Featured Application
Zirconia implants present success rates similar to titanium implants and seem to have a better soft tissue response and less material corrosion.
Abstract
This review aims to discuss the advantages and disadvantages of zirconia implants compared with titanium implants. Moreover, it intends to review the relevant available long-term literature of these two materials regarding osteointegration, soft-tissue, microbiota, and peri-implantitis, focusing on clinical results. Briefly, titanium implants are a reliable alternative for missing teeth; however, they are not incapable of failure. In an attempt to provide an alternative implant material, implants made from ceramic-derivate products were developed. Owing to its optimal osseointegration competence, biocompatibility, and esthetic proprieties, zirconium dioxide (ZrO2), also known as zirconia, has gained popularity among researchers and clinicians, being a metal-free alternative for titanium implants with its main use in the anterior esthetic zones. This type of implant may present similar osseointegration as those noted on titanium implants with a greater soft-tissue response. Furthermore, this material does not show corrosion as its titanium analog, and it is less susceptible to bacterial adhesion. Lastly, even presenting a similar inflammatory response to titanium, zirconia implants offer less biofilm formation, suggesting less susceptibility to peri-implantitis. However, it is a relatively new material that has been commercially available for a decade; consequently, the literature still lacks studies with long follow-up periods.
1. Introduction
Since Brånemark’s first implant attempts until now, dental implant therapy has advanced remarkably with innovations on both surgical and prosthetic fronts. New techniques, surgical procedures, and materials have been developed, ensuring implant stability. However, in contrast to what most patients may think, dental implants are not an infallible solution for the edentulous area. Several complications can occur, reducing the implant success rates. The most common cause of titanium implant failure in the medium and long-term is peri-implantitis. Peri-implantitis is a pathological condition occurring in the peri-implant tissues, characterized by inflammation in the peri-implant mucosa and subsequent progressive loss of supporting bone. It is characterized by profuse bleeding on probing or suppuration, deep probing depth, and bone loss beyond the initial bone remodeling [1]. It is estimated that the peri-implantitis prevalence can be up to 34% [2].
In additional, other less common complications can be found such as fractures [3], titanium allergy [4], and corrosion [5]. According to Lee (2018), the implant fracture prevalence is 0.4% and can be related to prosthetic factors such as inappropriate occlusal, peri-implantitis, overloading, or even manufacturing defects [3]. Titanium allergies are a reaction to the titanium components and might result from ion absorption through the skin or mucosal contact or from implant corrosion processes. It is estimated that 0.6% of the population is allergic to titanium [4]. Corrosion can result from the presence of several corrosive species like hydrogen sulfide compounds dissolved oxygen, free radicals, and chloride ion, resulting in the metal surface breakdown. Even in a passive environment, the basal implant corrosion rate is 0.02 mm/year to 0.13 mm/year [5]. Moreover, another drawback of titanium implants is their dark color. This can be an issue in a patient with a thin gingival phenotype, mainly in the esthetic area [6,7,8]. The grey shadow of titanium may be visible through the peri-implant mucosa, thus impairing the esthetic outcome [9].
In order to overcome the drawbacks of titanium implants, zirconia implants were implemented. These implants have been used in Europe since the early 2000s and, subsequent to FDA approval in 2011, it has been more often used in the USA [10]. Furthermore, implants are a popular solution for edentulous areas, and with the plethora of information available, patients search for different implant alternatives and come to dental offices requesting other options to the conventional titanium. In this matter, clinicians are raising questions such as whether the zirconia implants are reliable? Are they a better option compared with titanium implants? This narrative review aims to clarify these questions and review the long-term results of zirconia implants compared with titanium implants. Furthermore, this article offers a concise review of the literature regarding osteointegration, soft-tissue, microbiota, and peri-implantitis of the two materials, mainly focusing on clinical outcomes. Lastly, this review’s ultimate objective is to provide qualified information to support the decision-making process of clinicians regarding implant material.
2. What Is a Zirconia Implant?
Ceramics are a common material used in dentistry owing to their biology compatibility and long-term durability. The ceramic composites currently in use in medical and dental devices originated from structural materials used in the aerospace and military industry [5]. The first ceramic implant attempt was made of aluminum oxide. However, because of its unsatisfactory toughness and low long-term survival rates, it was withdrawn from the market in the early 1990s [11]. Nowadays, among all dental ceramics, zirconium dioxide (ZrO2), also known as zirconia, has arisen as a multipurpose material thanks to its biological, mechanical, and optical properties. Zirconia does not exist as a pure oxide. Therefore, it needs to be extracted from minerals such as zirconate (ZrO2-SiO2, ZrSiO4) and baddeleyite (ZrO2) [12]. In order to meet structural demands, zirconia is doped with stabilizers to achieve high strength and fracture toughness. Zirconia structures are characterized in three crystal forms: monoclinic, cubic, and tetragonal. At room temperature, zirconia is in its monoclinic structure and changes into the tetragonal structure at 117 °C and into a cubic phase at 237 °C. The cubic phase is unstable and can break into pieces. However, to stabilize the zirconia, CaO, MgO, and Y2O3 (Yttrium) are added to its structure [13]. The properties that make tetragonal zirconia polycrystal a suitable biomedical material are low porosity, high bending, high density, compression strength including low thermal conductivity, high flexural strength, favorable fracture resistance, as well as wear and corrosion resistance [14,15]. Moreover, the opaque white color of zirconia, as well as its biocompatibility, osseointegration, and low affinity to bacterial plaque, make this composite a good candidate for dental implants [14].
The enhanced aesthetics of zirconia is attributed to its ability to mask dark substrates with good opacity in the visible and infrared spectrum and controlled translucency. The masking ability is due to its grain size being greater than the length of light, high refractive index, low absorption coefficient, and high density with low residual porosity, as well as the presence of various additives, stabilizers, and pigments [16].
On the other hand, zirconia also has some disadvantages as aging or degradation at low temperatures. In the occurrence of water or water vapor, there is a slow transformation from the tetragonal phase into the monoclinic phase. It may lead to slow development of roughness, thus producing progressive deterioration of the material. Aging occurs as a result of compressive stresses and microcracking. It may be predisposed by its manufacturing processes, such as the macroscopic form and the surface features of an implant, but this has not yet been fully explained [16,17].
The mechanical and physical properties of zirconia implants depend upon its composition, nature of crystals, metastable polymorphic structure, the ratio of the monoclinic to tetragonal phase, percentage of stabilizing metal oxide, the aging process, the macro and micro design of the implant, the nature of the finish line on the implant abutment, the characteristics of implant abutment, and the amount of occlusal load [16].
Likewise, the implants made of titanium, the zirconia implants, also undergo a surface modification to improve their biological responses. The most common are sandblasting, acid etching, selective infiltration technique (coating the surface with glass infiltration and then heating it in temperature higher than glass transition temperature followed by the infiltration of molten glass between the material grains), polishing, laser treatment, and ultraviolet light [13]. Figure 1 presents the advantages and disadvantages of zirconia implants.
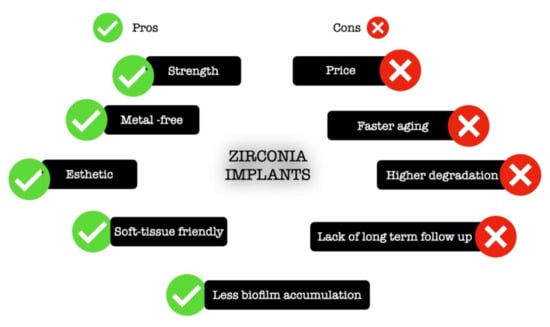
Figure 1.
Schematic illustration of the advantages and drawbacks of zirconia implants.
Nowadays, the main clinical indication of a zirconia implant is the anterior esthetic zone cases, especially in those with a scalloped, thin phenotype gingival architecture. Another indication is patients with metal allergies and chronic diseases resulting from this allergy. Further, owing to the lower bacteria accumulation propriety of zirconia implants, this ceramic might be a preferred material for patients with a history of peri-implantitis [7,18,19,20,21,22,23].
3. What Is a Titanium Implant?
Titanium is the ninth most common material found in nature [24]. The most common oxide is titanium dioxide (TiO2), which can be seen as a natural mineral source in three different crystalline forms called anatase, brookite, and rutile [24,25,26]. This material undergoes an allotropic transformation and changes from one crystallographic form to another. At room temperature, it has a hexagonal close-packed structure called the α phase. However, at high temperatures, or precisely at 883 °C, it changes to the β phase where it has a body-centered cubic structure [24,27]. Moreover, titanium can be combined with various other elements to increase its strength and provide resistance against corrosion [25]. Thanks to titanium biocompatibility, this material is broadly used in medicine and dentistry. Overall, titanium is considered inert and safe for human use with minimal side effects in the human body. Figure 2 presents the advantages and disadvantages of titanium implants.
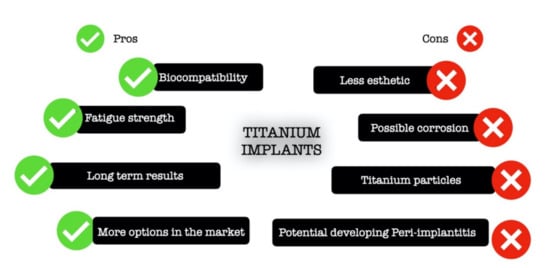
Figure 2.
Schematic illustration of advantages and drawbacks of titanium implants.
However, the true inertness of titanium has been questioned [28]. Lately, some reports of titanium allergy are described in the literature, and the prevalence of this allergic reaction is estimated to be 0.6% [4]. Likewise, one of the significant drawbacks of this metal is corrosion, leading to the release of titanium particles. Therefore, it is hypothesized that the oral cavity would be more susceptive to titanium corrosion than other parts of the body because of the oral environment condition such as the presence of some level of infection (gingivitis or periodontitis), the use of fluoride-containing toothpaste and mouthwashes, and higher glucose levels, as well as the presence of saliva containing corrosive compounds such as hydrogen, chloride ions, sulfide compounds, dissolved oxygen, and free radicals [29,30,31,32,33,34]. Besides, it has been shown that peri-implant tissues have 100–300 parts per million (ppm) of titanium concentration [26,35], and this number can be considerably higher if implantoplasty procedures are performed [36]. Nowadays, the effect of free titanium particles in the body is still not clear in the literature; however, some reports indicate that these particles could be harmful [37,38]. In the orthopedic field, for example, the most common reason for titanium prosthesis replacement is aseptic loosening, a local periprosthetic osteolysis around joint replacements triggered by wear debris in the absence of bacteria, an inherently mechanical problem [38,39]. More precisely, in the oral cavity, free titanium particles can trigger DNA damage response in oral epithelial cells and the release of titanium parts by ultrasonic scaling can activate the inflammatory response, by secretion of IL-1β, IL-6, and TNF-α [40,41,42]. Furthermore, titanium corrosion may lead to reduced implant strength, compromising its stability [36,43].
Titanium alloys are categorized into five micro-structure categories: α, near α, α + β, near β, and β. The alloying elements are classified as α-stabilizers, for instance, aluminum and oxygen, or β-stabilizers, such as vanadium, iron, nickel, and cobalt [44]. The most common titanium alloys are so-called commercially pure titanium and Ti-6Al-4V, also called TC4, Ti64, or ASTM Grade 5. These alloys are standard in dentistry owing to their high specific strength and excellent corrosion resistance [45]. The commercially pure titanium is classified as α micro-structure. It is available in four different grades numbered 1 to 4, according to the purity and the processing oxygen content, with 1 being the least in terms of oxygen content and 4 the highest. Moreover, the grades differ in corrosion resistance, ductility, and strength. On the other hand, Ti-6Al-4V is categorized as α + β alloy and is composite of 90% titanium, 6% aluminum, and 4% vanadium [46]. It has higher strength and elongation at failure compared with commercially pure titanium. However, aluminum and vanadium can be released by this alloy, causing problems in bone mineralization and allergy reactions [47,48].
4. Osteointegration
Osseointegration is a term from the Latin language where osseous means “bony” and integrare “to make whole”, and it is defined as a “function ankylosis” [49]. Similar to in titanium implants, the osseointegration of zirconia implants is a dynamic process composed of primary stability and secondary stability. Immediately after implant placement, mechanical fixation of the implant provides primary stability, which is direct contact between the bony walls and the implant. It is considered biomechanical stability as there is no biological activity. On the other hand, the secondary biological stability results from the cellular activity with new bone deposition on the implant surface, giving rise to the functional ankylosis [50,51].
At the histological level, osteointegration is an active process that evolves basically two phases: the establishment of the implant in the recipient site and its maintenance. A classical study performed by Berglundh and colleagues [52] elucidates the implant wound healing progression. Briefly, after the titanium is placed in the drilled site, the implant bed is filled with blood from the marginal tissue, and threads of the implant assure the primary stability in direct contact with the surrounding bone. A water molecule forms a pellicle in the implant surface, which facilitates the adhesion of intracellular matrix proteins provided by the blood cells and interstitial fluid [53]. These proteins assist cell adhesion, migration, and differentiation. Next, when hemostasis is achieved and the blood clot is formed, the inflammatory cells are attracted to the wound site, and give rise to the granulation tissues, and fibroblast-like cells can be encountered in the implant zone area. One week after the implant placement, the provisional matrix replaces the granulation tissues, and the first signs of woven bone are present. Between one and two weeks, the bone tissues between the threads, responsible for primary mechanical stability, began to be reabsorbed by osteoclasts, and new viable bone became deposited. Between eight to twelve weeks, the mature bone can be found to be surrounded by bone marrow [52,54,55].
Overall, titanium and zirconia implants present the same bone tissue integration [56]. However, microstructure and implant surface modification methods play a key role in osseointegration. These implant modifications are made to improve mechanical properties of the implants like strength, ductility, biological function, bacterial adhesion, wear, and fracture toughness. This has been well demonstrated in the literature for titanium implants [57,58,59,60,61]. Basically, the surface modifications can be prepared by physical or chemical methods. Among physical modifications can be cited maching, grit-blasting, acid-etching, laser ablation, and nanocomposite. Regarding the chemical modification, examples that can be mentioned are crystalline deposition, oxidation, fluoride treatment, UV treatment, and hydroxyapatite addition [62,63]. Surfaces with proper roughness and hydrophilicity are prone to promote more osseointegration than other surfaces. The most common surface modifications in clinical practice are SLA, SLActive, Osseotite, and TiUnite. SLA has a rough surface produced by sandblasting with large grit followed by mixed acid etching with hydrochloric and sulfuric materials. The SLActive surface is prepared by rinsing SLA-treated implants under a nitrogen atmosphere and storing them in NaCl solution rather than placing them in dry storage. The Osseotite surface is a rough surface with a uniform micro-texture produced by the dual-acid (HCl-H2SO4) etching method. Lastly, TiUnite results from the oxidation process in which implants are treated in a galvanic cell containing phosphoric acid electrolyte. The TiUnite surface is characterized by a thick TiO2 layer enriched with highly crystalline calcium phosphate [19,64,65,66]. A recent network meta-analysis [67] concluded that, regarding osseointegration in the early healing stages, SLActive shows better results. However, over time, TiUnite is the best option among the four implants evaluated. Concerning stability, all implants are eligible with ISQ values greater than 60. Additionally, a previous systematic review evaluation of 27 randomized clinical trials including 38 different implant types concluded that relatively smooth (turned) surfaces were less prone to lose bone as a result of chronic infection (peri-implantitis) than implants with much rougher surfaces (titanium-plasma-sprayed) [68]. Considering the effect of nanostructure at the cellular level, a systematic review concluded that this type of structure has a remarkable advantage in osteoblast proliferation [69].
Specifically regarding zirconia implants, according to Martins and colleagues [70], limited bone remodeling with machined-zirconia implants containing yttria over time can decrease the secondary stability of this type of implant compared with machined and resorbable blast media titanium ones.
In the same way as happens in the titanium implants, the surface modification of the zirconia implants may dictate differences in their osseointegration. Preclinical studies have revealed bone apposition on zirconia implants with various surface modifications, including sandblasting, etching [71], sintering, and coating [72], and even subtle alterations in the surface may have an enormous benefit in the osseointegration.
Owing to the increase in demand for zirconia implants, mainly in an esthetic-driven patient, clinicians and researchers question themselves about the efficiency of this type of implant and its non-inferiority in relation to conventional titanium implants. Mostly preclinical, but also some randomized clinical trials and systematic reviews have been published to answer these questions. Table 1 summarizes the results from existing systematic reviews analyzing the osseointegration of zirconia implants.

Table 1.
Summary of systematic reviews (SRs) analyzing the osseointegration of zirconia implants.
A study with human biopsies from 22 failed zirconia implants (failed from trauma or extensive bone loss compatible with peri-implantitis) showed dense bone with stable laminar structure in tight contact with the implant surface and no gaps. The bone–implant area ranged from 58.1 to 93.7% [77].
5. Soft Tissue Reaction
In natural dentition, the consensus is that there are not necessarily specific keratinized gingiva dimensions to maintain periodontal health under good oral hygiene [78,79,80]. On the other hand, the peri-implant soft tissue is different from that around a natural tooth. In implants, as its surface is directly attached to the bone, there are not periodontal fibers anchoring in the bone, and collagen fibers run parallel to the implant surface without direct anchorage [81,82]. This soft tissue architecture is considered unsteady and provides an inferior biologic seal [83]. As a consequence, the soft tissues surrounding the implants are more prone to develop inflammatory disease processes. Furthermore, implants with a thin phenotype and inadequate keratinized mucosa width (less than 2 mm) have a higher prevalence of peri-implantitis and peri-implant mucositis [83,84,85], demonstrating that soft tissue plays a crucial role in implant health.
Currently, several in vitro studies show that zirconia implants have better biological reactions in periodontal tissues when compared with titanium [13]. For instance, fibroblasts proliferate faster and are more equally distributed in zirconia disks compared with titanium [86]. Moreover, this material enhances collagen and extracellular matrix proteins’ release [87,88]. Furthermore, analyzing the results of zirconia interaction with epithelial cells, it looks like zirconia increases the differentiation and proliferation of these cells, facilitating the healing process and protective scarring around the implants [13]. Moreover, the zirconia implants present longer junctional epithelium and a higher density of collagen fibers, which might increase the soft-tissue closure and decrease the inflammatory infiltration around zirconia implants [56].
Regarding the orientation of collagen fibers around implants, the same way as in titanium pieces, the adherence of collagen fibers into zirconia implants occurs in a parallel and parallel-oblique way [89]. Furthermore, the supracrestal tissue adhesion (also formerly known as biology width or supracrestal tissue attachment) is more prolonged in titanium implants (mean of 5 mm) compared with zirconia implants (4.5 mm), meaning that zirconia implants have a significantly lower sulcus depth. Nevertheless, the epithelium length mean was 2.9 mm in both groups. On the other hand, a significant difference was found in the connective tissue of both materials (2.4 mm around zirconia implants and 1.5 mm around titanium implants), demonstrating a higher organization of collagen fibers. These results suggest that a more mature and pronounced soft tissue integration happens with zirconia implants compared with titanium [53].
6. Microbiota and Peri-Implantitis
Peri-implantitis is an inflammatory process around an osseointegrated implant, including soft tissue inflammation and progressive loss of supporting bone beyond biological bone remodeling [1]. This inflammatory process is the leading cause of loss of implants in the long term. Peri-implantitis results from biofilm dysbiosis deposits surrounding the peri-implants’ mucosa, meaning that bacteria deposition is essential to develop the inflammation reaction. In addition, research considering bacterial adhesion on titanium has revealed that the corrosion of titanium increases plaque accumulation. On this matter, zirconia implants have been suggested as a peri-implant mucosa-friendly alternative [90].
Recent studies show intriguing results promoting the advantage of zirconia implants in this matter regarding biofilm formation and bacterial adhesion in zirconia implants. An in vitro study aimed to compare the biofilm formation in the zirconia or titanium disks using three-species biofilm or human plaque samples in an anaerobic flow chamber mode. The researchers found that zirconia showed a reduction in biofilm thickness and mass compared with titanium, but similar biofilm metabolism was found. These results suggest that zirconia implants can form less plaque, leading to less peri-implant inflammation than titanium implants [20]. Considering the bacterial adhesion in zirconia, the Grossner–Schreiber group [22] found that bacterial counts were higher with titanium discs than zirconia, while Scarano et al. [23] showed that bacterial adhesion was significantly higher with pure titanium surfaces as compared with zirconium oxide surfaces, as data also supported by Sadid-Zadeh and collaborators find [87]. Moreover, these results are supported by an animal model. That is, a study comparing ligature-induced peri-implantitis around zirconia or titanium implants in dogs. They found that the first presented less crestal peri-implant bone loss and no implant failure. In contrast, the second shows one implant loss due to peri-implantitis [21]. Additionally, a recent systematic review with meta-analysis indicated that zirconia accumulated less initial oral biofilm parameters and less roughness in the zirconia surface [91].
On the other hand, taking into consideration the role of host response in peri-implantitis, a recent study [92] compared the expression of immunoinflammatory markers in the tissues around ceramic and titanium implants. The authors found a similar number of inflammatory cells (T- and B-cells) in mucosa surrounding ceramic implants when compared with titanium ones, concluding that the cellular response to the inflammatory challenge provoked by the biofilm around implants is the same, independent of the material with which the implant is made.
Besides, the hypothesis has been raised in the literature that not only biofilm is responsible for peri-implantitis in titanium implants, but also foreign body reaction, allergies, cement excess, or even metallosis [93]. Considering all the factors discussed above, it could be assumed that zirconia implants present lower prevalence rates when compared with titanium implants. Nevertheless, according to a systematic review and meta-analysis [94], owing to the heterogeny of assessment and short-term evaluation of the zirconia implants’ clinical studies, non-conclusive results are found regarding inflammatory parameters in peri-implant mucosa around zirconia implants. On the other hand, one prospective study that followed up zirconia implants for 7.8 years found no difference between teeth (control group) and implant regarding bleeding index, attachment level, or bacterial colonization. Interestingly, zirconia implants presented less plaque accumulation and recession, but more probing depths when compared with natural dentition [95]. Moreover, another systematic review [96] concluded that zirconia implants had less plaque accumulation and less inflammation around the peri-implant mucosa.
7. Long-Term Results
The objective of a dental implant is to replace a missing tooth in a functional and esthetic way. Thus, for an implant to be considered successful, it needs to be permanently osseointegrated and healthy. Thus, long-term results are essential to determine which material is superior. In this way, to compare titanium versus zirconia implants is an unfair evaluation because, on one side, it encounters a long-term result of the stabilized treatment option—titanium implants—against a novel material with short-term results and specific indications—zirconia.
Regarding the overall success of the implants, both materials present a satisfactory survival rate. According to Buser et al. [97], titanium implants show an overall 10-year implant survival rate of 98.8% and a success rate of 97.0%. On the other hand, the recent systematic review and meta-analysis, which evaluate randomized clinical trials of at least 1 year follow-up showed that zirconia implants present a survival rate of 91.5% to 98.3%, a success rate of 91.6%, and marginal bone loss ranging from 0.7 mm to 0.98 mm [94].
Table 2 presents the main findings of systematic reviews that analyzed titanium versus zirconia implants.

Table 2.
Summary of systematic reviews comparing zirconia and titanium implants.
8. Conclusions
To conclude, answering the question posed in the title of this review, it is unlikely that zirconia implants will replace the titanium implants’ use entirely owing to the high success rates, long-term stability, and satisfactory osseointegration of the titanium implants found on the market nowadays. However, based on the literature, it seems like zirconia implants are reliable substitutes for cases in which titanium is not an option or in cases where aesthetics is in high demand. Moreover, it is essential for the clinician to be aware of the advantages and drawbacks of this implant option to make the best decision for the treatment of the edentulous area. Furthermore, possibly, in the future, zirconia implants can be the first choice for a patient with a history of periodontitis/peri-implantitis, but more studies with longer follow-up are necessary to prove that zirconia has better performance against bacterial challenges.
Based on the findings of this review, it is possible to conclude that
- Titanium implants are still the standard material for the replacement of a missing tooth;
- Titanium particles are potentially harmful to the peri-implant tissue; however, more studies are necessary for definitive conclusions;
- Osteointegration of zirconia and titanium implants are similar and are influenced by microstructure and the treatment of the surface;
- Some clinical studies show better soft tissue response with zirconia implants, suggesting that this material can provide a protective effect against inflammation;
- Some studies suggest that zirconia implant seems to be less susceptive to peri-implantitis than titanium implants;
- There is a lack of studies with a long-term follow up of zirconia implants.
Author Contributions
L.P.W., H.-L.C. and H.-L.W. contributed to the conception, acquisition, and interpretation of the review data. L.P.W. was responsible for initial draft preparation. H.-L.C. and H.-L.W. were responsible for the final review and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef] [Green Version]
- Kordbacheh Changi, K.; Finkelstein, J.; Papapanou, P.N. Peri-implantitis prevalence, incidence rate, and risk factors: A study of electronic health records at a U.S. dental school. Clin. Oral Implants Res. 2019, 30, 306–314. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.T.; Jeong, S.N.; Kim, N.H.; Lee, D.W. Incidence and pattern of implant fractures: A long-term follow-up multicenter study. Clin. Implant Dent. Relat. Res. 2018, 20, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, V.; Sabane, A.V.; Tejas, K. Hypersensitivity to titanium: A less explored area of research. J. Indian Prosthodont. Soc. 2012, 12, 201–207. [Google Scholar] [CrossRef]
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Lu, Q.; Fan, Z. Changes in the esthetic, physical, and biological properties of a titanium alloy abutment treated by anodic oxidation. J. Prosthet. Dent. 2019, 121, 156–165. [Google Scholar] [CrossRef]
- Kim, A.; Campbell, S.D.; Viana, M.A.; Knoernschild, K.L. Abutment Material Effect on Peri-implant Soft Tissue Color and Perceived Esthetics. J. Prosthodont. 2016, 25, 634–640. [Google Scholar] [CrossRef]
- Thoma, D.S.; Ioannidis, A.; Cathomen, E.; Hämmerle, C.H.; Hüsler, J.; Jung, R.E. Discoloration of the Peri-implant Mucosa Caused by Zirconia and Titanium Implants. Int. J. Periodontol. Restor. Dent. 2016, 36, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontol. 2000 2017, 73, 241–258. [Google Scholar] [CrossRef]
- Hashim, D.; Cionca, N.; Courvoisier, D.S.; Mombelli, A. A systematic review of the clinical survival of zirconia implants. Clin. Oral Investig. 2016, 20, 1403–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koth, D.L.; McKinney, R.V.; Steflik, D.E.; Davis, Q.B. Clinical and statistical analyses of human clinical trials with the single crystal aluminum oxide endosteal dental implant: Five-year results. J. Prosthet. Dent. 1988, 60, 226–234. [Google Scholar] [CrossRef]
- Vagkopoulou, T.; Koutayas, S.O.; Koidis, P.; Strub, J.R. Zirconia in dentistry: Part 1. Discovering the nature of an upcoming bioceramic. Eur. J. Esthet. Dent. 2009, 4, 130–151. [Google Scholar] [PubMed]
- Kunrath, M.F.; Gupta, S.; Lorusso, F.; Scarano, A.; Noumbissi, S. Oral Tissue Interactions and Cellular Response to Zirconia Implant-Prosthetic Components: A Critical Review. Materials 2021, 14, 2825. [Google Scholar] [CrossRef]
- Covacci, V.; Bruzzese, N.; Maccauro, G.; Andreassi, C.; Ricci, G.A.; Piconi, C.; Marmo, E.; Burger, W.; Cittadini, A. In vitro evaluation of the mutagenic and carcinogenic power of high purity zirconia ceramic. Biomaterials 1999, 20, 371–376. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Sivaraman, K.; Chopra, A.; Narayan, A.I.; Balakrishnan, D. Is zirconia a viable alternative to titanium for oral implant? A critical review. J. Prosthodont. Res. 2018, 62, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Lughi, V.; Sergo, V. Low temperature degradation -aging- of zirconia: A critical review of the relevant aspects in dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Apratim, A.; Eachempati, P.; Krishnappa Salian, K.K.; Singh, V.; Chhabra, S.; Shah, S. Zirconia in dental implantology: A review. J. Int. Soc. Prev. Commun. Dent. 2015, 5, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornstein, M.M.; Valderrama, P.; Jones, A.A.; Wilson, T.G.; Seibl, R.; Cochran, D.L. Bone apposition around two different sandblasted and acid-etched titanium implant surfaces: A histomorphometric study in canine mandibles. Clin. Oral. Implants Res. 2008, 19, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Roehling, S.; Astasov-Frauenhoffer, M.; Hauser-Gerspach, I.; Braissant, O.; Woelfler, H.; Waltimo, T.; Kniha, H.; Gahlert, M. In Vitro Biofilm Formation on Titanium and Zirconia Implant Surfaces. J. Periodontol. 2017, 88, 298–307. [Google Scholar] [CrossRef]
- Roehling, S.; Schlegel, K.A.; Woelfler, H.; Gahlert, M. Zirconia compared to titanium dental implants in preclinical studies—A systematic review and meta-analysis. Clin. Oral Implants Res. 2019, 30, 365–395. [Google Scholar] [CrossRef]
- Grössner-Schreiber, B.; Teichmann, J.; Hannig, M.; Dörfer, C.; Wenderoth, D.F.; Ott, S.J. Modified implant surfaces show different biofilm compositions under in vivo conditions. Clin. Oral Implants Res. 2009, 20, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Barksdale, J. Titanium. In The Encyclopedia of the Chemical Elements; Hampel, C.A., Ed.; Reinhold Book Corporation: New York, NY, USA, 1968; pp. 732–738. [Google Scholar]
- Fage, S.W.; Muris, J.; Jakobsen, S.S.; Thyssen, J.P. Titanium: A review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Dermat. 2016, 74, 323–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Donachie, M.J. Titanium a Technical Guide; ASM International: Columbus, OH, USA, 1988. [Google Scholar]
- Kotsakis, G.A.; Olmedo, D.G. Peri-implantitis is not periodontitis: Scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontol. 2000 2021, 86, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Koike, M.; Fujii, H. The corrosion resistance of pure titanium in organic acids. Biomaterials 2001, 22, 2931–2936. [Google Scholar] [CrossRef]
- Barão, V.A.; Mathew, M.T.; Assunção, W.G.; Yuan, J.C.; Wimmer, M.A.; Sukotjo, C. The role of lipopolysaccharide on the electrochemical behavior of titanium. J. Dent. Res. 2011, 90, 613–618. [Google Scholar] [CrossRef]
- Barão, V.A.; Mathew, M.T.; Yuan, J.C.; Knoernschild, K.L.; Assunção, W.G.; Wimmer, M.A.; Sukotjo, C. Influence of corrosion on lipopolysaccharide affinity for two different titanium materials. J. Prosthet. Dent. 2013, 110, 462–470. [Google Scholar] [CrossRef]
- Nakagawa, M.; Matsuya, S.; Udoh, K. Effects of fluoride and dissolved oxygen concentrations on the corrosion behavior of pure titanium and titanium alloys. Dent. Mater. J. 2002, 21, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Noguti, J.; de Oliveira, F.; Peres, R.C.; Renno, A.C.; Ribeiro, D.A. The role of fluoride on the process of titanium corrosion in oral cavity. Biometals 2012, 25, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Lindholm-Sethson, B.; Ardlin, B.I. Effects of pH and fluoride concentration on the corrosion of titanium. J. Biomed. Mater. Res. A 2008, 86, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Suárez-López Del Amo, F.; Garaicoa-Pazmiño, C.; Fretwurst, T.; Castilho, R.M.; Squarize, C.H. Dental implants-associated release of titanium particles: A systematic review. Clin. Oral Implants Res. 2018, 29, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, A.; Bertl, K.; Eren, S.; Gotfredsen, K. Mechanical and biological complications after implantoplasty—A systematic review. Clin. Oral Implants Res. 2019, 30, 833–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.X.; Fan, Y.B.; Gao, Y.; Hu, Q.H.; Wang, T.C. TiO2 nanoparticles translocation and potential toxicological effect in rats after intraarticular injection. Biomaterials 2009, 30, 4590–4600. [Google Scholar] [CrossRef] [PubMed]
- Fretwurst, T.; Nelson, K.; Tarnow, D.P.; Wang, H.L.; Giannobile, W.V. Is Metal Particle Release Associated with Peri-implant Bone Destruction? An Emerging Concept. J. Dent. Res. 2018, 97, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.; Ries, M.D.; Paprosky, W. How prevalent are implant wear and osteolysis, and how has the scope of osteolysis changed since 2000? J. Am. Acad. Orthop. Surg. 2008, 16, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Suárez-López Del Amo, F.; Rudek, I.; Wagner, V.P.; Martins, M.D.; O’Valle, F.; Galindo-Moreno, P.; Giannobile, W.V.; Wang, H.L.; Castilho, R.M. Titanium Activates the DNA Damage Response Pathway in Oral Epithelial Cells: A Pilot Study. Int. J. Oral Maxillofac. Implants 2017, 32, 1413–1420. [Google Scholar] [CrossRef] [Green Version]
- Pettersson, M.; Kelk, P.; Belibasakis, G.N.; Bylund, D.; Molin Thorén, M.; Johansson, A. Titanium ions form particles that activate and execute interleukin-1β release from lipopolysaccharide-primed macrophages. J. Periodont. Res. 2017, 52, 21–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eger, M.; Sterer, N.; Liron, T.; Kohavi, D.; Gabet, Y. Scaling of titanium implants entrains inflammation-induced osteolysis. Sci. Rep. 2017, 7, 39612. [Google Scholar] [CrossRef]
- Okazaki, Y.; Rao, S.; Ito, Y.; Tateishi, T. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials 1998, 19, 1197–1215. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 11. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Tsoi, J.K.H.; Matinlinna, J.P. Binary titanium alloys as dental implant materials—A review. Regen. Biomater. 2017, 4, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.L. Aluminum toxicity to bone: A multisystem effect? Osteoporos. Sarcopenia 2019, 5, 2–5. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Jakobsen, S.S.; Engkilde, K.; Johansen, J.D.; Søballe, K.; Menné, T. The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop. 2009, 80, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Listgarten, M.A.; Lang, N.P.; Schroeder, H.E.; Schroeder, A. Periodontal tissues and their counterparts around endosseous implants. Clin. Oral Implants Res. 1991, 2, 1–19. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontol. 2000 2017, 73, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Ravidà, A.; Wang, H.L.; Helms, J.A.; Brunski, J.B. Relationship Between Primary/Mechanical and Secondary/Biological Implant Stability. Int. J. Oral Maxillofac. Implants 2019, 34, s7–s23. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Abrahamsson, I.; Lang, N.P.; Lindhe, J. De novo alveolar bone formation adjacent to endosseous implants. Clin. Oral Implants Res. 2003, 14, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Kazemian, M.; Hoseini, S.H.; Ghorbanzade, S. A brief overview of cellular and molecular mechanisms of osseointegration. Int. J. Contemp. Dent. Med. Rev. 2015. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implants Res. 2011, 22, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef] [PubMed]
- Liñares, A.; Grize, L.; Muñoz, F.; Pippenger, B.E.; Dard, M.; Domken, O.; Blanco-Carrión, J. Histological assessment of hard and soft tissues surrounding a novel ceramic implant: A pilot study in the minipig. J. Clin. Periodontol. 2016, 43, 538–546. [Google Scholar] [CrossRef]
- Buser, D.; Nydegger, T.; Oxland, T.; Cochran, D.L.; Schenk, R.K.; Hirt, H.P.; Snétivy, D.; Nolte, L.P. Interface shear strength of titanium implants with a sandblasted and acid-etched surface: A biomechanical study in the maxilla of miniature pigs. J. Biomed. Mater. Res. 1999, 45, 75–83. [Google Scholar] [CrossRef]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Carmo Filho, L.C.D.; Marcello-Machado, R.M.; Castilhos, E.D.; Del Bel Cury, A.A.; Faot, F. Can implant surfaces affect implant stability during osseointegration? A randomized clinical trial. Braz. Oral Res. 2018, 32, e110. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; Flores-Fraile, J.; Ramírez, J.M.; Sousa, B.M.; Herrero-Hernández, S.; López-Valverde, A. Bioactive Surfaces vs. Conventional Surfaces in Titanium Dental Implants: A Comparative Systematic Review. J. Clin. Med. 2020, 9, 47. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T.; Chrcanovic, B. Long-term clinical outcome of implants with different surface modifications. Eur. J. Oral Implantol. 2018, 11, S123–S136. [Google Scholar]
- Xue, T.; Attarilar, S.; Liu, S.; Liu, J.; Song, X.; Li, L.; Zhao, B.; Tang, Y. Surface Modification Techniques of Titanium and its Alloys to Functionally Optimize Their Biomedical Properties: Thematic Review. Front. Bioeng. Biotechnol. 2020, 8, 603072. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Xiropaidis, A.V.; Qahash, M.; Lim, W.H.; Shanaman, R.H.; Rohrer, M.D.; Wikesjö, U.M.; Hall, J. Bone-implant contact at calcium phosphate-coated and porous titanium oxide (TiUnite)-modified oral implants. Clin. Oral Implants Res. 2005, 16, 532–539. [Google Scholar] [CrossRef]
- Roccuzzo, M.; Aglietta, M.; Bunino, M.; Bonino, L. Early loading of sandblasted and acid-etched implants: A randomized-controlled double-blind split-mouth study. Five-year results. Clin. Oral Implants Res. 2008, 19, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Browaeys, H.; Defrancq, J.; Dierens, M.C.; Miremadi, R.; Vandeweghe, S.; Van de Velde, T.; De Bruyn, H. A retrospective analysis of early and immediately loaded osseotite implants in cross-arch rehabilitations in edentulous maxillas and mandibles up to 7 years. Clin. Implant Dent. Relat. Res. 2013, 15, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.P.; Cao, N.J.; Zhu, Y.H.; Wang, W. The osseointegration and stability of dental implants with different surface treatments in animal models: A network meta-analysis. Sci. Rep. 2021, 11, 13849. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Ardebili, Y.; Worthington, H.V. Interventions for replacing missing teeth: Different types of dental implants. Cochrane Database Syst. Rev. 2014, Cd003815. [Google Scholar] [CrossRef]
- Goldman, M.; Juodzbalys, G.; Vilkinis, V. Titanium surfaces with nanostructures influence on osteoblasts proliferation: A systematic review. J. Oral Maxillofac. Res. 2014, 5, e1. [Google Scholar] [CrossRef]
- Martins, R.; Cestari, T.M.; Arantes, R.V.N.; Santos, P.S.; Taga, R.; Carbonari, M.J.; Oliveira, R.C. Osseointegration of zirconia and titanium implants in a rabbit tibiae model evaluated by microtomography, histomorphometry and fluorochrome labeling analyses. J. Periodont. Res. 2018, 53, 210–221. [Google Scholar] [CrossRef]
- Hoffmann, O.; Angelov, N.; Zafiropoulos, G.G.; Andreana, S. Osseointegration of zirconia implants with different surface characteristics: An evaluation in rabbits. Int. J. Oral Maxillofac. Implants 2012, 27, 352–358. [Google Scholar] [PubMed]
- Lee, J.; Sieweke, J.H.; Rodriguez, N.A.; Schüpbach, P.; Lindström, H.; Susin, C.; Wikesjö, U.M. Evaluation of nano-technology-modified zirconia oral implants: A study in rabbits. J. Clin. Periodontol. 2009, 36, 610–617. [Google Scholar] [CrossRef]
- Wenz, H.J.; Bartsch, J.; Wolfart, S.; Kern, M. Osseointegration and clinical success of zirconia dental implants: A systematic review. Int. J. Prosthodont. 2008, 21, 27–36. [Google Scholar]
- Pieralli, S.; Kohal, R.J.; Jung, R.E.; Vach, K.; Spies, B.C. Clinical Outcomes of Zirconia Dental Implants: A Systematic Review. J. Dent. Res. 2017, 96, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, S.; Kohal, R.J.; Lopez Hernandez, E.; Doerken, S.; Spies, B.C. Osseointegration of zirconia dental implants in animal investigations: A systematic review and meta-analysis. Dent. Mater. 2018, 34, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Borges, H.; Correia, A.R.M.; Castilho, R.M.; de Oliveira Fernandes, G.V. Zirconia Implants and Marginal Bone Loss: A Systematic Review and Meta-Analysis of Clinical Studies. Int. J. Oral Maxillofac. Implants 2020, 35, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Schwindling, F.S.; Bächle, M.; Spies, B.C. Peri-implant bone response to retrieved human zirconia oral implants after a 4-year loading period: A histologic and histomorphometric evaluation of 22 cases. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1622–1631. [Google Scholar] [CrossRef]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S237–S248. [Google Scholar] [CrossRef]
- Kennedy, J.E.; Bird, W.C.; Palcanis, K.G.; Dorfman, H.S. A longitudinal evaluation of varying widths of attached gingiva. J. Clin. Periodontol. 1985, 12, 667–675. [Google Scholar] [CrossRef]
- Miyasato, M.; Crigger, M.; Egelberg, J. Gingival condition in areas of minimal and appreciable width of keratinized gingiva. J. Clin. Periodontol. 1977, 4, 200–209. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Gonzalez-Martin, O.; Couso-Queiruga, E.; Wang, H.L. The peri-implant phenotype. J. Periodontol. 2020, 91, 283–288. [Google Scholar] [CrossRef]
- Lindhe, J.; Berglundh, T. The interface between the mucosa and the implant. Periodontol. 2000 1998, 17, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Carcuac, O.; Abrahamsson, I.; Albouy, J.P.; Linder, E.; Larsson, L.; Berglundh, T. Experimental periodontitis and peri-implantitis in dogs. Clin. Oral Implants Res. 2013, 24, 363–371. [Google Scholar] [CrossRef]
- Carcuac, O.; Berglundh, T. Composition of human peri-implantitis and periodontitis lesions. J. Dent. Res. 2014, 93, 1083–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharpure, A.S.; Latimer, J.M.; Aljofi, F.E.; Kahng, J.H.; Daubert, D.M. Role of thin gingival phenotype and inadequate keratinized mucosa width (<2 mm) as risk indicators for peri-implantitis and peri-implant mucositis. J. Periodontol. 2021. [Google Scholar] [CrossRef]
- Yamano, S.; Ma, A.K.; Shanti, R.M.; Kim, S.W.; Wada, K.; Sukotjo, C. The influence of different implant materials on human gingival fibroblast morphology, proliferation, and gene expression. Int. J. Oral Maxillofac. Implants 2011, 26, 1247–1255. [Google Scholar] [PubMed]
- Atsuta, I.; Ayukawa, Y.; Kondo, R.; Oshiro, W.; Matsuura, Y.; Furuhashi, A.; Tsukiyama, Y.; Koyano, K. Soft tissue sealing around dental implants based on histological interpretation. J. Prosthodont. Res. 2016, 60, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, M.; Liao, Y.; Zhou, J.; Li, H.; Tan, J. Different behavior of human gingival fibroblasts on surface modified zirconia: A comparison between ultraviolet (UV) light and plasma. Dent. Mater. J. 2019, 38, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Tetè, S.; Mastrangelo, F.; Bianchi, A.; Zizzari, V.; Scarano, A. Collagen fiber orientation around machined titanium and zirconia dental implant necks: An animal study. Int. J. Oral Maxillofac. Implants 2009, 24, 52–58. [Google Scholar] [PubMed]
- Sadid-Zadeh, R.; Willis, J.; Forgo, G.; Haraszthy, V. Comparative Analysis of Biofilm Formation on Materials Used for the Fabrication of Implant-Supported Prostheses. Braz. Dent. J. 2020, 31, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Kniha, K.; Heussen, N.; Modabber, A.; Hölzle, F.; Möhlhenrich, S.C. The effect of zirconia and titanium surfaces on biofilm formation and on host-derived immunological parameters. Int. J. Oral Maxillofac. Surg. 2021. [Google Scholar] [CrossRef]
- Fretwurst, T.; Müller, J.; Larsson, L.; Bronsert, P.; Hazard, D.; Castilho, R.M.; Kohal, R.; Nelson, K.; Iglhaut, G. Immunohistological composition of peri-implantitis affected tissue around ceramic implants—A pilot study. J. Periodontol. 2021, 92, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.G., Jr. Bone loss around implants—Is it metallosis? J. Periodontol. 2021, 92, 181–185. [Google Scholar] [CrossRef]
- Afrashtehfar, K.I.; Del Fabbro, M. Clinical performance of zirconia implants: A meta-review. J. Prosthet. Dent. 2020, 123, 419–426. [Google Scholar] [CrossRef]
- Lorenz, J.; Giulini, N.; Hölscher, W.; Schwiertz, A.; Schwarz, F.; Sader, R. Prospective controlled clinical study investigating long-term clinical parameters, patient satisfaction, and microbial contamination of zirconia implants. Clin. Implant. Dent. Relat. Res. 2019, 21, 263–271. [Google Scholar] [CrossRef]
- Comisso, I.; Arias-Herrera, S.; Gupta, S. Zirconium dioxide implants as an alternative to titanium: A systematic review. J. Clin. Exp. Dent. 2021, 13, e511–e519. [Google Scholar] [CrossRef]
- Buser, D.; Janner, S.F.; Wittneben, J.G.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef]
- Andreiotelli, M.; Wenz, H.J.; Kohal, R.J. Are ceramic implants a viable alternative to titanium implants? A systematic literature review. Clin. Oral Implants Res. 2009, 20, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Manzano, G.; Herrero, L.R.; Montero, J. Comparison of clinical performance of zirconia implants and titanium implants in animal models: A systematic review. Int. J. Oral Maxillofac. Implants 2014, 29, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafezeqoran, A.; Koodaryan, R. Effect of Zirconia Dental Implant Surfaces on Bone Integration: A Systematic Review and Meta-Analysis. Biomed Res. Int. 2017, 2017, 9246721. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Khan, A.S.; Zafar, S. Thirty Years of Translational Research in Zirconia Dental Implants: A Systematic Review of the Literature. J. Oral Implantol. 2017, 43, 314–325. [Google Scholar] [CrossRef] [PubMed]
- ArRejaie, A.S.; Al-Hamdan, R.S.; Basunbul, G.I.; Abduljabbar, T.; Al-Aali, K.A.; Labban, N. Clinical performance of one-piece zirconia dental implants: A systematic review. J. Investig. Clin. Dent. 2019, 10, e12384. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).