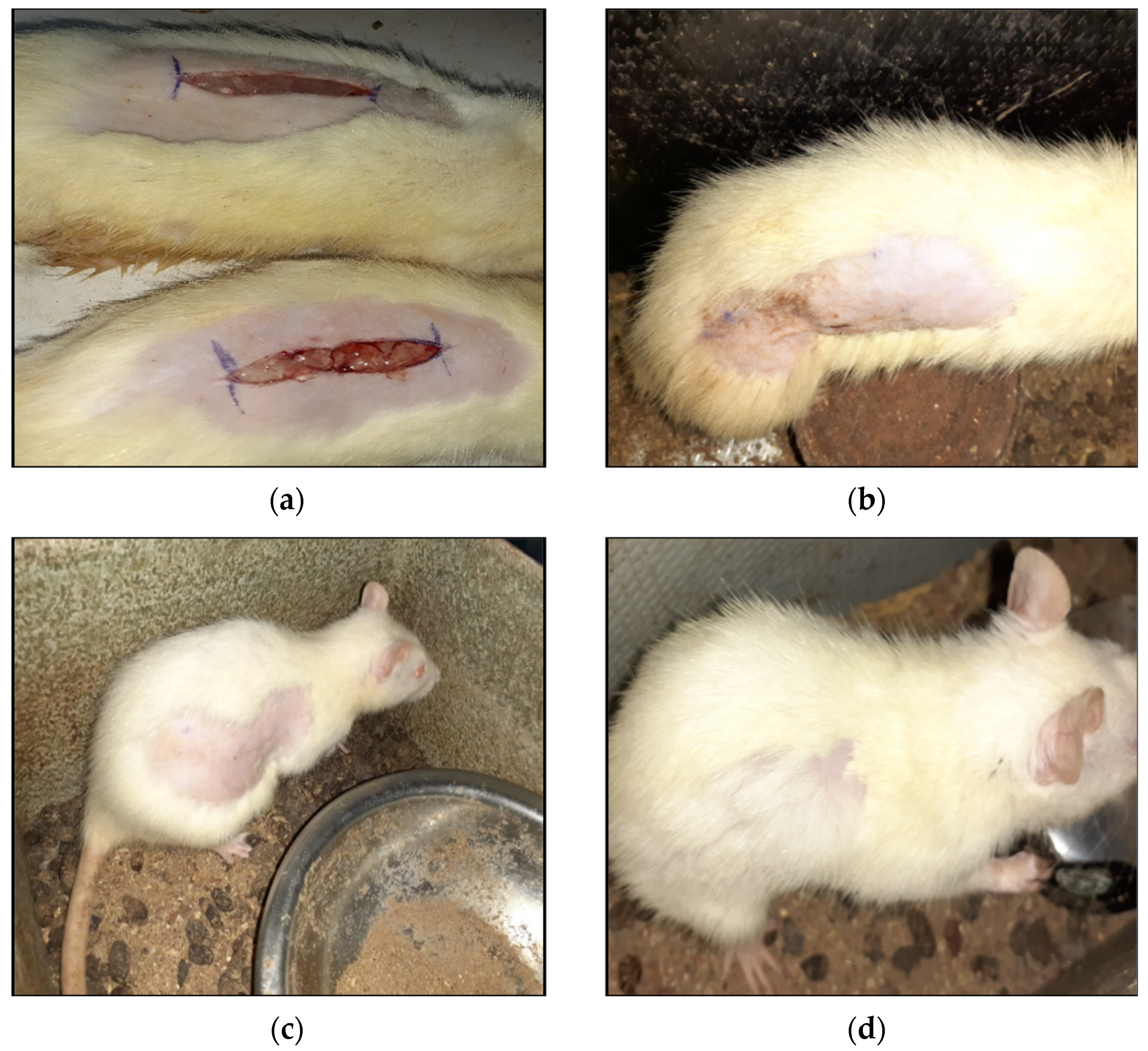
2.9. Effect of PBA-Alg Hydrogel on Wound Healing
3. Results and Discussion
3.1. Formulation and Relevant Characterization of Hydrogels
3.1.1. Interfacial Tension between Fluids and Hydrogel
3.1.2. Moisture Uptake and Loss of Films
3.1.3. FTIR Spectroscopy
3.1.4. Self-Healing Properties
3.1.5. Blood Clotting Time
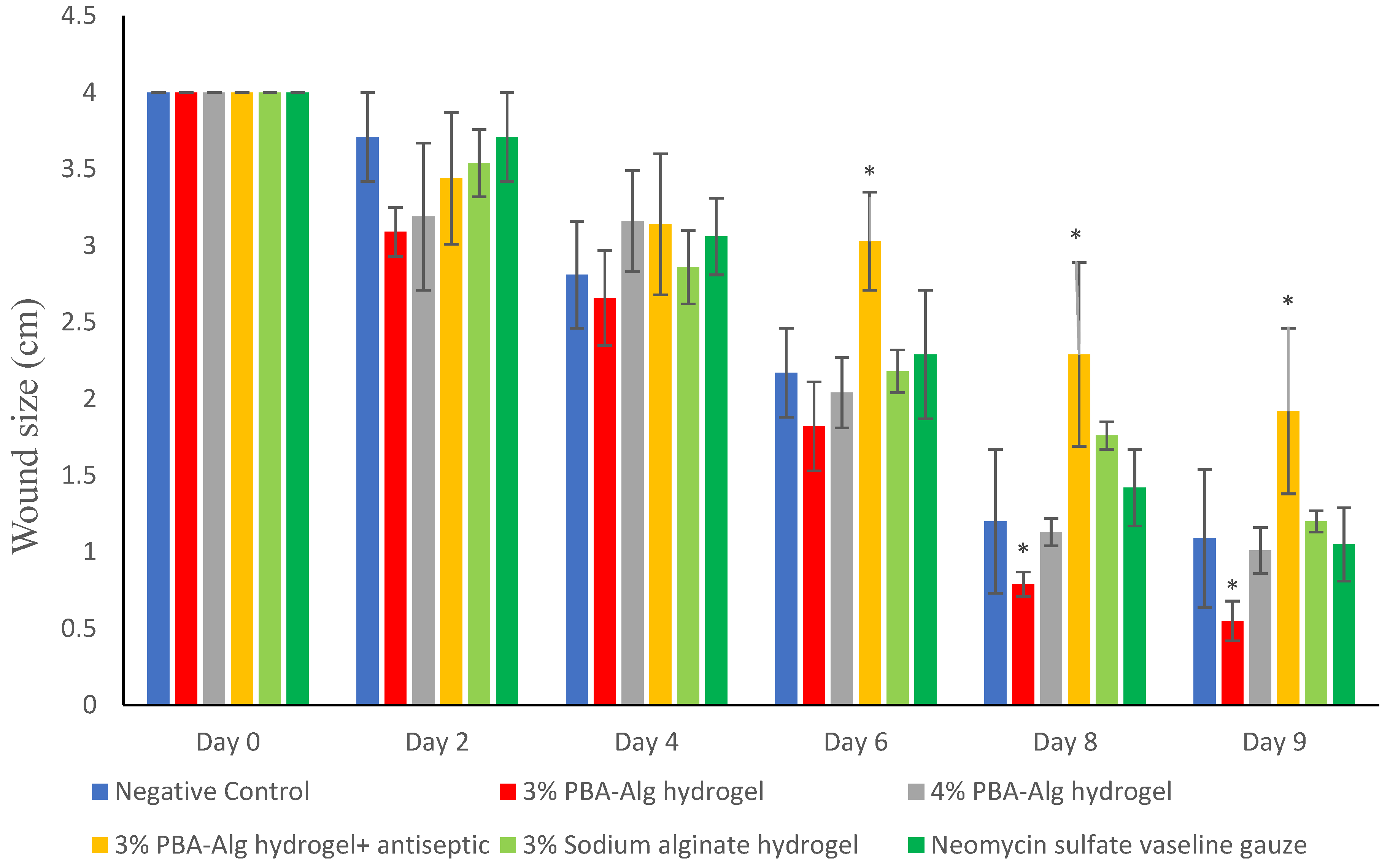
3.1.6. Wound Size Reduction
3.1.7. Wound Closure and Scar Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Meneghetti, M. Self-healing at the nanoscale. Nanoscale 2009, 1, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P. Biomimetic materials research: What can we really learn from nature’s structural materials? J. R. Soc. Interface 2007, 4, 637–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davami, K.; Mohsenizadeh, M.; Mitcham, M.; Damasus, P.; Williams, Q.; Munther, M. Additively manufactured self-healing structures with embedded healing agent reservoirs. Sci. Rep. 2019, 9, 7474. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.H.; Zhou, J.; Xu, F.; Zrínyi, M.; Dussault, P.H.; Osada, Y.; Chen, Y.M. Self-healing gels based on constitutional dynamic chemistry and their potential applications. Chem. Soc. Rev. 2014, 43, 8114–8131. [Google Scholar] [CrossRef]
- Agubata, C.O.; Ezeanya, N.I.; Nzekwe, I.T.; Uzondu, S.W.; Mbah, C.C. Characterization and sustainable anti-ulcer activity of hydrogels based on acid-diol reactions. Sustain. Chem. Pharm. 2021, 21, 100414. [Google Scholar] [CrossRef]
- Cambre, J.N.; Sumerlin, B.S. Biomedical applications of boronic acid polymers. Polymer 2011, 52, 4631–4643. [Google Scholar] [CrossRef] [Green Version]
- Marco-Dufort, B.; Tibbitt, M.W. Design of moldable hydrogels for biomedical applications using dynamic covalent boronic esters. Mater. Today Chem. 2019, 12, 16–33. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Sun, K.; Fan, J.; Zhang, P.; Meng, J.; Wang, S.; Jiang, L. Dual-responsive surfaces modified with phenylboronic acid-containing polymer brush to reversibly capture and release cancer cells. J. Am. Chem. Soc. 2013, 135, 7603–7609. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, G.J.; Shih, Y.V.; Kim, T.; Varghese, S. Phenylboronic acid-polymers for biomedical applications. Curr. Med. Chem. 2019, 26, 6797–6816. [Google Scholar] [CrossRef]
- Aeridou, E.; Díaz, D.D.; Alemán, C.; Pérez-Madrigal, M.M. Advanced functional hydrogel biomaterials based on dynamic B-O bonds and polysaccharide building blocks. Biomacromolecules 2020, 21, 3984–3996. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Pettignano, A.; Grijalvo, S.; Häring, M.; Eritja, R.; Tanchoux, N.; Quignard, F.; Díaz, D.D. Boronic acid-modified alginate enables direct formation of injectable, self-healing and multistimuli-responsive hydrogels. Chem. Commun. 2017, 53, 3350–3353. [Google Scholar] [CrossRef] [Green Version]
- Suresh, K.; Häring, M.; Kumaraswamy, G.; Díaz, D.D. On the sensitivity of alginate rheology to composition. Soft Matter 2019, 15, 159–165. [Google Scholar] [CrossRef]
- Mishra, D.K.; Yadav, K.S.; Prabhakar, B.; Gaud, R.S. Nanocomposite for cancer targeted drug delivery. In Applications of Nanocomposite Materials in Drug Delivery; Inamuddin Asiri, A.M., Mohammed, A., Eds.; Woodhead publishing: Sawston, UK, 2018; pp. 323–337. [Google Scholar]
- Hári, J.; Pukánszky, B. Nanocomposites: Preparation, structure and properties. In Applied Plastics Engineering Handbook: Processing and Materials; Kutz, M., Ed.; William Andrew Publishing: New York, NY, USA, 2011; pp. 109–142. [Google Scholar]
- Gaharwar, A.K.; Kishore, V.; Rivera, C.; Bullock, W.; Wu, C.J.; Akkus, O.; Schmidt, G. Physically crosslinked nanocomposites from silicate-crosslinked PEO: Mechanical properties and osteogenic differentiation of human mesenchymal stem cells. Macromol. Biosci. 2012, 12, 779–793. [Google Scholar] [CrossRef]
- Meng, H.; Xiao, P.; Gu, J.; Wen, X.; Xu, J.; Zhao, C.; Zhang, J.; Chen, T. Self-healable macro-/microscopic shape memory hydrogels based on supramolecular interactions. Chem. Commun. 2014, 50, 12277–12280. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 1–495. [Google Scholar]
- Lyklema, J. Fundamentals of Interface and Colloid Science: Liquid-Fluid Interfaces; Elsevier: Amsterdam, The Netherlands, 2000; pp. 1–751. [Google Scholar]
- Henriksen, K.; Karsdal, M.A. Type 1 Collagen. In Biochemistry of Collagens, Laminins and Elastin. Structure, Function and Biomarkers; Karsdal, M.A., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–11. [Google Scholar]
- Kumar, S.S.; George, J.; Mukkadan, J.K. Bleeding time and clotting time in healthy male and female college students of Karukutty village, Kerala. Health Prospect. 2013, 12, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Tetley, R.J.; Staddon, M.F.; Heller, D.; Hoppe, A.; Banerjee, S.; Mao, Y. Tissue fluidity promotes epithelial wound healing. Nat. Phys. 2019, 15, 1195–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyman, J.H.P.; Bruce, I.E. Surfactant properties and biodegradation of polyethoxylates from phenolic lipids. J. Surfactants Deterg. 2004, 7, 169–173. [Google Scholar] [CrossRef]
- Kang, W.; Lim, H.; Kim, B.; Lee, Y.; Hahm, K.; Kim, S. Assessment of the effects of sugammadex on coagulation profiles using thromboelastographic parameters. Sci. Rep. 2020, 10, 19860. [Google Scholar] [CrossRef]
- Singh, S.; Dodt, J.; Volkers, P.; Hethershaw, E.; Philippou, H.; Ivaskevicius, V.; Imhof, D.; Oldenburg, J.; Biswas, A. Structure functional insights into calcium binding during the activation of coagulation factor XIII A. Sci. Rep. 2019, 9, 11324. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Litvinov, R.I.; Weisel, J.W. What is the biological and clinical relevance of fibrin? Semin. Thromb. Hemost. 2016, 42, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Manju, S.; Antonty, M.; Sreenivasan, K. Synthesis and evaluation of a hydrogel that binds glucose and releases ciprofloxacin. J. Mater. Sci. 2010, 45, 4006–4012. [Google Scholar] [CrossRef]
- Uçan, U.S.; Sayin, Z. Proliferation effects of phenylboronic acid and boric acid on canine peripheral blood mononuclear cells. Turk. J. Vet. Anim. Sci. 2019, 43, 229–234. [Google Scholar] [CrossRef]
- Brooks, W.L.A.; Deng, C.C.; Sumerlin, B.S. Structure–reactivity relationships in boronic acid–diol complexation. ACS Omega 2018, 3, 17863–17870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elshaarani, T.; Yu, H.; Wang, L.; Ullah, R.S.; Haroon, M.; Khan, R.U.; Fahad, S.; Khan, A.; Nazir, A.; Usman, M. Synthesis of hydrogel-bearing phenylboronic acid moieties and their applications in glucose sensing and insulin delivery. J. Mater. Chem. B 2018, 6, 3831–3854. [Google Scholar] [CrossRef] [PubMed]
- Beyranvand, S.; Pourghobadi, Z.; Sattari, S.; Soleymani, K.; Donskyi, I.; Gharabaghi, M.; Unger, W.E.S.; Farjanikish, G.; Nayebzadeh, H.; Adeli, M. Boronic acid functionalized graphene platforms for diabetic wound healing. Carbon 2020, 158, 327–336. [Google Scholar] [CrossRef]
- Thomas, G.W.; Rael, L.T.; Bar-Or, R.; Shimonkevitz, R.; Mains, C.W.; Slone, D.S.; Craun, M.L.; Bar-Or, D. Mechanisms of delayed wound healing by commonly used antiseptics. J. Trauma 2009, 66, 82–91. [Google Scholar] [CrossRef]
- Junker, J.P.E.; Kamel, R.A.; Caterson, E.J.; Eriksson, E. Clinical impact upon wound healing and inflammation in moist, wet, and dry environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouro, C.; Gomes, A.P.; Ahonen, M.; Fangueiro, R.; Gouveia, I.C. Chelidonium majus L. Incorporated emulsion electrospun PCL/PVA_PEC nanofibrous meshes for antibacterial wound dressing applications. Nanomaterials 2021, 11, 1785. [Google Scholar] [CrossRef]
- Xu, R.; Xia, H.; He, W.; Li, Z.; Zhao, J.; Liu, B.; Wang, Y.; Lei, Q.; Kong, Y.; Bai, Y.; et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci. Rep. 2016, 6, 24596. [Google Scholar] [CrossRef] [Green Version]
- Patel, G.K.; Wilson, C.H.; Harding, K.G.; Finlay, A.Y.; Bowden, P.E. Numerous keratinocyte subtypes involved in wound re-epithelialization. J. Investig. Dermatol. 2006, 126, 497–502. [Google Scholar] [CrossRef] [Green Version]
| Treatment | Clotting Time (min) |
|---|---|
| 3% PBA-Alg | 9.09 ± 0.04 * |
| 4% PBA-Alg | 9.34 ± 0.03 * |
| 3% PBA-Alg + Savlon | 10.75 ± 0.04 * |
| none (negative control) | 4.20 ± 0.02 |
| nanocomposite (20 mg silicate) | 4.08 ± 0.02 |
| nanocomposite (80 mg silicate) | 5.55 ± 0.03 * |
| nanocomposite (140 mg silicate) | 11.50 ± 0.05 * |
| nanocomposite (200 mg silicate) | 180.00 ± 0.12 ** |
| Drug | Wound Closure Day |
|---|---|
| 3% PBA-Alg hydrogel | day 14 |
| 4% PBA-Alg hydrogel | day 14 |
| 3% PBA-Alg hydrogel + Savlon | day 16 |
| 3% sodium alginate hydrogel | day 15 |
| Negative control | day 15 |
| Neomycin sulfate Vaseline gauze | day 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agubata, C.O.; Mbaoji, C.C.; Nzekwe, I.T.; Saldías, C.; Díaz Díaz, D. Biohydrogel Based on Dynamic Covalent Bonds for Wound Healing Applications. Appl. Sci. 2021, 11, 6945. https://doi.org/10.3390/app11156945
Agubata CO, Mbaoji CC, Nzekwe IT, Saldías C, Díaz Díaz D. Biohydrogel Based on Dynamic Covalent Bonds for Wound Healing Applications. Applied Sciences. 2021; 11(15):6945. https://doi.org/10.3390/app11156945
Chicago/Turabian StyleAgubata, Chukwuma O., Cynthia C. Mbaoji, Ifeanyi T. Nzekwe, César Saldías, and David Díaz Díaz. 2021. "Biohydrogel Based on Dynamic Covalent Bonds for Wound Healing Applications" Applied Sciences 11, no. 15: 6945. https://doi.org/10.3390/app11156945
APA StyleAgubata, C. O., Mbaoji, C. C., Nzekwe, I. T., Saldías, C., & Díaz Díaz, D. (2021). Biohydrogel Based on Dynamic Covalent Bonds for Wound Healing Applications. Applied Sciences, 11(15), 6945. https://doi.org/10.3390/app11156945







