Abstract
Ectoine, a heterocyclic amino acid produced by various bacteria, was widely used in the fields of cosmetics and medicine. In this study, a novel ectoine synthesis cluster from marine bacterium Salinicola salarius 1A01339 was firstly introduced into Escherichia coli BL21(DE3) for heterologous production of ectoine. The bioinformatic analysis proved the function of these ectoine synthesis enzymes, and showed the highest identities of 83.3–87.7% with enzymes from other microorganisms. Using the whole-cell biocatalytic method, 3.28 g/L ectoine was synthesized and excreted into the medium with the substrate of 200 mM sodium aspartate at 25 °C, pH 6.5 in flask-level. Further bioconversion was performed in the fermentor system at the high cell density of 20 OD/mL, and the concentration of extracellular ectoine was increased to 22.5 g/L in 24 h (equivalent to the specific productivity of 0.94 g/L·h), achieving over 6 times of production compared with that in flasks. Significantly, the recombinant strain demonstrated a lower catalytic temperature with the optimum of 25 °C, and a stronger tolerance to the substrate aspartate of 300 mM. These results might provide a compelling case for ectoine synthesis as well as potential applications in large-scale industrial production.
1. Introduction
In order to survive in extreme salty habitat, various halophilic microorganisms have applied two different strategies to maintain osmotic balance. One of the ways is through inorganic salts in the cytosol, which could counterbalance the high extracellular salt concentrations [1,2]. Another method is to synthesize small organic molecules called compatible solutes (including sugars, polyols, methylamines, betaines, amino acids and their derivatives) as osmotic counterweights, such as Halorhodospim halochloris [3], Halomonas elongata [4] and Marinococcus halophilus [5,6]. Ectoine (1,4,5,6-tetrahydro-2-methyl-4-pyrimdine-carboxylic acid), a heterocyclic amino acid derived from aspartate, is one of the widely used compatible solutes which could be functional as a protective extremolyte under environmental stresses, e.g., extreme temperature, high osmolarity and dryness. Besides being an osmotic protector, ectoine was found to act as skin protector against aging and cell damage and medicine to treat diseases such as Alzheimer’s Disease, atopic dermatitis, colitis, allergic rhinitis, and lung inflammation [7,8,9].
Initially, ectoine was found in the extremely halophilic phototrophic bacterium Halorhodospim halochloris [3], and the biosynthetic pathway of ectoine has subsequently been fully presented in following studies [10,11,12]. The first two enzymatic steps: the synthesis of L-aspartate-phosphate through the ATP-dependent phosphorylation by L-aspartate-4-phosphotransferase (Ask) and the synthesis of L-aspartate-beta-semialdehyde through an NADPH-dependent reaction by L-2, 4-diaminobutyrate:2-oxoglutarate-4-aminotransferase (Asd) were similar to the biosynthetic pathway of amino acids of the aspartate family [13]. The following biosynthesis was carried out by a cascade of three biochemical conversions catalyzed by diaminobutyrate-2-oxoglutarate transaminase (EctB), L-2, 4-diamino-butyrate acetyltransferase (EctA), and L-ectoine synthase (EctC), and finally led to the formation of tetrahydropyrimidine, ectoine (Figure 1).
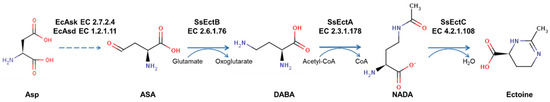
Figure 1.
The engineered ectoine biosynthetic pathway in E. coli using ectABCS. salarius cluster from Salinicola salarius 1A01339. EcAsk: L-aspartate-4-phosphotransferase in Escherichia coli; EcAsd: L-2, 4-diaminobutyrate:2-oxoglutarate-4-aminotransferase in Escherichia coli; Asp: L-aspartate; ASA: L-aspartate-beta-semialdehyde; DABA: L-2, 4-diaminobutyric acid; NADA: N-γ-acetyldiaminobutyric acid.
It was reported that a number of species which carried the ectABC cluster have been shown to synthesize ectoine in salt stress habitat [14,15]. In recent years, the widely producing and marketing of ectoine used in pharmaceuticals products and cosmetics had led to the increasing commercial demand of this bio-functional compound [16,17,18]. In order to satisfy the increasing commercial needs of ectoine, numerous biosynthesis strains for ectoine production have been chosen and built in the past two decades [19,20,21]. A bioprocess technique called “bacterial milking” was conducted using the halophilic eubacterium Halomonas elongata and employing kinds of biology strategies [22,23]. However, relatively high purity of ectoine required specific fermentation with more complicated and costly process because of high salt addition and elimination during this bioprocess. To avoid these drawbacks, a series of heterologous expressions of the cluster ectABC in non-halophilic bacterium E. coli had been attempted and the final recombinant E. coli strain BW (pBAD-ectABC) could accumulate and secrete 25.1 g/L ectoine by fed-batch fermentation [19]. In another report, utilizing transcriptional balancing, the engineered strain Corynebacterium glutamicum ectABCopt has proven to achieve high production of ectoine to a final titer of more than 65 g/L within 56 h [24]. These results suggested that heterologous expression of the ectoine biosynthetic pathway could be an attractive strategy for industrial production of pharmaceutical organic molecules.
In this study, we designed a synthetic cell factory by recruiting the novel ectABCS. salarius (see Appendix A) cluster from Salinicola salarius 1A01339, which was isolated from seawater of Indian Ocean and might produce enzymes with desirable characteristics. The ectABCS. salarius cluster, which had not been reported and studied, was firstly gene-mined from the Genbank Database and then successfully expressed in E. coli BL21(DE3) to obtained the recombinant strain BL-SsEct. We further optimized the condition of ectoine biosynthesis and preformed an efficient whole-cell bioconversion at a lower temperature of 25 °C. These results provide a promising potentiality for large-scale ectoine production in industrial applications using a more economical and effective bioprocess.
2. Materials and Methods
2.1. Chemicals, Bacterial Strains, and Cultivation
Isopropyl-β-d-thiogalactoside (IPTG), kanamycin (Km), methanol (HPLC grade) and ectoine used in the experiment were supplied by Macklin Biochemical Co., Ltd. (Shanghai, China). All other chemicals were purchased from Sinopharm Group Co. Ltd. (Shanghai, China). The strains and plasmids constructed and used in this study are listed in Table 1. The host strains for cloning, protein expression and ectoine production, were cultivated in LB medium containing 1.0% tryptone, 0.5% yeast extract, and 1.0% NaCl. The recombinant E. coli harboring ectoine production genes were cultured and expressed in LB media with 50 µg/mL Km. For overproduction of ectoine synthetic enzymes, fermentation medium (FM) was used for fed-batch cultures which contained per liter: 0.8% yeast extract, 1.2% tryptone, 0.7% K2HPO4, 0.5% NaCl, 0.3% (NH4)2SO4, 0.05% MgSO4·7H2O, 0.5% glycerol and 0.5% defoamer. The feeding solution for fed-batch cultures contained 10% yeast extract, 2.5% tryptone and 40% glycerol.

Table 1.
Bacterial strains and plasmids used in this study.
2.2. Gene Cloning, and Expression for EctAS. salarius, EctBS. salarius, EctCS. salarius
The ectoine biosynthetic cluster used for this study was obtained by bioinformatic analysis of the genome sequence from S. salarius 1A01339. Firstly, the gene-mining and prediction of the novel ectABC cluster were performed by Basic Local Alignment Search Tool (BLAST, https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 30 June 2021)) using typical ectABC sequence from Halomonas elongata DSM 2581 [21]. According to the whole genome shotgun sequence of S. salarius 1A01339 (GenBank assembly accession NZ_NHOU01000014), the predicted ectoine synthetic cluster, named as ectABCS. salarius, was synthesized artificially by GenScript Biotech Corporation (Nanjing, China). Then, the DNA fragments were ligated into plasmid pDK6 to construct the plasmid pDK-ectABCS. salarius and was further transformed into the competent cells of E. coli BL21(DE3) for protein expression. The plasmid DNA extraction, PCR, ligation, plasmid construction, and transformation were conducted following the method described previously by Lin et al. [25].
2.3. Bioinformatic Analysis and Molecular Modeling of EctABCS. salarius Structure
Sequence alignments of EctAS. salarius, EctBS. salarius and EctCS. salarius with other different ectoine production proteins were performed using ClustalX software and ESPript 3.0 program (http://espript.ibcp.fr/ESPript/ESPript/ (accessed on 30 June 2021)), respectively. The phylogenetic tree was inferred using the Neighbor-Joining method and conducted in MEGA7 [26]. In order to reveal the interaction between the substrate and all three ectoine biosynthetic enzymes, the three-dimensional structural modelling of EctAS. salarius, EctBS. salarius and EctCS. salarius were modelled by “SWISS-MODEL Workspace” (https://swissmodel.expasy.org/ (accessed on 30 June 2021)) using the reported crystal data of (Pl)EctA:CoA:DAB tertiary crystal structure from Paenibacillus lautus (PDB code 6SLL) [27], (Cs)EctB:PLP tertiary crystal structure from Chromohalobacter salexigens (PDB code 6RL5) [28] and (Pl)EctC:ADABA crystal structure from Paenibacillus lautus (PDB code 5ONO) [29] as the template, respectively. The hypothetical surface graphics, cartoon diagrams and stick models were illustrated by PyMOL (The PyMOL Molecular Graphics System, Version 2.0, Schrödinger, LLC, New York, NY, USA).
2.4. Preparation of E. coli for Biocatalysis
Precultures of E. coli BL21 harboring the recombinant plasmid pDK-ectABCS. salarius were conducted in shaking flasks (37 °C, 180 rpm) with LB broth containing Km and incubated for 24 h. Subsequently, 1% (v/v) of the bacterial culture was inoculated in fresh LB medium and cultured at 37 °C. When the optical density of the culture at 600 nm reached 1.0, IPTG was added to a final concentration of 0.1 mM, and the culture was further incubated at 25 °C for 16 h. Before and after induction, 1 mL samples were taken for protein analysis. Cells from the culture were harvested by cold centrifugation at 16 °C and 2132× g (Rotor F0850, Beckman, Brea, CA, USA) for 20 min and then resuspended in 50 mM of potassium phosphate buffer (pH 7.0). The cell suspension was sonicated (Soniprep 150 sonifier) at a frequency of 40 Hz for 10 min and centrifuged at 4 °C and 8528× g (Rotor F0850, Beckman) for 10 min. Afterward, the supernatant and precipitation were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Batch fermentations were performed in a 5-L fermentor (Biotech-5BG, Shanghai Baoxing Bio-Engineer Equipment Co., Ltd., Shanghai, China) with 3 L of FM. The temperature was maintained at 37 °C and the pH was controlled at 7.0 ± 0.05 by automatic feeding of 95% (v/v) NH4OH. The dissolved oxygen level was maintained above 30% air saturation by supplying air at 1 vvm (air volume/working-volume/minute) as measured using a polarization pO2 electrode. The feeding solution was added to the medium to maintain the continuous growth of the cells. Then, expression of ectoine synthetic enzymes were performed by adding IPTG to the final concentration of 0.2 mM when the culture reached around 30 optical-density at 600 nm (OD600). After another incubation at 25 °C for 10 h, cells from the culture were harvested and subjected to perform further metabolic transformation of ectoine.
2.5. Bioconversion Conditions
The whole-cell transformation was performed using cell suspensions in a 100-mL flask with the final liquid volume of 20 mL at 25 °C and 180 rpm on a rotary shaker for 24 h. The reaction mixture containing 100 mM sodium phosphate buffer (pH 6.5), 200 mM sodium aspartate, 50 mM KCl, and 100 mM glucose to form a cell suspension (OD600 = 10).
To enhance ectoine yield and determine the optimal conditions of ectoine production, various concentrations of sodium aspartate (50, 100, 200 and 300 mM) in the reaction mixture were compared in biosynthesis test. It had previously been reported that the apparently inactivated EctB was reactivated partially by the addition of KCl [4,30], therefore various concentrations of KCl (0, 50, 100, 200 and 300 mM) were added into the reaction mixture to estimate the effect on ectoine bioconversion. To investigate the influence of pH, the reaction mixture was adjusted to pH levels of 6.0, 6.5, 7.0, 7.5 and 8.0, and incubated at 25 °C. To examine the effect of temperature on the bioconversion of ectoine, the reactions were carried out at 20, 25, 30, 35 and 40 °C at pH 6.5. The biosynthesis experiments were performed in triplicates.
Ectoine bioconversion was also performed under high-density cells in a fermentor. Fermentation broth after induction from a fed-batch fermentation was centrifugated at 16 °C and 2132× g (Rotor F0850, Beckman) for 20 min and then resuspended in the reaction mixture containing 100 mM sodium phosphate buffer (pH 6.5), 200 mM sodium aspartate, 200 mM glucose and the whole-cell biocatalysts, 100 mM KCl. 1 L of cell suspension (OD600 = 20) was added into the 5 L fermentor. H2SO4 (0.5 M) were used to keep the pH at 6.5 ± 0.05. The reaction was performed at 25 °C with agitation speed of 600 rpm and the aeration rate at 1 vvm. When the substrates were consumed, feed solution containing 2 M sodium aspartate, 2 M glucose and 100 mM KCl was added periodically.
2.6. HPLC Analytical Methods
The concentration of extracellular ectoine was quantified by isocratic HPLC (LC-20AD, Shimadzu International Trading Co., Ltd., Shanghai, China) and LC-HRESIMS. The Shim-pack HRC-NH2 column (4.6 × 250 mm, Shimadzu) with methanol/water (90:10 v/v) at a flow rate of 0.8 mL/min as the mobile phase was used for HPLC analysis. UV detection at a wavelength of 210 nm was used to measure ectoine. The purity and concentration of ectoine was determined by using commercially standard sample (Macklin Biochemical Co., Ltd., Shanghai, China) as control check according to a reported method with slight modification [19]. LC–MS was acquired using a Waters 2695 HPLC coupled to a Thermo LCQTM Deca XP plus ion trap mass spectrometer in the negative ionization.
3. Results
3.1. Gene Mining of Potential Ectoine Biosynthesis Cluster
The predicted ectoine biosynthetic gene, referred as ectABCS. salarius, from the marine bacterium S. salarius 1A01339 using draft genome mining analysis was cloned and then sequenced. The sequencing data showed that ectABCS.salarius was 2416 bp in length and consisted of ectAS. salarius (588 bp), ectBS. salarius (1,266 bp), and ectCS. salarius (393 bp), encoding three proteins with 195 amino acids, 421 amino acids and 130 amino acids, respectively. The nucleotide sequences of EctABCS. salarius were previously reported and deposited in the GenBank database under the accession numbers NZ_NHOU01000014(REGION: 85664..86251 for ectA, REGION: 86336..87601 for ectB and REGION: 87687..88079 for ectC).
3.2. Bioinformatic Analysis and the Molecular Modeling of EctABCS. salarius
Multiple sequence alignments were carried out with ClustalX and ESPript 3.0 [31,32] (Figures S1–S3). The result showed that EctAS. salarius, EctBS. salarius and EctCS. salarius respectively belonged to the acetyltransferase (GNAT) family (PF00583) [33], aminotransferase class-III family (PF00202) [34] and ectoine synthase family (PF06339) [35], which had been identified and characterized from various species of microorganism.
As shown in Tables S1–S3, EctAS. salarius, EctBS. salarius and EctCS. salarius from S. salarius 1A01339 shared high identities with widely used ectoine enzymes from H. elongata DSM 2581, as 83.3% identities with EctAH. elongata, 85.7% identities with EctBH. elongata and 73.6% identities with EctCH. elongata, respectively. Furthermore, we also determined the phylogenetic relationships between ectoine synthesis enzymes and other reviewed proteins from UniProtKB database. As shown in Figures S4–S6, the phylogenetically closest proteins to EctAS. salarius were the EctA from Halomonas elongata DSM 2581 and Chromohalobacter salexigens DSM 3043, as 83.3% identities. While EctB from Halomonas elongata DSM 2581 and Chromohalobacter salexigens DSM 3043 had closest relationship with EctBS. salarius and shared highest identities of 85.7% and 84.8%. Similarly, EctC from Halomonas elongata DSM 2581 and Chromohalobacter salexigens DSM 3043 had closest relationship with EctCS. salarius and shared highest identities of 73.6% and 87.7%, respectively. These results proved that EctAS. salarius, EctBS. salarius and EctCS. salarius could be the functional proteins involved in the ectoine synthesis in S. salarius 1A01339 and help the host adapt to the disadvantaged environmental condition.
To analyze the molecular structures of the ectoine-synthesis enzymes, three crystal models were predicted and elucidated by using the solved data as template, respectively. As shown in Figure 2, the EctAS. salarius protein adopted the classical fold shared in acetyltransferase family (PF00583), which usually formed an inner parallel/antiparallel twisted β-sheet and four flanked α-helices. Further inspection revealed that the functional enzyme was assembled as an asymmetric homodimer, in which EctAS. salarius monomers were rotated against each other by about 180° and included an aromatic tyrosine (Tyr60) from the other opposite monomer. Notably, the strictly conserved amino acid residues, Asp55, Tyr60 (other opposite monomer), Trp102, Gln103, His176, and Glu179, formed an architecture of the substrate-binding pocket to contribute to the binding interaction and catalytic reaction with L-2,4-diaminobutyrate (DAB) (Figure S7) [27].
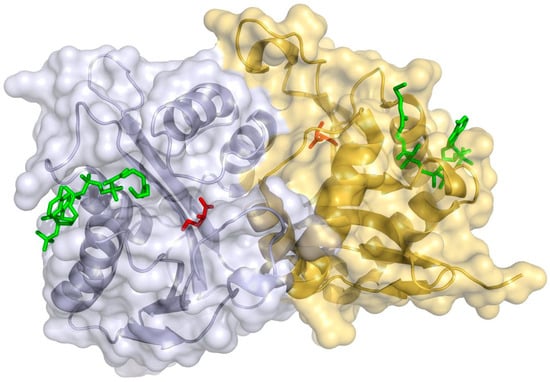
Figure 2.
Dimer assembly of the EctAS. salarius enzyme from Salinicola salarius 1A01339. Surface presentation of the dimer assembly of the EctAS. salarius protein displaying the binding site with the ligands CoA (green sticks) and the substrate DAB (red sticks). This graphical representation of the dimer assembly was rendered by using the (Pl)EctA:CoA:DAB tertiary crystal structure (PDB code 6SLL) as the template. The figure was generated by SWISS-MODEL and PyMOL.
The results of sequence alignments and crystallized analysis showed that EctBS. salarius model contained eight polypeptide chains forming two functional tetramers, which was a dimer of dimers reported as a member of the class III PLP-dependent aminotransferases (PF00202). Each dimer consisted of two monomers, in which a central region was folded by seven-stranded antiparallel β-sheets core and surrounded by nine α-helices, and another small region, consisted of three-stranded β-sheets core and surrounded by three α-helices, was situated on the surface of the monomer (Figure 3). Previous reports revealed that the active site of class III DABA aminotransferases has two binding pockets with conserved recognition residues involved in forming bonds with the neighboring dimer to maintain structural integrity of the tetrameric model, binding PLP to shape the active site, and forming strong directional bonds to the substrate. It was also proved in the graphical representation of EctBS. salarius shown in Figure S8 that Lys393, Glu210, Glu383, Tyr16, Leu76, Arg298, Asn294 and Lys267, which were commonly conserved in the sequence alignments and considered as fingerprint residues of DABA aminotransferases, shaped the suggested active site cavities and thus interacted with the carboxylic group of the substrates. Furthermore, the conserved R299 provided by the neighboring chain in the dimer could complete the gating loop and appeared to be important for specificity in the substrate-binding. And Lys267 could build covalent linkage with the coenzyme PLP through a Schiff-base intermediate and contribute to the substrate specificity and enzyme activity during catalysis [28,36].
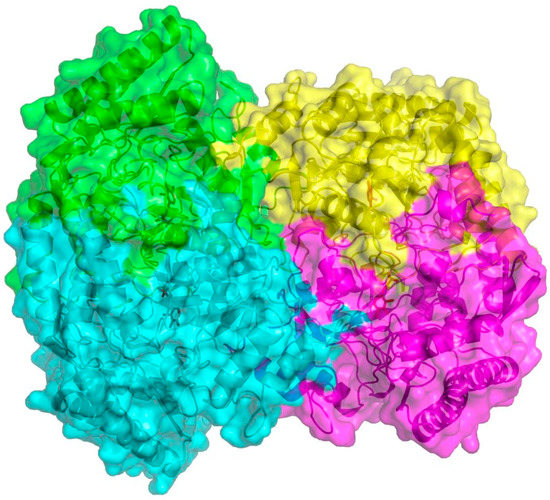
Figure 3.
The tetramer modelling of the EctBS. salarius from Salinicola salarius 1A01339. The overall EctB tetramer was described by the tetrameric interface between the two dimers, and the dimeric interface between the two chains in a dimer. The coenzyme PLP (red sticks) was captured in the crystal structure and accordingly positioned into all chains. This graphical representation of the dimer assembly was rendered by using the (Cs)EctB:PLP tertiary crystal structure from Chromohalobacter salexigens (PDB code 6RL5). The figure was generated by SWISS-MODEL and PyMOL.
Finally, we demonstrated the structural data of ectoine synthase from S. salarius 1A01339 to provide a deeper understanding of the reaction mechanism catalyzed by the EctC enzymes (Figure 4). It was suggested that EctCS. salarius was a typical protein that belonged to the cupin superfamily (PF06339), of which enzyme was a functional dimer composed of two monomers. Each monomer commonly consisted of two anti-parallel β-sheet regions forming a cup-shaped sandwich domain and was further packed against each other in a head-to-tail arrangement to assemble the actively topological structure. Similar to the crystal data of template protein from Paenibacillus lautus (PDB code 5ONN), EctCS. salarius was a divalent-metal dependent enzyme and three conserved amino acid residues (Glu59, Tyr87, and His95) involved in metal coordination were found in close proximity to the catalytic center, in which the substrate N-γ-acetyl-L-2,4-diaminobutyric acid (N-γ-ADABA) was tetrahedrally surrounded and ligated via interactions with five conserved residues (Trp23, Arg27, Thr42, Tyr54, and Glu59) located on the internal surface of the cupin barrel (Figure S9). And these results were consistent with earlier research that Trp23, Arg27, Thr42, Tyr54, Glu59, Tyr87 and His95, which were completely conserved in sequence alignment, could play a critical role in anchoring metal catalyst, substrate-binding and stabilizing the functional group in the catalytic core of the ectoine synthases [29].
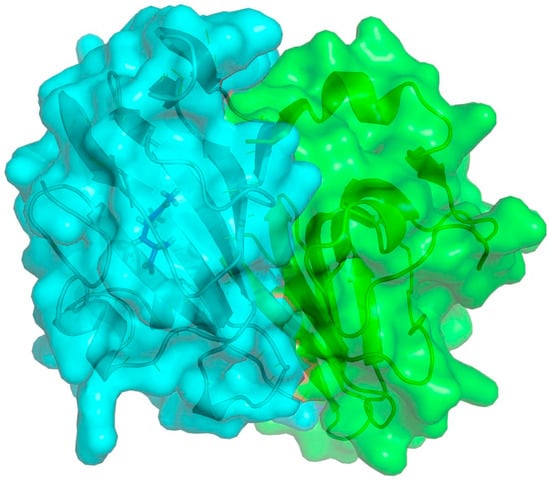
Figure 4.
Crystal modelling of the EctCS. salarius protein from Salinicola salarius 1A01339. The overall fold of the EctCS. salarius shown as a head-to-tail dimer was rendered by using the (Pl)EctC:ADABA crystal structure from Paenibacillus lautus (PDB code 5ONO). Two monomers were depicted in surface and cartoon representation marked in blue and green, respectively. The substrate ADABA was represented as blue sticks located in the center of the monomer. The figure was generated by SWISS-MODEL and PyMOL.
3.3. Production of Ectoine by the Recombinant E. coli
To evaluate the expression level of the ectABCS. salarius genes in Escherichia coli, the predicted gene cluster was synthesized artificially and subsequently introduced into E. coli BL21 strain using pDK6 as a heterologous expression vector. After an induced incubation, the supernatant and precipitation of cell extracts from recombinant strain E. coli BL21(DE3)-pDK6-ectABCS. salarius (referred to BL-SsEct) were evaluated by SDS-PAGE analysis. The results showed three clear bands of EctAS. salarius, EctBS. salarius and EctCS. salarius with molecular masses of about 25 kDa, 48 kDa and 18 kDa as revealed by Figure 5.
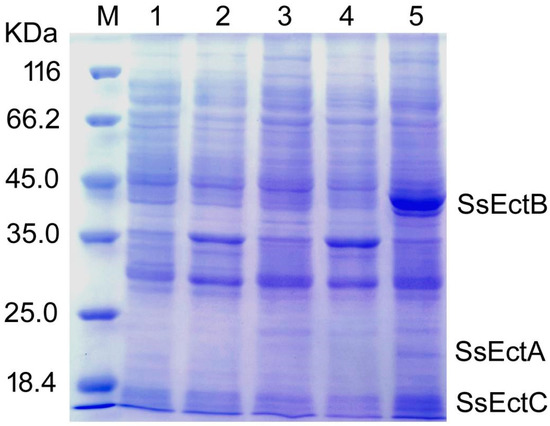
Figure 5.
SDS-PAGE analysis of EctABCS. salarius expressed in E. coli BL21(DE3). Lane M, protein molecular markers. Lane 1, the supernatant of cell extracts from induced E. coli BL21(DE3)-pDK6; Lane 2, the precipitation of cell extracts from induced E. coli BL21(DE3)-pDK6; Lane 3, the supernatant of cell extracts from the uninduced recombinant strain E. coli BL21(DE3)-pDK6-ectABCS. salarius; Lane 4, the precipitation of cell extracts from the uninduced recombinant strain E. coli BL21(DE3)-pDK6-ectABCS. salarius; Lane 5, the supernatant of cell extracts from the induced recombinant strain E. coli BL21(DE3)-pDK6-ectABCS. salarius.
In the following studies, ectoine was successfully synthesized by the recombinant strain BL-SsEct using a whole-cell biocatalytic method. In the reaction mixture, aspartate provided the substrate to synthesize L-aspartate-β-semialdehyde (ASA) and glucose could offer energy and the acetyl group for ectoine synthesis. KCl was added to the mixture to improve the activity and stability of EctBS. salarius. The culture samples were analyzed by HPLC and LC-MS, and the chromatogram showed a new peak possessing the same retention time as the authentic ectoine (Figure 6). After 24 h, the concentration of extracellular ectoine reached 3.28 g/L in the flask-level conversion. Furthermore, the molecular weight was confirmed as 142 (C6H10N2O2) utilizing HRESIMS (m/z [141.1]–) (Figure 7).
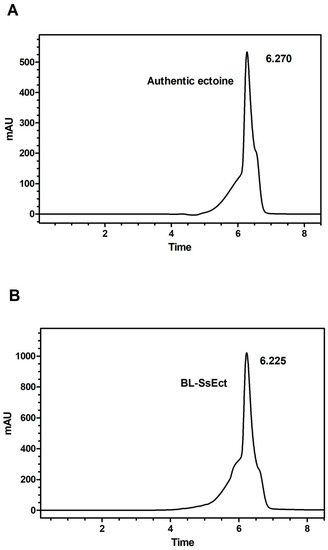
Figure 6.
HPLC analysis of biosynthetic ectoine from whole-cell catalysis. HPLC chromatograms obtained from authentic ectoine (A) and extracts (B) from conversion by engineered strain BL-SsEct.
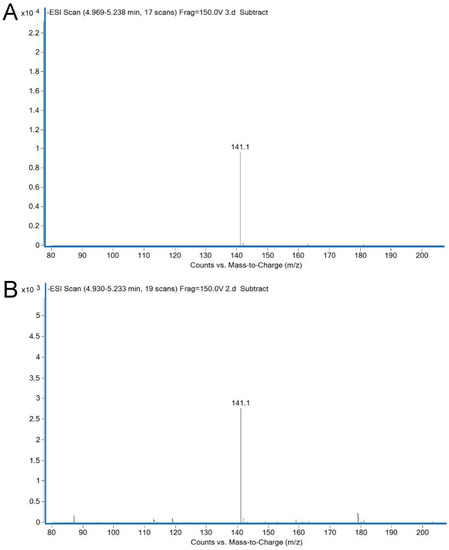
Figure 7.
HRESIMS spectrum of biosynthetic ectoine from whole-cell catalysis. LC-MS profile of authentic ectoine (A) and extracts (B) from conversion by engineered strain BL-SsEct.
3.4. Optimization of Ectoine Production Conditions
In order to determine the optimal conditions of ectoine biosynthesis by BL-SsEct, we further investigated some key factors affecting ectoine biosynthesis in flask-level. The optimization of ectoine production by the pre-cultured cell with the density of 10 OD/mL was performed on four factors: the level of pH value, sodium aspartate concentration, KCl content and incubation temperature. As shown in Figure 8A, the optimal concentration of sodium aspartate was 200 mM and over 3.28 g/L of ectoine was detected in the reaction mixture, while lower and higher concentration (100 and 300 mM) of substrate would lead to obviously decreased productions of 2.93 and 2.54 g/L (Figure 8B). The effect on KCl concentration was investigated and the results indicated that addition of 50–200 mM KCl could significantly improve the synthetic efficiency of ectoine, resulting in a maximum ectoine yield of 2.94 g/L. In incubation temperature test, the bioconversion was carried out between 20–40 °C, and the highest yield was obtained at 25 °C (Figure 8C). In addition, the effect of pH on ectoine synthesis was tested in the range of 6.0–8.0 and it showed that the optimal pH is 6.5 (Figure 8D). Therefore, the optimal ectoine transformation conditions in Erlenmeyer flask were determined as follows: 200 mM sodium aspartate, 200 mM KCl, pH 6.5 and 25 °C. Under the optimal conditions, the production of extracellular ectoine increased from 1.98 mg/mL to 3.28 mg/mL in 24 h.
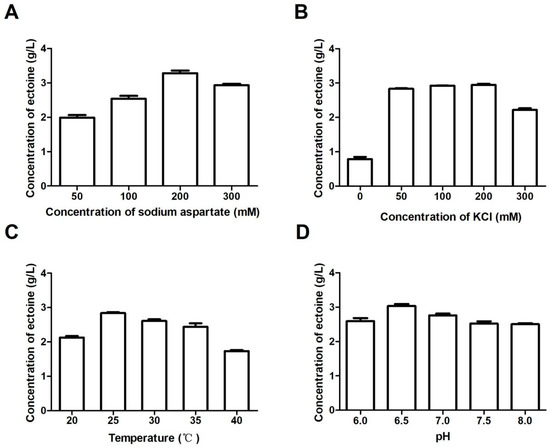
Figure 8.
Optimization of ectoine production. The initial reaction mixture containing 100 mM sodium phosphate buffer (pH 7.0), 200 mM sodium aspartate, 100 mM KCl, 100 mM glucose, and recombinant E. coli cells of 10 OD/mL was incubated at 25 °C for 24 h. (A) Effects of sodium aspartate concentration on the production of ectoine. (B) Effects of KCl concentration on the production of ectoine (the concentration of sodium aspartate was 200 mM). (C) Effects of temperature on the production of ectoine (the concentration of sodium aspartate was 200 mM and KCl was 100 mM). (D) Effects of pH on the production of ectoine (the concentration of sodium aspartate was 200 mM and KCl was 100 mM). Error bars represent standard deviations from triplicate biological replicates.
3.5. Production of Ectoine Using High Density Cells in a Fermentor
To evaluate the productivity and capability of engineered BL-SsEct in commercial application, ectoine bioconversion was also performed with higher density cells (OD600 = 20) in a 5-L fermentor (Figure 9). Under the initial biosynthesis condition of 200 mM sodium aspartate, 200 mM KCl and 100 mM sodium phosphate buffer (pH 6.5) at 25 °C, the concentration of extracellular ectoine exhibited a linear increase and obtained the yield of 3.30 g/L in 2 h. In order to maintain the supply of aspartate in the reaction mixture, the feeding solution (2 M sodium aspartate, 2 M glucose and 100 mM KCl) was added into the bioreactor with a flow speed of 20 mL/h at the end of first 2-h bioprocess. As expected, this whole-cell catalysis system operated successfully in the fermentation, and a significant quantity of ectoine was synthesized and excreted at a rate of 0.94 g/L·h. After a reaction time of 24 h, the ectoine concentration reached 22.5 g/L in the medium, thus increased at over six times compared with that in the flasks reactions.
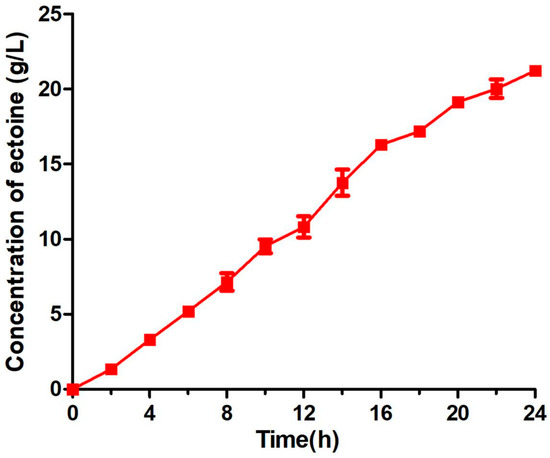
Figure 9.
Time profiles of ectoine increasement during fed-batch fermentation. The 2 L-scale bioconversion was carried out in a 5 L-scale bioreactor using engineered strain BL-SsEct (20 OD/mL). Optimized reaction was performed under a flow feed of 200 mM sodium aspartate and the lower temperature of 25 °C for 24 h. Error bars represent standard deviations from triplicate biological replicates.
4. Discussion
Previous studies reported several ectoine biosynthesis clusters from different microorganisms, however only the genetically engineered strain of Halomonas elongata or engineered Escherichia coli with the gene clusters derived from this organism were successfully applied in current production and marketing at large scale. And the novel hyperproducers and heterologous production strains with improved characteristics, such as higher catalytic efficiency at low temperature, stronger substrate tolerance and at a certain pH, were suggested to be generated to meet the demand for more efficient production of ectoines in coming research [19,30,37].
In this study, we have firstly characterized the novel ectoine biosynthesis genes in S. salarius 1A01339 isolated from the Indian Ocean. The bioinformatical analyses showed that the three enzymes EctA, EctB and EctC shared the highest identities of 83.3%, 85.7% and 87.7% with reported proteins, respectively. As expected, EctAS. salarius had six strictly conserved amino acid residues (Asp55, Tyr60 Trp102, Gln103, His176, and Glu179) and belonged to the typical acetyltransferase family (PF00583). Likewise, EctBS. salarius was classified to aminotransferase class-III family (PF00202) containing the fingerprint residues of Lys394, Glu211, Glu384, Try17, Leu77, Arg299, Asn295 and Lys268. And EctCS. salarius was a typical dimer protein belonged to the cupin superfamily (PF06339) in which Trp23, Arg27, Thr42, Tyr54, Glu59, Tyr87 and His95 were completely conserved. These bioinformatical analyses suggested that these novel ectoine biosynthetic proteins EctAS. salarius, EctBS. salarius and EctCS. salarius most likely have similar activities and properties to the ectoine biosynthetic enzymes from Halomonas elongata and Chromohalobacter salexigens and thus, have great potential for industrial production of ectoine.
In order to characterize the enzymatic properties of the EctAS. salarius, EctBS. salarius and EctCS. salarius, and estimate their potentiality in manufacturing output, the engineered strain Escherichia coli BL21(DE3) harboring ectABCS. salarius operon was cultured in flask and all three proteins corresponding to ectoine synthesis achieved successful expression under the control of tac promoter. Afterwards, this transgenic E. coli BL-SsEct was subjected to whole-cell catalysis reaction for biosynthesis of ectoines using aspartate as substrates.
Compared with the reported heterologous productions by different engineered strains as shown in Table 1, E. coli BL21-pDK-ectABCS. salarius exhibited an excellent capability in ectoine biosynthesis and accumulated high extracellular ectoine concentrations (3.28 g/L) at the optimal condition in shaking flasks. Interestingly, this recombinant strain adapted a lower catalytic temperature with the optimum of 25 °C, and even maintained 80% bioconversion production at 20 °C, which was a clear difference from the optimal temperature (30–40 °C) for the bio-catalysis process utilizing recombinant strain harboring ectoine synthesis genes from other microorganisms (Table 2). This was in part because these enzymes were originated from S. salarius 1A01339, which were isolated from the seawater and could produce evolved enzymes with desirable characteristics to survive in this environment. The same conclusion had been reached through exploration and application of temperature-adapted phytases, pectinases, proteases, amylases, xylanases and cellulases described in previous studies [38,39,40].

Table 2.
Comparison of bioconversion conditions for ectoine heterologous production.
Besides, BL-SsEct also demonstrated a stronger tolerance to the substrate aspartate, and maintained 90% ectoines production at the concentration of 300 mM, under which Escherichia coli K12/BW25113 and E. coli MG1655 ECT2 only showed the approximately 55% and 40%, respectively [7,19]. Given that aspartate also could be utilized by the glutamate-aspartate transamination system to form glutamate, which could act as an important osmoregulatory solute in vivo and circularly provide the amino donor group for the transamination catalyzed by EctBS. salarius participating in the first reaction of ectoine synthesis [42,43], thereby it was confirmed to be an ideal substrate for ectoine bioconversion, and increasing concentration of this dual functional substrate was thought to be rather advantageous for driving ectoine synthesis and accumulation in the BL-SsEct cells at maximum-tolerated concentrations.
Finally, to investigate the potential of strain BL-SsEct to become a promising candidate for commercial production, bioconversion was performed in a 5 L fermentor under the optimum condition of 25 °C. By process controlling of the pH and substrate feeding, the concentration of the extracellular ectoine maintained a linear accumulation during the whole bioreactor cultivation of 24 h, and achieved highest yield of 22.5 g/L, of which exhibited a significant improvement of catalysis efficiency compared with the reaction in flask level. However, it was slightly lower than ectoine yield of 25.1 g/L produced by Escherichia coli K12/BW25113 [19] and Escherichia coli W3110 ECT05 [30] harboring the ectoine biosynthesis genes from H. elongata, BL-SsEct demonstrated a more excellent adaptability under the lower catalytic temperature of 25 °C. It is widely acknowledged that lower reaction temperature could lead to some obvious and unique advantages compared to mesophilic and thermophilic manufacture process, including reducing the energy consumption in the heating process, avoiding thermo-inactivation of the heat-labile enzymes and preventing the configural and functional transformations of the thermosensitive flavors and drugs [25,38]. Accordingly, efficient bioconversion under lower catalytic temperature using this producer strain meant a competitive advantage for commercialized production of pharmaceutical ectoine.
5. Conclusions
In summary, we reported a recombinant E. coli strain BL-SsEct harboring a novel ectABCS. salarius gene of ectoine biosynthesis from marine organism S. salarius. The engineered strain demonstrated a high efficiency in whole-cell biocatalysis of ectoine and led to an extracellular accumulation of 3.28 g/L in the flask using fed-batch culture method. Further bioconversion was performed in the fermentor system, and the total ectoine production of 22.5 g/L with the specific ectoine productivity of 0.94 g/L·h was achieved under the distinctive temperature condition of 25 °C. It should be noted that the requirement of lower temperature in the bioconversion process made the BL-SsEct more competitive and more capable in commercial and economical production of ectoine.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11156873/s1, Figure S1: Multiple sequence alignment of EctAS. salarius with other L-2, 4-diaminobutyric acid acetyltransferases, Figure S2: Multiple sequence alignment of EctBS. salarius with other Diaminobutyrate-2-oxoglutarate transaminases, Figure S3: Multiple sequence alignment of EctCS. salarius with other L-ectoine synthases, Table S1: The comparation of EctAS. salarius with other EctAs from different microorganisms, Table S2: The comparation of EctBS. salarius with other EctBs from different microorganisms, Table S3: The comparation of EctCS. salarius with other EctCs from different microorganisms, Figure S4: Phylogenetic tree of diaminobutyrate acetyltransferase (EctAS. salarius) from marine bacterium Salinicola salarius 1A01339, Figure S5: Phylogenetic tree of diaminobutyrate-2-oxoglutarate transaminase (EctBS. salarius) from marine bacterium Salinicola salarius 1A01339, Figure S6: Phylogenetic tree of ectoine synthase (EctCS. salarius) from marine bacterium Salinicola salarius 1A01339, Figure S7: The interaction of the substrate within the catalytic centre of EctAS. salarius, Figure S8: The proposed configuration of active site cavity with key residues in the EctBS. salarius module, Figure S9. Crystal modelling views into the catalytic center of the EctAS. salarius.
Author Contributions
Conceptualization, Y.S. and L.L.; methodology, Y.S., W.P., T.W.; software, Y.S.; validation, W.P., T.W. and L.L.; formal analysis, Y.S., Y.L. (Yanhui Li), L.Z. and X.W.; data curation, Y.L. (Ying Li), T.W. and L.L.; writing—original draft preparation, Y.S.; writing—review and editing, Y.S. and L.L.; supervision, L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Natural Science Research for Colleges and Universities of Anhui Province of China (KJ2020A0066) and Anhui Provincial Key Laboratory of the Conservation and Exploitation Research of Biological Resources.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Summary of the main abbreviations and terms.
Table A1.
Summary of the main abbreviations and terms.
| Abbreviation or Terms | Meaning | Comments |
|---|---|---|
| Ask | L-aspartate-4-phosphotransferase | EC 2.7.2.4 |
| Asd | L-2, 4-diaminobutyrate:2-oxoglutarate-4-aminotransferase | EC 1.2.1.11 |
| EctB | Diaminobutyrate-2-oxoglutarate transaminase | EC 2.6.1.76 |
| EctA | L-2, 4-diaminobutyric acid acetyltransferase | EC 2.3.1.178 |
| EctC | L-ectoine synthase | EC 4.2.1.108 |
| ASA | L-aspartate-β-semialdehyde | Substrate of EctB |
| DABA | L-2, 4-diaminobutyric acid | Substrate of EctA |
| ADABA | Nγ-acetyl-L-2, 4-diaminobutyric acid | Substrate of EctC |
| CoA | Coenzyme A | |
| IPTG | Isopropyl-β-d-thiogalactoside | Inducer |
| Asp | Aspartate | Substrate of BL-SsEct |
| PLP | Pyridoxal-5-phosphate | Coenzyme for EctB |
| BL-SsEct | E. coli BL21(DE3)-pDK6- ectABCS. salarius | engineered strain |
| ectABCS. salarius | Ectoine biosynthetic cluster from Salinicola salarius 1A01339 | gene |
| ectABC | Ectoine biosynthetic gene cluster | gene |
References
- Oren, A.; Heldal, M.; Norland, S.; Galinski, E.A. Intracellular ion and organic solute concentrations of the extremely halophilic bacterium Salinibacter ruber. Extremophiles 2002, 6, 491–498. [Google Scholar] [CrossRef]
- Fenizia, S.; Thume, K.; Wirgenings, M.; Pohnert, G. Ectoine from Bacterial and Algal Origin is a Compatible Solute in Microalgae. Mar. Drugs 2020, 18, 42. [Google Scholar] [CrossRef] [Green Version]
- Galinski, E.A.; Pfeiffer, H.P.; Truper, H.G. 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid. A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur. J. Biochem. 1985, 149, 135–139. [Google Scholar] [CrossRef]
- Ono, H.; Sawada, K.; Khunajakr, N.; Tao, T.; Yamamoto, M.; Hiramoto, M.; Shinmyo, A.; Takano, M.; Murooka, Y. Characterization of biosynthetic enzymes for ectoine as a compatible solute in a moderately halophilic eubacterium, Halomonas elongata. J. Bacteriol. 1999, 181, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Galinski, E.A. Characterization of genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoregulated expression in Escherichia coli. Microbiology 1997, 143, 1141–1149. [Google Scholar] [CrossRef] [Green Version]
- Gregory, G.J.; Boyd, E.F. Stressed out: Bacterial response to high salinity using compatible solute biosynthesis and uptake systems, lessons from Vibrionaceae. Comput. Struct. Biotechnol. 2021, 19, 1014–1027. [Google Scholar] [CrossRef]
- Chen, J.; Liu, P.; Chu, X.; Chen, J.; Zhang, H.; Rowley, D.C.; Wang, H. Metabolic Pathway Construction and Optimization of Escherichia coli for High-Level Ectoine Production. Curr. Microbiol. 2020, 77, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Bilstein, A.; Sonnemann, U. Nasal spray and eye drops containing ectoine, a novel natural, non-drug anti-allergic substance are not less effective than azelastine nasal spray and eye drops in improving the symptoms of allergic rhinitis and conjunctivitis. Allergy 2011, 66, 132. [Google Scholar] [CrossRef]
- Liu, M.; Liu, H.; Shi, M.; Jiang, M.; Li, L.; Zheng, Y. Microbial production of ectoine and hydroxyectoine as high-value chemicals. Microb. Cell. Fact. 2021, 20, 33771157. [Google Scholar] [CrossRef]
- Peters, P.; Galinski, E.; Trüper, H.G. The Biosynthesis of Ectoine. FEMS Microbiol. Lett. 1990, 71, 157–162. [Google Scholar] [CrossRef]
- Bursy, J.; Pierik, A.J.; Pica, N.; Bremer, E. Osmotically induced synthesis of the compatible solute hydroxyectoine is mediated by an evolutionarily conserved ectoine hydroxylase. J. Biol. Chem. 2007, 282, 31147–31155. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Zhao, Y.; Huang, W.; Zhang, L.; Wu, F.; Ye, J.; Chen, G.Q. Rational flux-tuning of Halomonas bluephagenesis for co-production of bioplastic PHB and ectoine. Nat. Commun. 2020, 11, 32620759. [Google Scholar] [CrossRef]
- Li, Y.; Wei, H.; Wang, T.; Xu, Q.; Zhang, C.; Fan, X.; Ma, Q.; Chen, N.; Xie, X. Current status on metabolic engineering for the production of l-aspartate family amino acids and derivatives. Bioresour. Technol. 2017, 245, 1588–1602. [Google Scholar] [CrossRef] [PubMed]
- Hermann, L.; Mais, C.-N.; Czech, L.; Smits, S.; Bange, G.; Bremer, E. The ups and downs of ectoine: Structural enzymology of a major microbial stress protectant and versatile nutrient. Biol. Chem. 2020, 401, 1443–1468. [Google Scholar] [CrossRef]
- Mais, C.N.; Hermann, L.; Altegoer, F.; Seubert, A.; Richter, A.A.; Wernersbach, I.; Czech, L.; Bremer, E.; Bange, G. Degradation of the microbial stress protectants and chemical chaperones ectoine and hydroxyectoine by a bacterial hydrolase-deacetylase complex. J. Biol. Chem. 2020, 295, 9087–9104. [Google Scholar] [CrossRef]
- Parwata, I.P.; Wahyuningrum, D.; Suhandono, S.; Hertadi, R. Heterologous Ectoine Production in Escherichia coli: Optimization Using Response Surface Methodology. Int. J. Microbiol. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hans Jorg, K.; Georg, L.; Erwin, A.G. Industrial Production of the Cell Protectant Ectoine: Protection Mechanisms, Processes, and Products. Curr. Biotech. 2014, 3, 10–25. [Google Scholar] [CrossRef] [Green Version]
- Vandrich, J.; Pfeiffer, F.; Alfaro-Espinoza, G.; Kunte, H.J. Contribution of mechanosensitive channels to osmoadaptation and ectoine excretion in Halomonas elongata. Extremophiles 2020, 24, 421–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.Z.; Gong, J.; Yu, H.Y.; Tao, Y.; Zhang, S.; Dong, Z.Y. High production of ectoine from aspartate and glycerol by use of whole-cell biocatalysis in recombinant Escherichia coli. Microb. Cell Fact. 2015, 14, 55. [Google Scholar] [CrossRef] [Green Version]
- Grammann, K.; Volke, A.; Kunte, H.J. New type of osmoregulated solute transporter identified in halophilic members of the Bacteria domain: TRAP transporter TeaABC mediates uptake of ectoine and hydroxyectoine in Halomonas elongata DSM 2581(T). J. Bacteriol. 2002, 184, 3078–3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwibbert, K.; Marin-Sanguino, A.; Bagyan, I.; Heidrich, G.; Lentzen, G.; Seitz, H.; Rampp, M.; Schuster, S.C.; Klenk, H.P.; Pfeiffer, F.; et al. A blueprint of ectoine metabolism from the genome of the industrial producer Halomonas elongata DSM 2581(T). Environ. Microbiol. 2011, 13, 1973–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, T.; Galinski, E.A. Bacterial milking: A novel bioprocess for production of compatible solutes. Biotechnol. Bioeng. 1998, 57, 306–313. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, J.; Han, R.; Shen, G.; Long, Q.; Wei, X.; Liu, D. Identification and characterization of ectoine biosynthesis genes and heterologous expression of the ectABC gene cluster from Halomonas sp. QHL1, a moderately halophilic bacterium isolated from Qinghai Lake. J. Microbiol. 2014, 52, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Giesselmann, G.; Dietrich, D.; Jungmann, L.; Kohlstedt, M.; Jeon, E.J.; Yim, S.S.; Sommer, F.; Zimmer, D.; Muhlhaus, T.; Schroda, M.; et al. Metabolic Engineering of Corynebacterium glutamicum for High-Level Ectoine Production: Design, Combinatorial Assembly, and Implementation of a Transcriptionally Balanced Heterologous Ectoine Pathway. Biotechnol. J. 2019, 14, 1800417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Qin, N.; Guan, L.Y. A Novel Cold-adapted Endoglucanase (M6A) from Microbacterium kitamiense S12 Isolated from Qinghai-Tibetan Plateau. Biotechnol. Bioprocess Eng. 2019, 24, 544–551. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Richter, A.A.; Kobus, S.; Czech, L.; Hoeppner, A.; Zarzycki, J.; Erb, T.J.; Lauterbach, L.; Dickschat, J.S.; Bremer, E.; Smits, S.H.J. The architecture of the diaminobutyrate acetyltransferase active site provides mechanistic insight into the biosynthesis of the chemical chaperone ectoine. J. Biol. Chem. 2020, 295, 2822–2838. [Google Scholar] [CrossRef]
- Hillier, H.T.; Altermark, B.; Leiros, I. The crystal structure of the tetrameric DABA-aminotransferase EctB, a rate-limiting enzyme in the ectoine biosynthesis pathway. FEBS J. 2020, 287, 4641–4658. [Google Scholar] [CrossRef]
- Czech, L.; Hoppner, A.; Kobus, S.; Seubert, A.; Riclea, R.; Dickschat, J.S.; Heider, J.; Smits, S.H.J.; Bremer, E. Illuminating the catalytic core of ectoine synthase through structural and biochemical analysis. Sci. Rep. 2019, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Ning, Y.; Wu, X.; Zhang, C.; Xu, Q.; Chen, N.; Xie, X. Pathway construction and metabolic engineering for fermentative production of ectoine in Escherichia coli. Metab. Eng. 2016, 36, 10–18. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Neuwald, A.F.; Landsman, D. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem. Sci. 1997, 22, 154–155. [Google Scholar] [CrossRef]
- Shen, B.W.; Hennig, M.; Hohenester, E.; Jansonius, J.N.; Schirmer, T. Crystal structure of human recombinant ornithine aminotransferase. J. Mol. Biol. 1998, 277, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, A.U.; Bremer, E. Osmotically regulated synthesis of the compatible solute ectoine in Bacillus pasteurii and related Bacillus spp. Appl. Environ. Microbiol. 2002, 68, 772–783. [Google Scholar] [CrossRef] [Green Version]
- Czech, L.; Hermann, L.; Stoveken, N.; Richter, A.A.; Hoppner, A.; Smits, S.H.J.; Heider, J.; Bremer, E. Role of the Extremolytes Ectoine and Hydroxyectoine as Stress Protectants and Nutrients: Genetics, Phylogenomics, Biochemistry, and Structural Analysis. Genes 2018, 9, 177. [Google Scholar] [CrossRef] [Green Version]
- Schubert, T.; Maskow, T.; Benndorf, D.; Harms, H.; Breuer, U. Continuous synthesis and excretion of the compatible solute ectoine by a transgenic, nonhalophilic bacterium. Appl. Environ. Microbiol. 2007, 73, 3343–3347. [Google Scholar] [CrossRef] [Green Version]
- Margesin, R.; Schinner, F.; Marx, J.C.; Gerday, C. Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Ed.; Springer: Cham, Switzerland, 2017; pp. 285–303. [Google Scholar] [CrossRef]
- Dou, S.H.; Chi, N.Y.; Zhou, X.S.; Zhang, Q.F.; Pang, F.; Xiu, Z.L. Molecular cloning, expression, and biochemical characterization of a novel cold-active alpha-amylase from Bacillus sp. dsh19-1. Extremophiles 2018, 22, 739–749. [Google Scholar] [CrossRef]
- Merin, M.G.; de Ambrosini, V.I.M. Highly cold-active pectinases under wine-like conditions from non-Saccharomyces yeasts for enzymatic production during winemaking. Lett. Appl. Microbiol. 2015, 60, 467–474. [Google Scholar] [CrossRef]
- Bethlehem, L.; Moritz, K.D. Boosting Escherichia coli’s heterologous production rate of ectoines by exploiting the non-halophilic gene cluster from Acidiphilium cryptum. Extremophiles 2020, 24, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Czech, L.; Wilcken, S.; Czech, O.; Linne, U.; Brauner, J.; Smits, S.H.J.; Galinski, E.A.; Bremer, E. Exploiting Substrate Promiscuity of Ectoine Hydroxylase for Regio- and Stereoselective Modification of Homoectoine. Front. Microbiol. 2019, 10, 2745–2762. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.A.; Mais, C.N.; Czech, L.; Geyer, K.; Hoeppner, A.; Smits, S.H.J.; Erb, T.J.; Bange, G.; Bremer, E. Biosynthesis of the Stress-Protectant and Chemical Chaperon Ectoine: Biochemistry of the Transaminase EctB. Front. Microbiol. 2019, 10, 2811–2830. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).