Evolution of Composting Process in Maize Biomass Revealed by Analytical Pyrolysis (Py-GC/MS) and Pyrolysis Compound Specific Isotope Analysis (Py-CSIA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composting Samples and Preparation
2.2. Pyrolysis Gas Chromatography Mass Spectrometry (Py-GC/MS)
2.3. Pyrolysis Compound Specific Isotopic Analysis (Py-GC-C/IRMS)
2.4. Elemental Analyzer (EA-Isolink)
2.5. Statistical Analysis
3. Results and Discussion
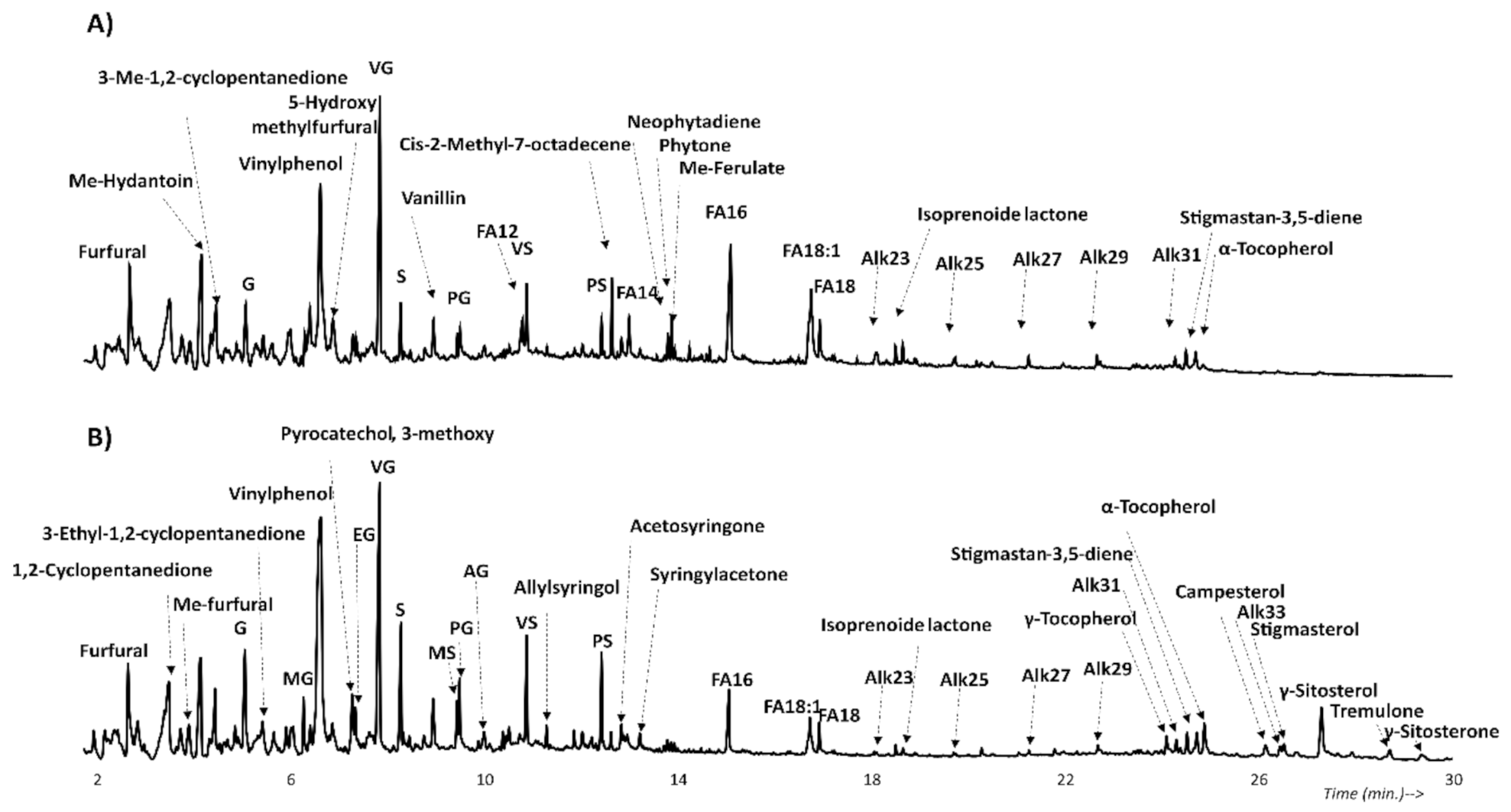
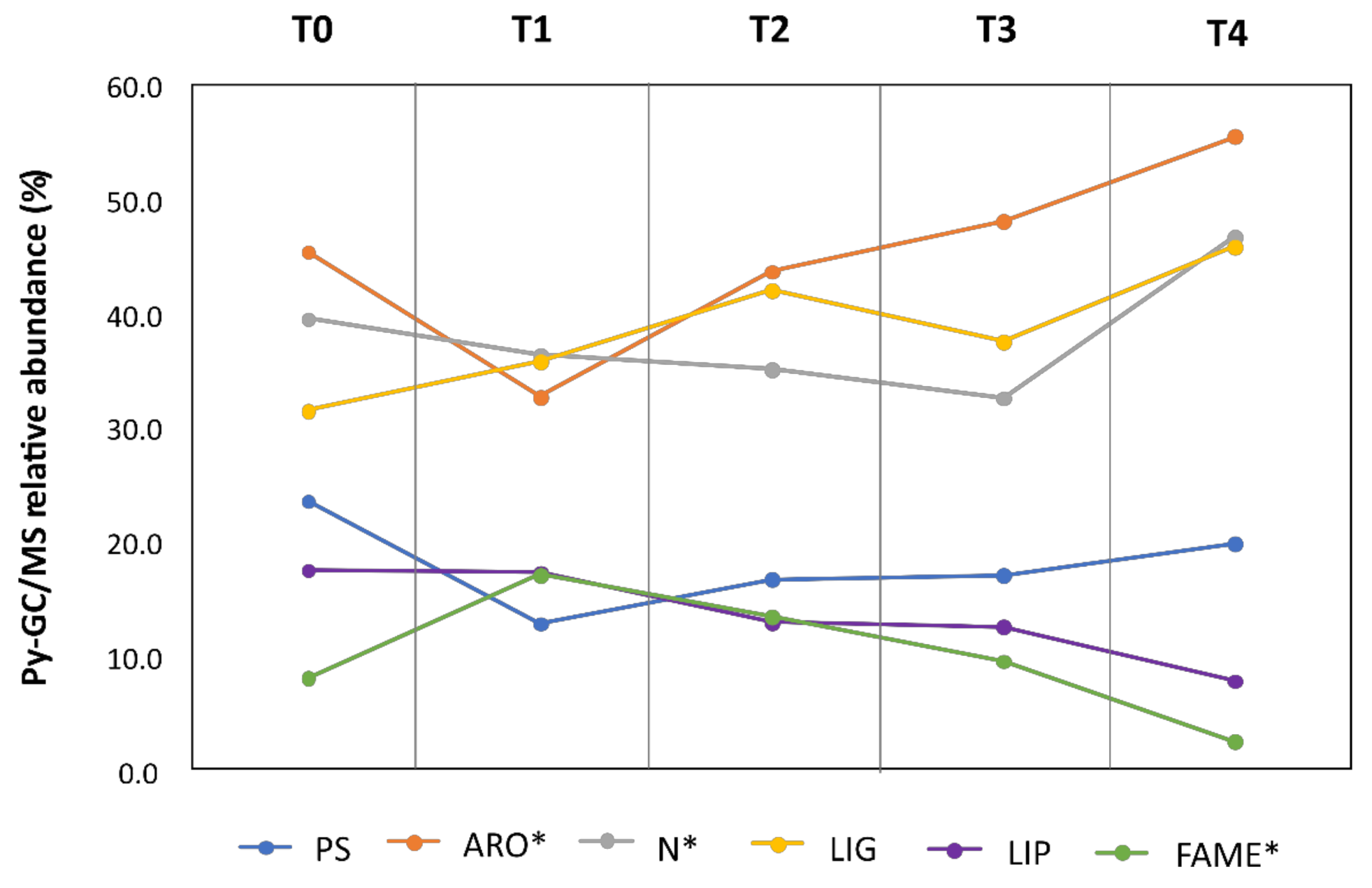
3.1. Pyrolysis Gas Chromatography Mass Spectrometry (Py-GC/MS)
3.1.1. Lignocellulosic Compounds
3.1.2. Other Compounds
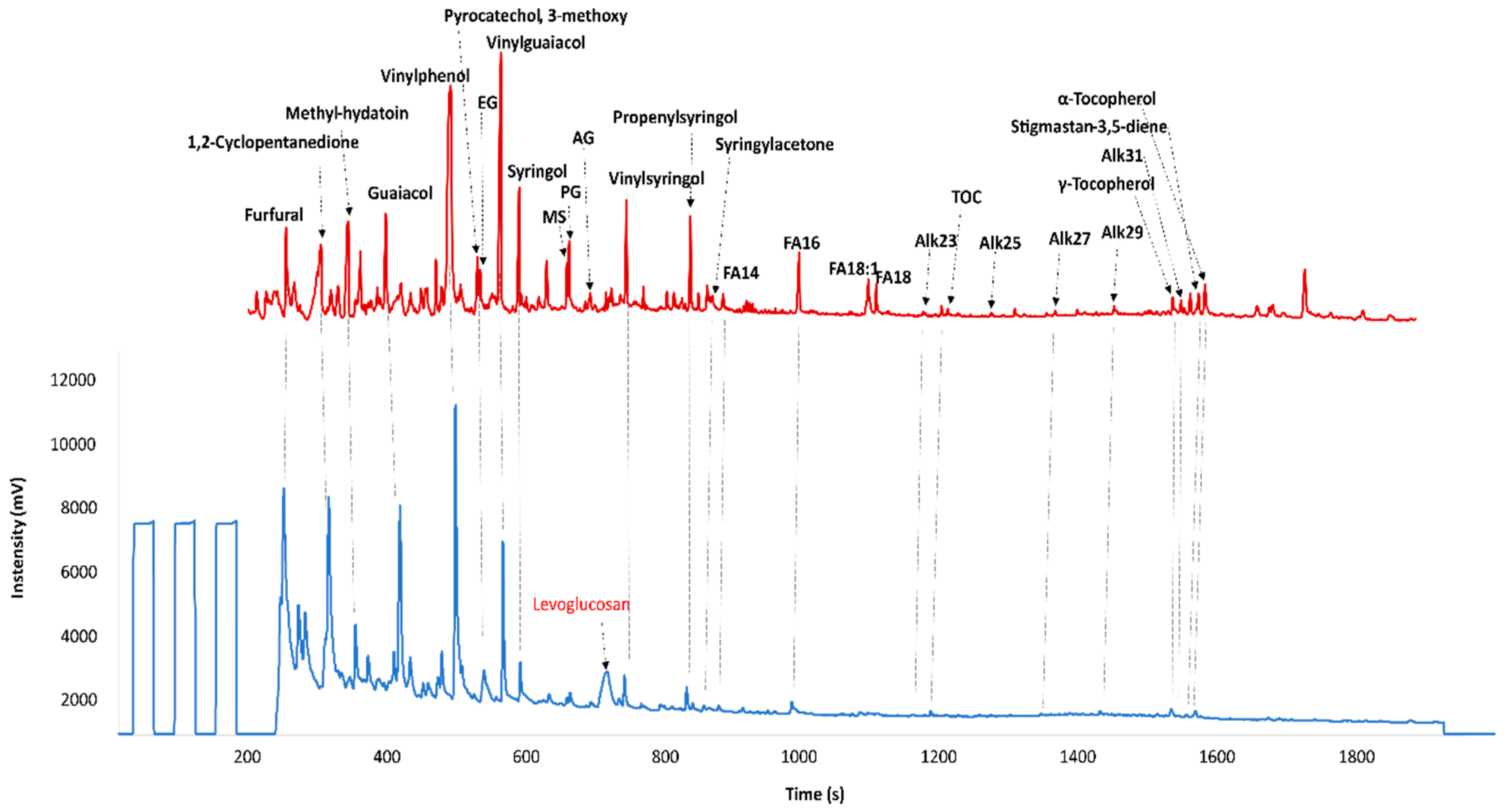
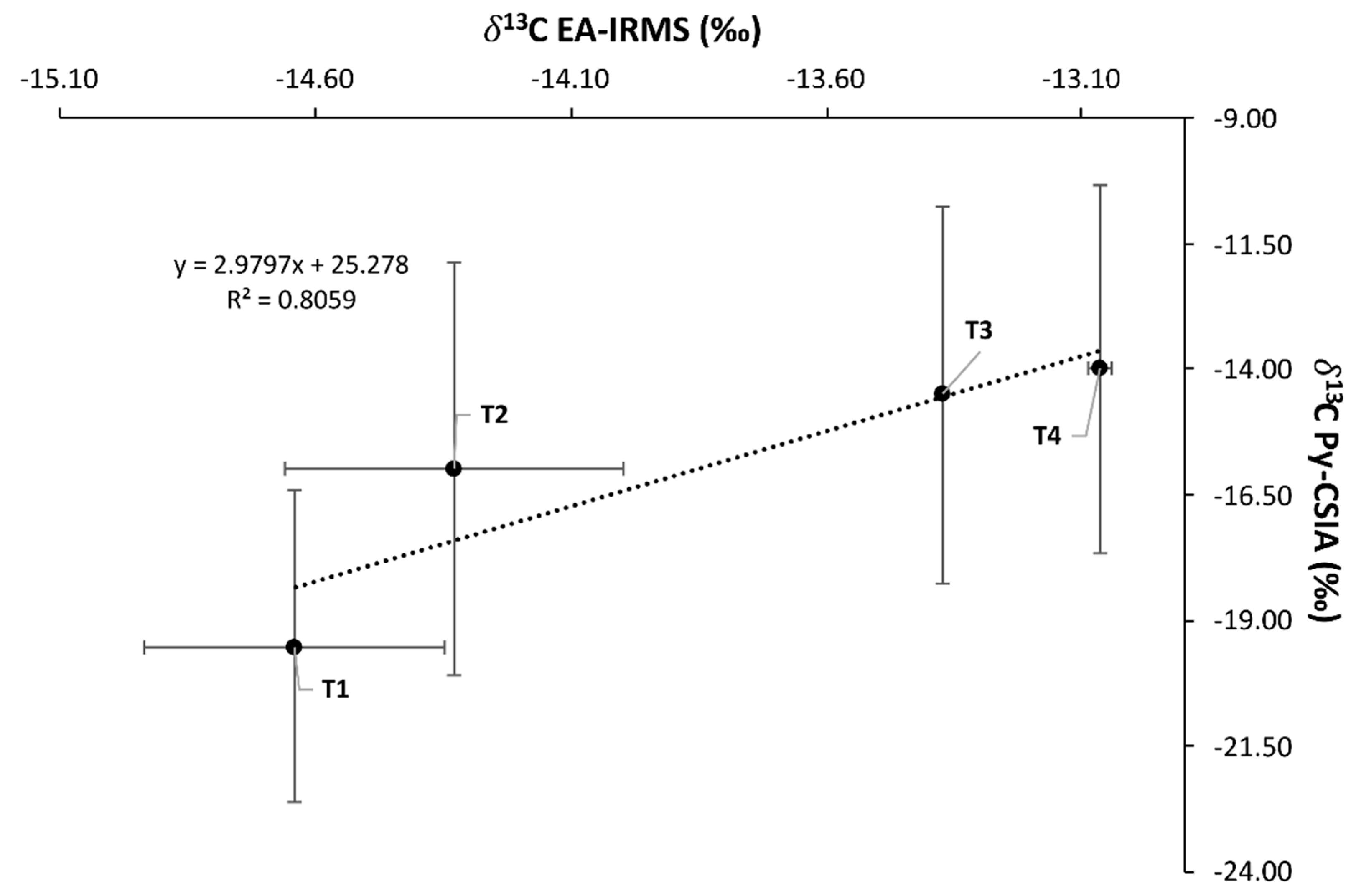
3.2. Pyrolysis Compound Specific Isotopic Analysis (Py-CSIA)
3.3. Bulk δ13C, δ15N IRMS, and C, N Content Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Fernández, J.J.; Galea, Z.; Álvarez, J.M.; Hormaza, J.I.; López, R. Evaluation of composition and performance of composts derived from guacamole production residues. J. Environ. Manag. 2015, 147, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Sayara, T.; Basheer-Salimia, R.; Hawamde, F.; Sánchez, A. Recycling of organic wastes through composting: Process performance and compost application in agriculture. Agronomy 2020, 10, 1838. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Pasian, C.; Lal, R.; Lopez-Nuñez, R.; Fernández, M. A biotic strategy to sequester carbon in the ornamental containerized bedding plant production: A review. Span. J. Agric. Res. 2018, 16, 4. [Google Scholar] [CrossRef]
- Freibauer, A.; Rounsevell, M.D.; Smith, P.; Verhagen, J. Carbon sequestration in the agricultural soils of Europe. Geoderma 2004, 122, 1–23. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Tserkezou, P. Corn and rice waste: A comparative and critical presentation of methods and current and potential uses of treated waste. Int. J. Food Sci. Technol. 2008, 43, 958–988. [Google Scholar] [CrossRef]
- Maucieri, C.; Barco, A.; Borin, M. Compost as a substitute for mineral N fertilization? Effects on crops, soil and N leaching. Agronomy 2019, 9, 193. [Google Scholar] [CrossRef] [Green Version]
- Abdelhamid, M.T.; Horiuchi, T.; Oba, S. Composting of rice straw with oilseed rape cake and poultry manure and its effects on faba bean (Vicia faba L.) growth and soil properties. Bioresour. Technol. 2004, 93, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Jurak, E.; Punt, A.M.; Arts, W.; Kabel, M.A.; Gruppen, H. Fate of carbohydrates and lignin during composting and mycelium growth of Agaricus bisporus on wheat straw based compost. PLoS ONE 2015, 10, e0138909. [Google Scholar] [CrossRef] [PubMed]
- Dignac, M.F.; Houot, S.; Francou, C.; Derenne, S. Pyrolytic study of compost and waste organic matter. Org. Geochem. 2005, 36, 1054–1071. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Belmonte-Urena, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Balesdent, J.; Mariotti, A.; Guillet, B. Natural 13C abundance as a tracer for studies of soil organic matter dynamics. Soil Biol. Biochem. 1987, 19, 25–30. [Google Scholar] [CrossRef]
- Aita, C.; Recous, S.; Angers, D.A. Short-term kinetics of residual wheat straw C and N under field conditions: Characterization by 13C15N tracing and soil particle size fractionation. Eur. J. Soil Sci. 1997, 48, 283–294. [Google Scholar] [CrossRef]
- Spaccini, R.; Piccolo, A.; Haberhauer, G.; Gerzabek, M.H. Transformation of organic matter from maize residues into labile and humic fractions of three European soils as revealed by 13C distribution and CPMAS-NMR spectra. Eur. J. Soil Sci. 2000, 51, 583–594. [Google Scholar] [CrossRef]
- Lynch, D.H.; Voroney, R.P.; Warman, P.R. Use of 13C and 15N natural abundance techniques to characterize carbon and nitrogen dynamics in composting and in compost-amended soils. Soil Biol. Biochem. 2006, 38, 103–114. [Google Scholar] [CrossRef]
- González-Vila, F.J.; González-Pérez, J.A.; Akdi, K.; Gómis, M.D.; Pérez-Barrera, F.; Verdejo, T. Assessing the efficiency of urban waste biocomposting by analytical pyrolysis (Py–GC/MS). Bioresour. Technol. 2009, 100, 1304–1309. [Google Scholar] [CrossRef]
- Akalın, M.K.; Karagöz, S. Analytical pyrolysis of biomass using gas chromatography coupled to mass spectrometry. TrAC Trends Anal. Chem. 2014, 61, 11–16. [Google Scholar] [CrossRef]
- Miller, A.Z.; de la Rosa, J.M.; Jiménez-Morillo, N.T.; Pereira, M.F.; González-Pérez, J.A.; Calaforra, J.M.; Saiz-Jimenez, C. Analytical pyrolysis and stable isotope analyses reveal past environmental changes in coralloid speleothems from Easter Island (Chile). J. Chromatogr. A 2016, 1461, 144–152. [Google Scholar] [CrossRef]
- Girona-García, A.; Badía-Villas, D.; Jiménez-Morillo, N.T.; González-Pérez, J.A. Changes in soil organic matter composition after Scots pine afforestation in a native European beech forest revealed by analytical pyrolysis (Py-GC/MS). Sci. Total Environ. 2019, 691, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.E.; Polvillo, O.; Rodríguez, J.; Hernández, M.; González-Pérez, J.A.; González-Vila, F.J. Thermal transformations of pine wood components under pyrolysis/gas chromatography/mass spectrometry conditions. J. Anal. Appl. Pyrolysis 2006, 77, 63–67. [Google Scholar] [CrossRef]
- Babinszki, B.; Jakab, E.; Terjék, V.; Sebestyén, Z.; Várhegyi, G.; May, Z.; Mahakhant, A.; Attanatho, L.; Suemanotham, A.; Thanmongkhon, Y.; et al. Thermal decomposition of biomass wastes derived from palm oil production. J. Anal. Appl. Pyrolysis 2021, 155, 105069. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Analytical Pyrolysis of Natural Organic Polymers. Techniques and Instrumentation in Analytical Chemistry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 20, 510p. [Google Scholar]
- Quénéa, K.; Derenne, S.; González-Vila, F.J.; González-Pérez, J.A.; Mariotti, A.; Largeau, C. Double-shot pyrolysis of the non-hydrolysable organic fraction isolated from a sandy forest soil (Landes de Gascogne, South-West France): Comparison with classical Curie point pyrolysis. J. Anal. Appl. Pyrolysis 2006, 76, 271–279. [Google Scholar] [CrossRef]
- Parsi, Z.; Hartog, N.; Górecki, T.; Poerschmann, J. Analytical pyrolysis as a tool for the characterization of natural organic matter—A comparison of different approaches. J. Anal. Appl. Pyrolysis 2007, 79, 9–15. [Google Scholar] [CrossRef]
- Sáez, J.A.; Flores, P.; Bustamante, M.Á.; Sánchez-Hernández, J.C.; Moral, R.; Pérez-Murcia, M.D. Nitrogen isotope fractionation during composting of sewage and agri-food sludge with pruning waste. Agronomy 2020, 10, 1954. [Google Scholar] [CrossRef]
- Inácio, C.T.; Magalhães, A.M.; Souza, P.O.; Chalk, P.M.; Urquiaga, S. The relative isotopic abundance (δ13C, δ15N) during composting of agricultural wastes in relation to compost quality and feedstock. Isot. Environ. Health Stud. 2018, 54, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Choi, W.J.; Lim, S.S.; Kwak, J.H.; Chang, S.X.; Kim, H.Y.; Yoon, K.S.; Ro, H.M. Changes in nitrogen isotopic compositions during composting of cattle feedlot manure: Effects of bedding material type. Bioresour. Technol. 2008, 99, 5452–5458. [Google Scholar] [CrossRef] [PubMed]
- Zech, W.; Senesi, N.; Guggenberger, G.; Kaiser, K.; Lehmann, J.; Miano, T.M.; Miltner, A.; Schroth, G. Factors controlling humification and mineralization of soil organic matter in the tropics. Geoderma 1997, 79, 117–161. [Google Scholar] [CrossRef]
- Schweizer, M.; Fear, J.; Cadisch, G. Isotopic (13C) fractionation during plant residue decomposition and its implications for soil organic matter studies. Rapid Commun. Mass Spectrom. 1999, 13, 1284–1290. [Google Scholar] [CrossRef]
- Benner, R.; Fogel, M.L.; Sprague, E.K.; Hodson, R.E. Depletion of 13C in lignin and its implications for stable carbon isotope studies. Nature 1987, 329, 708–710. [Google Scholar] [CrossRef]
- Gerzabek, M.H.; Haberhauer, G.; Kirchmann, H. Soil organic matter pools and carbon-13 natural abundances in particle-size fractions of a long-term agricultural field experiment receiving organic amendments. Soil Sci. Soc. Am. J. 2001, 65, 352–358. [Google Scholar] [CrossRef]
- Chamberlain, P.M.; Bull, I.D.; Black, H.I.; Ineson, P.; Evershed, R.P. Lipid content and carbon assimilation in Collembola: Implications for the use of compound-specific carbon isotope analysis in animal dietary studies. Oecologia 2004, 139, 325–335. [Google Scholar] [CrossRef]
- Upadhayay, H.R.; Bodé, S.; Griepentrog, M.; Huygens, D.; Bajracharya, R.M.; Blake, W.H.; Dercon, G.; Mabit, L.; Gibbs, M.; Semmens, B.X.; et al. Methodological perspectives on the application of compound-specific stable isotope fingerprinting for sediment source apportionment. J. Soils Sediments 2017, 17, 1537–1553. [Google Scholar] [CrossRef]
- Chikaraishi, Y.; Naraoka, H. Compound-specific δD–δ13C analyses of n-alkanes extracted from terrestrial and aquatic plants. Phytochemistry 2003, 63, 361–371. [Google Scholar] [CrossRef]
- Dungait, J.A.; Bol, R.; Lopez-Capel, E.; Bull, I.D.; Chadwick, D.; Amelung, W.; Granger, S.J.; Manning, D.A.C.; Evershed, R.P. Applications of stable isotope ratio mass spectrometry in cattle dung carbon cycling studies. Rapid Commun. Mass Spectrom. 2010, 24, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Dungait, J.; Ateerear, N.A.; van Dongen, B.E.; Bol, R.; Evershed, R.P. Off-line pyrolysis and compound-specific stable carbon isotope analysis of lignin moieties: A new method for determining the fate of lignin residues in soil. Rapid Commun. Mass Spectrom. 2008, 22, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Treignier, C.; Tolosa, I.; Grover, R.; Reynaud, S.; sa Ferrier-Pagé, C. Carbon isotope composition of fatty acids and sterols in the scleractinian coral Turbinaria reniformis: Effect of light and feeding. Limnol. Oceanogr. 2009, 54, 1933–1940. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Schmidt, C.M.; Heide, K.; Gleixner, G. δ13C values of pyrolysis products from cellulose and lignin represent the isotope content of their precursors. J. Anal. Appl. Pyrolysis 2006, 75, 19–26. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; Jiménez-Morillo, N.T.; de la Rosa, J.M.; Almendros, G.; González-Vila, F.J. Compound-specific stable carbon isotopic signature of carbohydrate pyrolysis products from C3 and C4 plants. J. Sci. Food Agric. 2016, 96, 948–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llana-Ruíz-Cabello, M.; Pichardo, S.; Jiménez-Morillo, N.T.; Bermúdez, J.M.; Aucejo, S.; González-Vila, F.J.; Cameán, A.M.; González-Pérez, J.A. Molecular characterization of a bio-based active packaging containing Origanum vulgare L. essential oil using pyrolysis gas chromatography-mass spectrometry (Py-GC/MS). J. Sci. Food Agric. 2016, 96, 3207–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleixner, G.; Bol, R.; Balesdent, J. Molecular insight into soil carbon turnover. Rapid Commun. Mass Spectrom. 1999, 13, 1278–1283. [Google Scholar] [CrossRef]
- Jiménez-González, M.A.; Álvarez, A.M.; Carral, P.; González-Pérez, J.A.; Almendros, G. Climate variability in Mediterranean ecosystems is reflected by soil organic matter pyrolytic fingerprint. Geoderma 2020, 374, 114443. [Google Scholar] [CrossRef]
- Ralph, J.; Hatfield, R.D. Pyrolysis-GC-MS characterization of forage materials. J. Agric. Food Chem. 1991, 39, 1426–1437. [Google Scholar] [CrossRef]
- Schimmelmann, A.; Qi, H.; Coplen, T.B.; Brand, W.A.; Fong, J.; Meier-Augenstein, W.; Kemp, H.F.; Toman, B.; Ackermann, A.; Assonov, S.; et al. Organic reference materials for hydrogen, carbon, and nitrogen stable isotope-ratio measurements: Caffeines, -alkanes, fatty acid methyl esters, glycines, L-valines, polyethylenes, and oils. Anal. Chem. 2016, 88, 4294–4302. [Google Scholar] [CrossRef] [Green Version]
- Coplen, T.B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 2011, 25, 2538–2560. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, O.; de la Rosa, J.M.; González-Pérez, J.A.; Knicker, H.; Pezzolla, D.; Gigliotti, G.; Provenzano, M.R. Molecular characterization of digestates from solid-state anaerobic digestion of pig slurry and straw using analytical pyrolysis. J. Anal. Appl. Pyrolysis 2018, 134, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Song, H.; Pan, S.; Dong, N.; Wang, X.; Sun, S. Initial pyrolysis mechanism and product formation of cellulose: An Experimental and Density functional theory (DFT) study. Sci. Rep. 2020, 10, 3626. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Zühlke, D.; Becher, D.; Riedel, K.; Baldrian, P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 2016, 6, 25279. [Google Scholar] [CrossRef]
- Khiyami, M.A.; Pometto, A.L.; Brown, R.C. Detoxification of corn stover and corn starch pyrolysis liquors by Pseudomonas putida and Streptomyces setonii suspended cells and plastic compost support biofilms. J. Agric. Food Chem. 2005, 53, 2978–2987. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Cheng, S.; Fang, H.; Pei, J.; Xu, M.; Lu, M.; Yang, Y.; Cao, Z.; Li, Y. Different molecular characterization of soil particulate fractions under N deposition in a subtropical forest. Forests 2019, 10, 914. [Google Scholar] [CrossRef] [Green Version]
- Thevenot, M.; Dignac, M.F.; Rumpel, C. Fate of lignins in soils: A review. Soil Biol. Biochem. 2010, 42, 1200–1211. [Google Scholar] [CrossRef]
- Ertel, J.R.; Hedges, J.I. The lignin component of humic substances: Distribution among soil and sedimentary humic, fulvic, and base-insoluble fractions. Geochim. Cosmochim. Acta 1984, 48, 2065–2074. [Google Scholar] [CrossRef]
- José, C.; Gutiérrez, A.; Rodríguez, I.M.; Ibarra, D.; Martinez, A.T. Composition of non-woody plant lignins and cinnamic acids by Py-GC/MS, Py/TMAH and FT-IR. J. Anal. Appl. Pyrolysis 2007, 79, 39–46. [Google Scholar] [CrossRef]
- Buranov, A.U.; Mazza, G. Lignin in straw of herbaceous crops. Ind. Crop. Prod. 2008, 28, 237–259. [Google Scholar] [CrossRef]
- Arias, M.E.; Polvillo, O.; Rodríguez, J.; Hernández, M.; Molina, J.M.; González-Pérez, J.A.; González-Vila, F.J. Effect of different Streptomyces strains on pine wood as seen by Py–GC/MS: Implications for mechanical pulping. J. Anal. Appl. Pyrolysis 2005, 74, 138–144. [Google Scholar] [CrossRef]
- Dignac, M.F.; Pechot, N.; Thevenot, M.; Lapierre, C.; Bahri, H.; Bardoux, G.; Rumpel, C. Isolation of soil lignins by combination of ball-milling and cellulolysis: Evaluation of purity and isolation efficiency with pyrolysis/GC/MS. J. Anal. Appl. Pyrolysis 2009, 85, 426–430. [Google Scholar] [CrossRef]
- Bracewell, J.M.; Robertson, G.W. Quantitative comparison of the nitrogen-containing pyrolysis products and amino acid composition of soil humic acids. J. Anal. Appl. Pyrolysis 1984, 6, 19–29. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; Martin-Sanchez, P.M.; González-Pérez, J.A.; Hermosín, B. Analytical pyrolysis of the fungal melanins from Ochroconis spp. isolated from Lascaux Cave, France. Appl. Sci. 2021, 11, 1198. [Google Scholar] [CrossRef]
- Fabbri, D.; Adamiano, A.; Falini, G.; de Marco, R.; Mancini, I. Analytical pyrolysis of dipeptides containing proline and amino acids with polar side chains. Novel 2, 5-diketopiperazine markers in the pyrolysates of proteins. J. Anal. Appl. Pyrolysis 2012, 95, 145–155. [Google Scholar] [CrossRef]
- Tsuge, S.; Matsubara, H. High-resolution pyrolysis-gas chromatography of proteins and related materials. J. Anal. Appl. Pyrolysis 1985, 8, 49–64. [Google Scholar] [CrossRef]
- Gleixner, G.; Poirier, N.; Bol, R.; Balesdent, J. Molecular dynamics of organic matter in a cultivated soil. Org. Geochem. 2002, 33, 357–366. [Google Scholar] [CrossRef]
- Fukushima, M.; Tu, X.; Aneksampant, A.; Tanaka, A. Analysis of branched-chain fatty acids in humic substances as indices for compost maturity by pyrolysis–gas chromatography/mass spectrometry with tetramethylammonium hydroxide (TMAH-py–GC/MS). J. Mater. Cycles Waste Manag. 2018, 20, 176–184. [Google Scholar] [CrossRef]
- Angst, G.; John, S.; Mueller, C.W.; Kögel-Knabner, I.; Rethemeyer, J. Tracing the sources and spatial distribution of organic carbon in subsoils using a multi-biomarker approach. Sci. Rep. 2016, 6, 29478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, C.M.; Eglinton, T.I.; Palić, R.; Benitez-Nelson, B.C.; Stojanović, G.; Palić, I.; Djordjevic, S.; Eglinton, G. Even carbon number predominance of plant wax n-alkanes: A correction. Org. Geochem. 2000, 31, 331–336. [Google Scholar] [CrossRef]
- Brooks, P.W.; Maxwell, J.R. Early-stage fate of phytol in a recently deposited lacustrine sediment. In Advances in Organic Geochemistry; Tissot, B., Bienner, F., Eds.; Editions Technip: Paris, France, 1973; pp. 977–991. [Google Scholar]
- Rajiv, P.; Rajeshwari, S.; Venckatesh, R. Fourier transform-infrared spectroscopy and gas chromatography–mass spectroscopy: Reliable techniques for analysis of Parthenium mediated vermicompost. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 116, 642–645. [Google Scholar] [CrossRef]
- Rossmann, A.; Butzenlechner, M.; Schmidt, H.L. Evidence for a nonstatistical carbon isotope distribution in natural glucose. Plant Physiol. 1991, 96, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Chikaraishi, Y.; Naraoka, H. δ13C and δD relationships among three n-alkyl compound classes (n-alkanoic acid, n-alkane and n-alkanol) of terrestrial higher plants. Org. Geochem. 2007, 38, 198–215. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Y.; Huang, X.; Qiu, R.; Zhang, Z.; Meyers, P.A. Comparison of n-alkane molecular, carbon and hydrogen isotope compositions of different types of plants in the Dajiuhu peatland, central China. Org. Geochem. 2018, 124, 1–11. [Google Scholar] [CrossRef]
- Kracht, O.; Gleixner, G. Isotope analysis of pyrolysis products from Sphagnum peat and dissolved organic matter from bog water. Org. Geochem. 2000, 31, 645–654. [Google Scholar] [CrossRef]
- Katsumi, N.; Yonebayashi, K.; Okazaki, M.; Nishiyama, S.; Nishi, T.; Hosaka, A.; Watanabe, C. Characterization of soil organic matter with different degrees of humification using evolved gas analysis-mass spectrometry. Talanta 2016, 155, 28–37. [Google Scholar] [CrossRef]
- Bezabih, M.; Pellikaan, W.F.; Tolera, A.; Hendriks, W.H. Evaluation of n-alkanes and their carbon isotope enrichments (δ13C) as diet composition markers. Animal 2011, 5, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werth, M.; Kuzyakov, Y. 13C fractionation at the root–microorganisms–soil interface: A review and outlook for partitioning studies. Soil Biol. Biochem. 2010, 42, 1372–1384. [Google Scholar] [CrossRef]
- Kufka, D.; Bucha, M.; Pleśniak, Ł.; Jędrysek, M.O. Stable isotopes of C and H in methane fermentation of agriculture substrates at different temperature conditions. Open Geosci. 2019, 11, 471–481. [Google Scholar] [CrossRef]
- Haubert, D.; Birkhofer, K.; Fließbach, A.; Gehre, M.; Scheu, S.; Ruess, L. Trophic structure and major trophic links in conventional versus organic farming systems as indicated by carbon stable isotope ratios of fatty acids. Oikos 2009, 118, 1579–1589. [Google Scholar] [CrossRef]
- Mathur, S.P.; Owen, G.; Dinel, H.; Schnitzer, M. Determination of compost biomaturity. I. Literature review. Biol. Agric. Hortic. 1993, 10, 65–85. [Google Scholar] [CrossRef]
- Golueke, C.G. Principles of biological resources recovery. Biocycle 1981, 22, 36–40. [Google Scholar]

| Origin 1 | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| PS | 27.3 b,* | 14.8 b | 19.3 b | 19.7 b | 23.0 c |
| ARO | 5.3 a,b,c | 3.8 b | 5.1 b | 5.6 b | 6.5 c |
| N | 4.6 b,c | 4.2 b | 4.1 b | 3.8 a,b | 5.4 c |
| LH | 9.9 d | 9.3 c | 12.0 c | 11.9 b | 17.5 d |
| LG | 15.2 b,c | 16.2 b | 18.02 b | 17.6 b | 20.6 c |
| LS | 11.5 c | 16.1 b | 18.8 b,* | 14.0 b | 15.2 c |
| LIP | 0.68 a | 0.54 a,b | 0.46 a | 0.37 a | 0.12 a |
| FA | 12.6 c | 9.3 b | 9.0 b | 7.8 a,b | 5.6 c |
| FAME | 0.9 a | 2.0 a,* | 1.6 a | 1.1 a | 0.3 a |
| ISO | 3.0 a,b | 2.8 a,b | 1.3 a,b | 1.7 a,b | 1.3 b |
| UNK | 1.1 a,b | 2.07 a,b | 1.1 a,b | 1.5 a,b | 0.5 a |
| ALK | 4.0 a,b | 7.4 a,b,* | 4.3 a,b | 4.7 a | 2.0 a |
| TOC | 0.8 a,b,c | 2.5 a,b,* | 1.1 a,b | 2.6 a | 0.3 a |
| EST | 0.69 a,b | 6.4 a,* | 1.8 a | 5.9 a | 0.7 a |
| T0 | T1 | T2 | T3 | T4 | |
|---|---|---|---|---|---|
| Polysaccharide peaks (%) | 27.3 | 14.8 | 19.3 | 19.7 | 23.0 |
| Total lignin peaks (%) | 37.2 | 42.2 | 49.5 | 44.0 | 53.5 |
| Total lignin/polysaccharides | 1.4 | 2.9 | 2.6 | 2.2 | 2.3 |
| LG a/polysaccharides | 0.79 | 1.1 | 1.0 | 0.92 | 0.9 |
| LG/LS b | 1.9 | 1.1 | 1.0 | 1.3 | 1.4 |
| Ph-C1 c | 1.5 | 2.4 | 2.6 | 2.0 | 3.6 |
| Ph-C2 d | 8.5 | 8.7 | 9.2 | 9.7 | 11.2 |
| Ph-C3 e | 5.0 | 7.8 | 7.8 | 7.2 | 5.6 |
| Ph-C1 + C2/Ph-C3 f | 2.0 | 1.4 | 1.5 | 1.6 | 2.6 |
| Ketones | 1.7 | 2.6 | 2.7 | 1.7 | 2.1 |
| Acids | 2.2 | 3.0 | 4.2 | 1.9 | 2.6 |
| Aldehydes | 2.4 | 2.1 | 3.2 | 3.1 | 2.3 |
| Al/K + Ac g | 0.62 | 0.38 | 0.47 | 0.86 | 0.48 |
| Origin 1 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|
| C content (%) | 21.4 (4.7) a | 25.8 (3.8) a | 33.6 (2.5) b | 30.1 (0.5) b |
| N content (%) | 1.2 (0.2) a | 1.4 (0.1) a | 1.7 (0.01) b | 1.4 (0.01) a |
| C:N ratio | 17.4 | 18.5 | 19.3 | 21.9 |
| δ13C bulk (‰) | −14.6 (0.3) a | −14.3 (0.3) a | −13.4 (0.01) b | −13.07 (0.02) c |
| δ15N bulk (‰) | 13.5 (0.3) b | 14.01 (0.6) bc | 15.0 (0.4) c | 9.7 (0.4) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San-Emeterio, L.M.; López-Núñez, R.; González-Vila, F.J.; González-Pérez, J.A. Evolution of Composting Process in Maize Biomass Revealed by Analytical Pyrolysis (Py-GC/MS) and Pyrolysis Compound Specific Isotope Analysis (Py-CSIA). Appl. Sci. 2021, 11, 6684. https://doi.org/10.3390/app11156684
San-Emeterio LM, López-Núñez R, González-Vila FJ, González-Pérez JA. Evolution of Composting Process in Maize Biomass Revealed by Analytical Pyrolysis (Py-GC/MS) and Pyrolysis Compound Specific Isotope Analysis (Py-CSIA). Applied Sciences. 2021; 11(15):6684. https://doi.org/10.3390/app11156684
Chicago/Turabian StyleSan-Emeterio, Layla M., Rafael López-Núñez, Francisco J. González-Vila, and José A. González-Pérez. 2021. "Evolution of Composting Process in Maize Biomass Revealed by Analytical Pyrolysis (Py-GC/MS) and Pyrolysis Compound Specific Isotope Analysis (Py-CSIA)" Applied Sciences 11, no. 15: 6684. https://doi.org/10.3390/app11156684
APA StyleSan-Emeterio, L. M., López-Núñez, R., González-Vila, F. J., & González-Pérez, J. A. (2021). Evolution of Composting Process in Maize Biomass Revealed by Analytical Pyrolysis (Py-GC/MS) and Pyrolysis Compound Specific Isotope Analysis (Py-CSIA). Applied Sciences, 11(15), 6684. https://doi.org/10.3390/app11156684









