Detection and Model of Thermal Traces Left after Aggressive Behavior of Laboratory Rodents
Abstract
:1. Introduction
- Investigate the influence of corner detector parameters on the detection of interesting points related to saliva traces.
- Investigate the influence of the properties of the simulated saliva traces on the detection results.
- Investigate the conditions when the cooling–heating model describing the temperature behavior of the saliva trace could be successfully fitted to the observable data.
- Answer the question if the cooling-heating model could be potentially used to estimate the exact bite time even, when the bite event is not directly recorded (e.g., as one animal covers another, due to the limited camera frame rate, etc.).
2. Materials and Methods
2.1. Thermal Data Acquisition
2.2. Data Simulation Methods
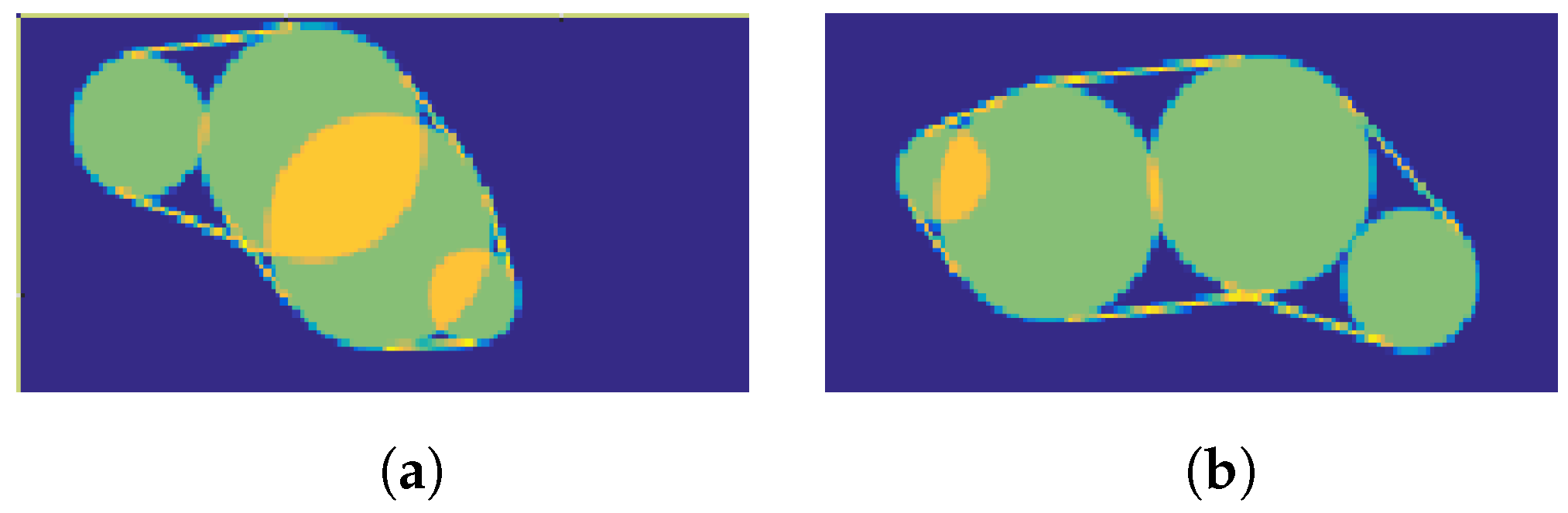
2.2.1. Shape Simulation
2.2.2. Texture Simulation
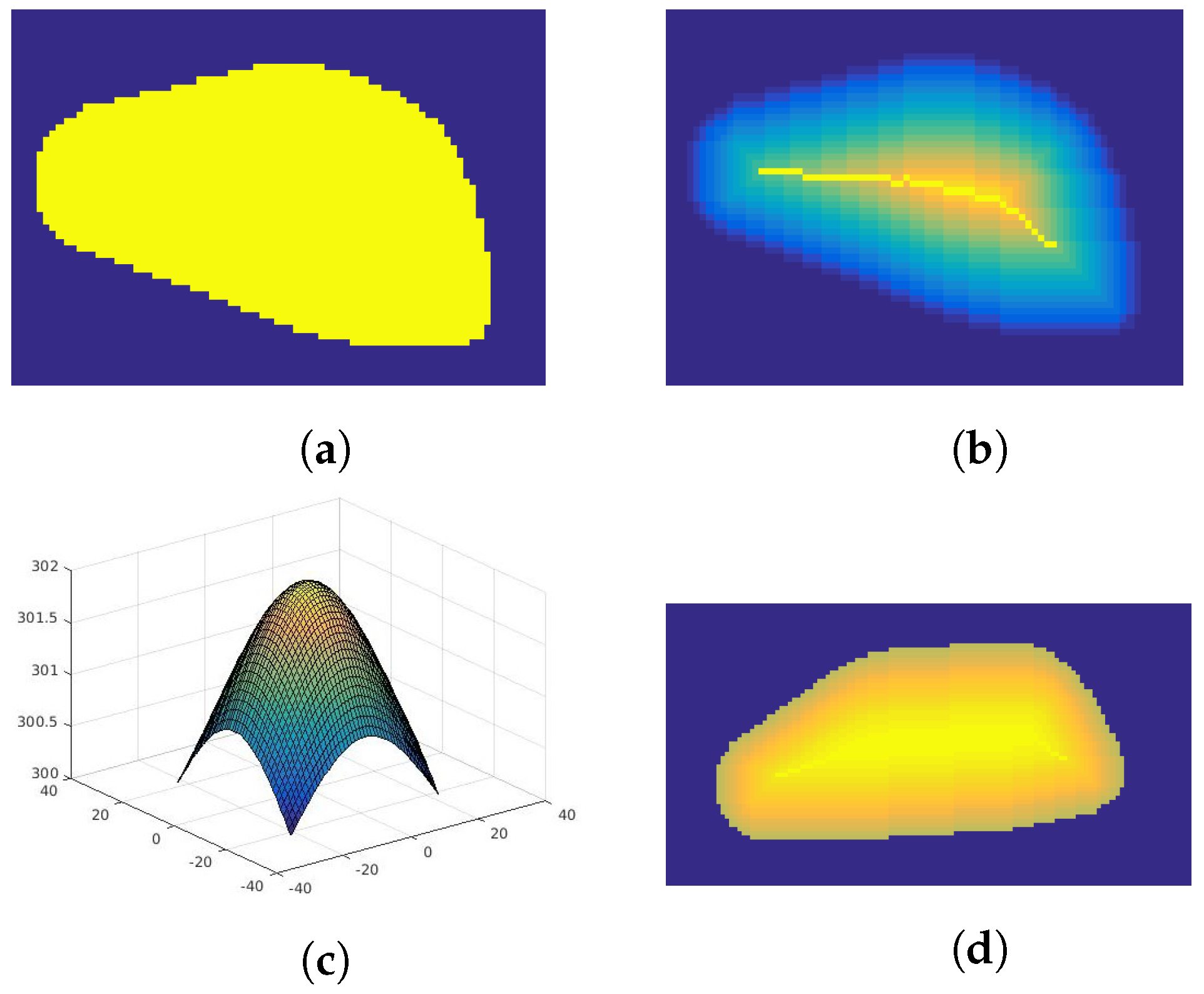
- Division of the area into subregions—uses skeletonization method based on the parallel thinning of the object described in [26]. This algorithm iteratively removes external pixels in such a way that the object (Figure 2a) shrinks to the middle line (dorsum). Figure 2b presents this method with each iteration marked with different color. The color is distributed linearly.
- Assigning temperature to each subregion—a process of sampling Gaussian distribution (Figure 2c) according to the number of iteration steps and normalization to the surface temperature of the body range. Each subsequent subdivision determined by the skeletonization algorithm is given the next temperature value according to the created distribution (Figure 2d). The degree of variability of the simulated texture can be adjusted by the value of the distribution variance.
2.2.3. Motion Simulation
2.2.4. Bite Event Simulation
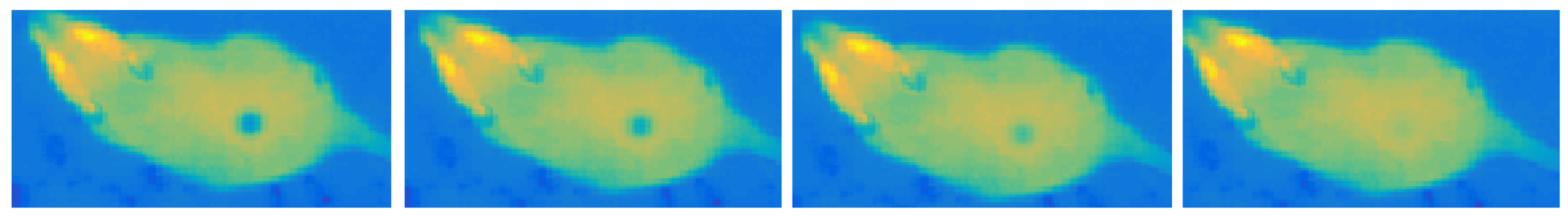
2.2.5. Temporal Changes Simulation
- cooling—very short and often unnoticeable, associated with the rapid heat dissipation by the liquid;
- heating—long-lasting temperature increase up to the ambient temperature caused by the drying, results in a gradual deterioration of visibility until the trace disappears completely.
3. Data Analysis Methods
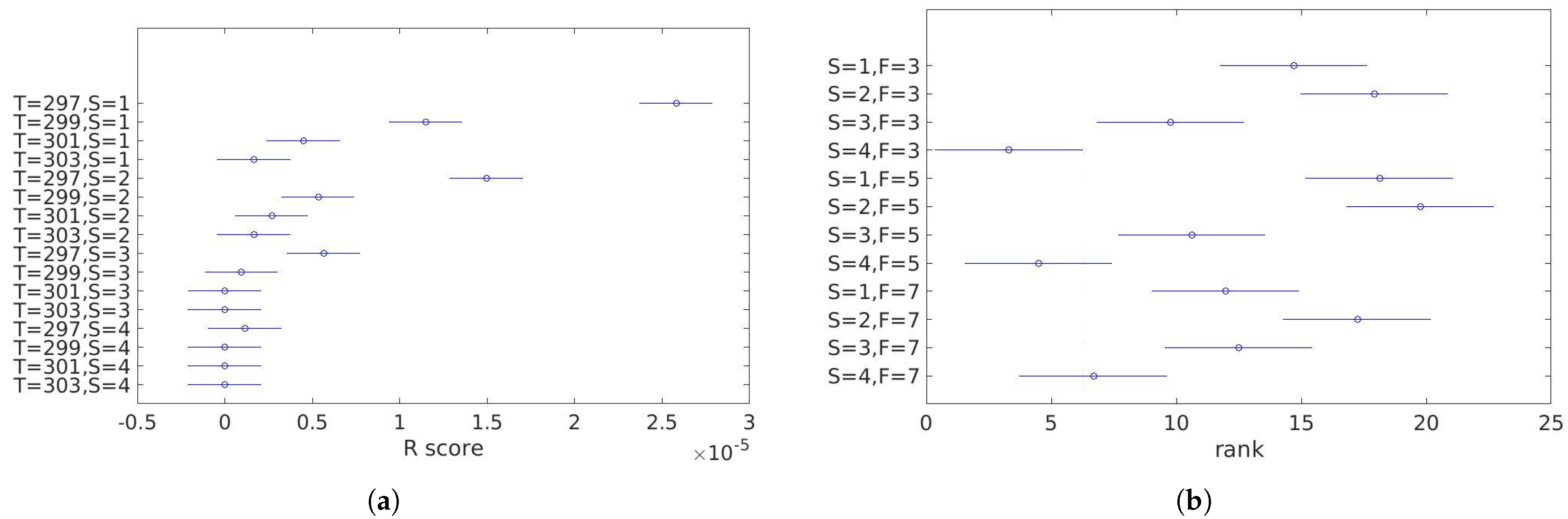
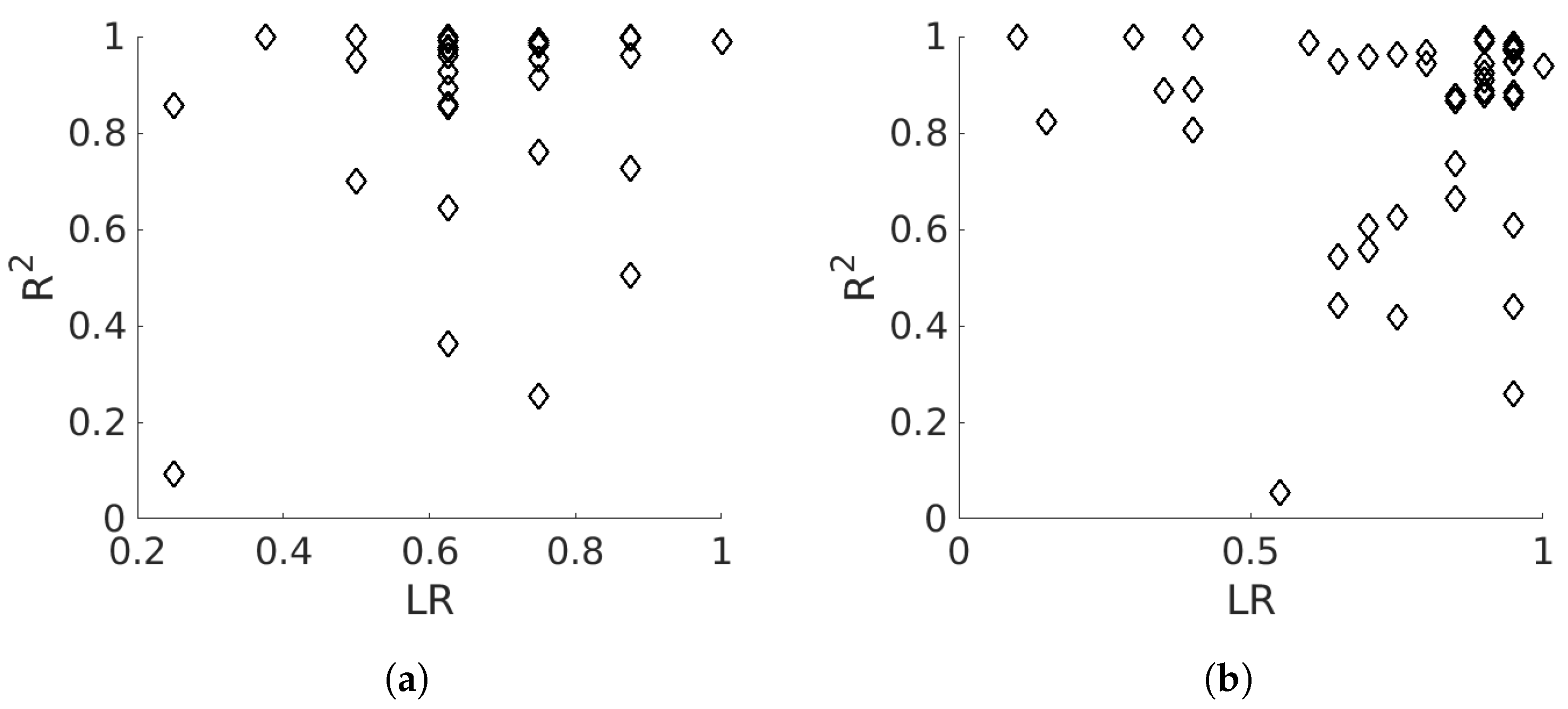
3.1. Corner Detection
- the Corner Response Score (R score),for Harris detector defined aswherei —eigenvalues of the covariance matrix of the pixel intensity gradient,k—tunable sensitivity parameter.
- rank—the position on the list of corners sorted in a descending order by the R score.
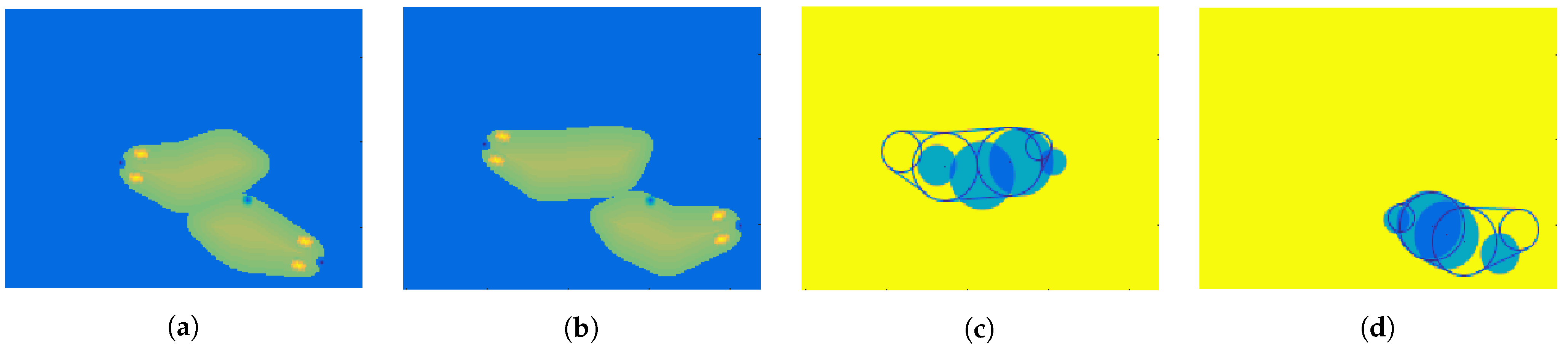
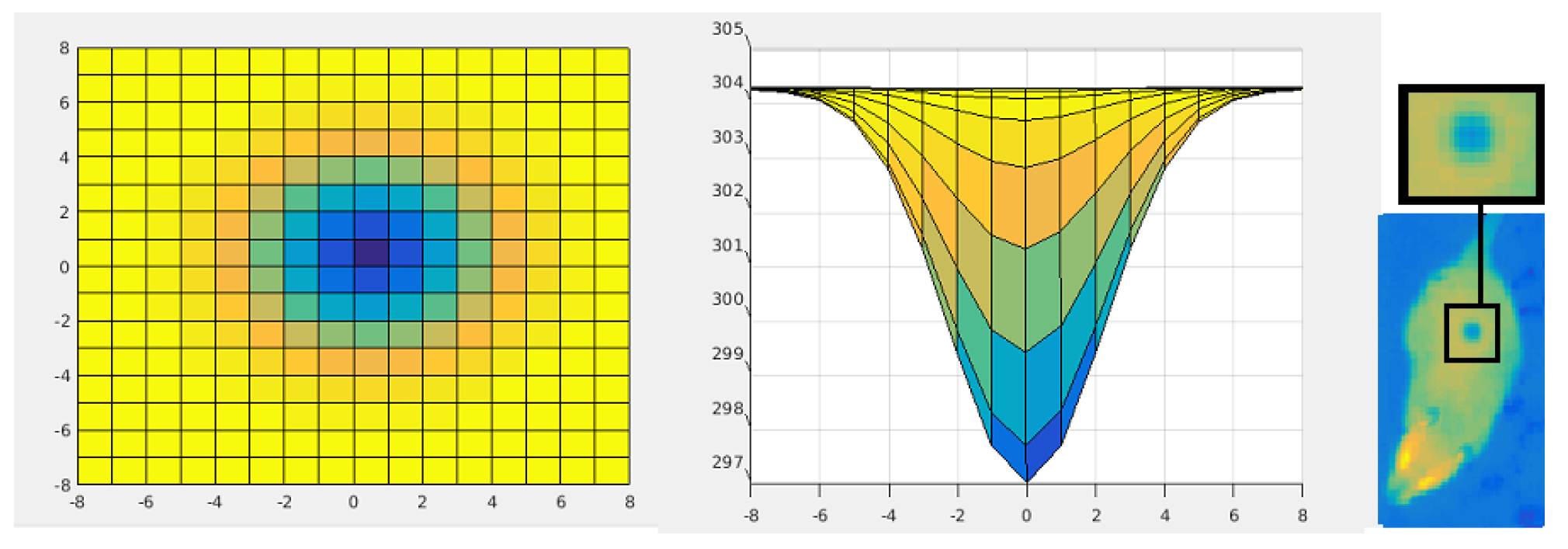
3.2. Static Analysis
- temperature: 297, 299, 301, and 303 K (Figure 5),
- size and pixel intensity gradient—four stages (Figure 6): radius equal 1, 2, 3, or 4 pixels.
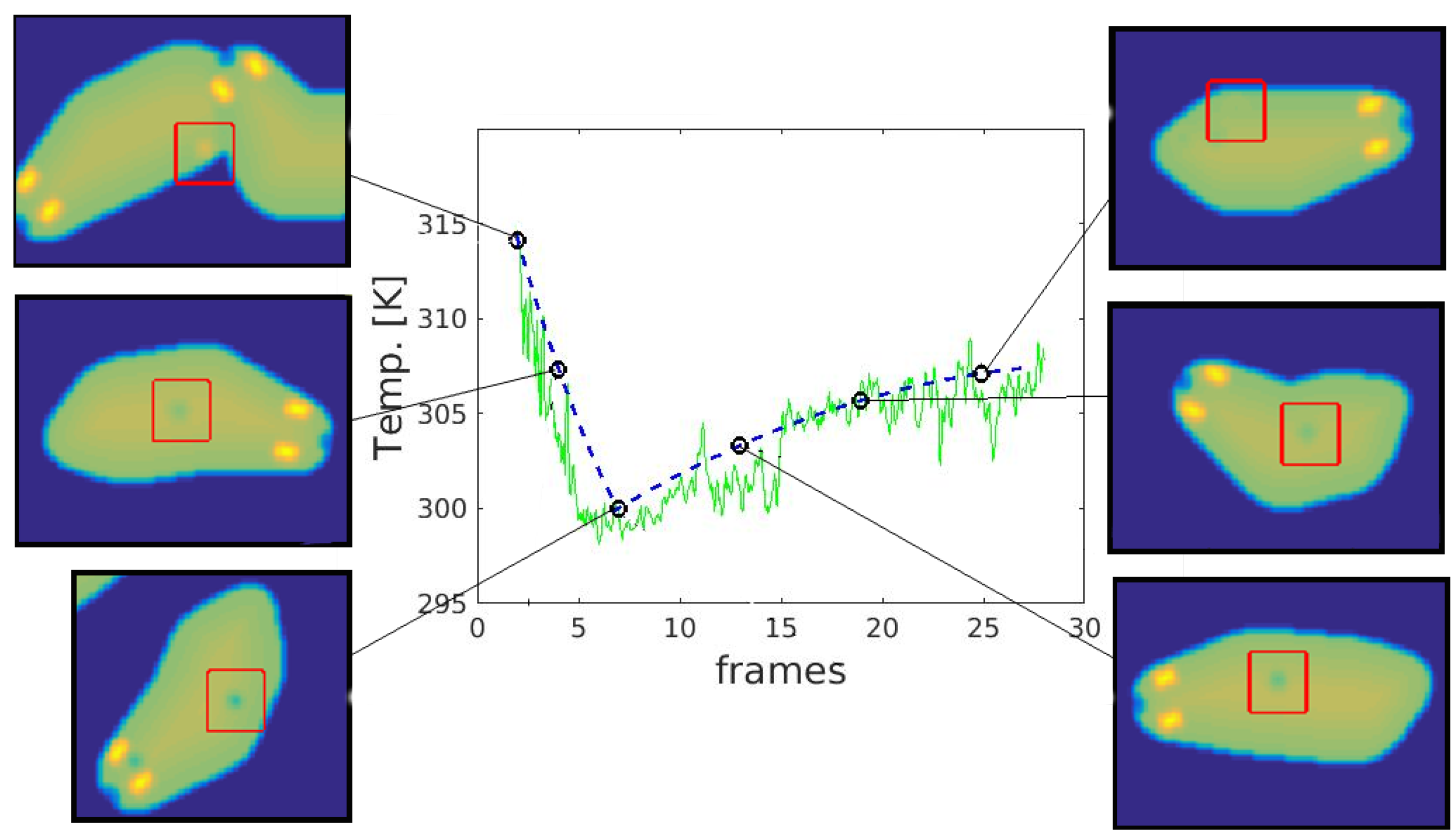
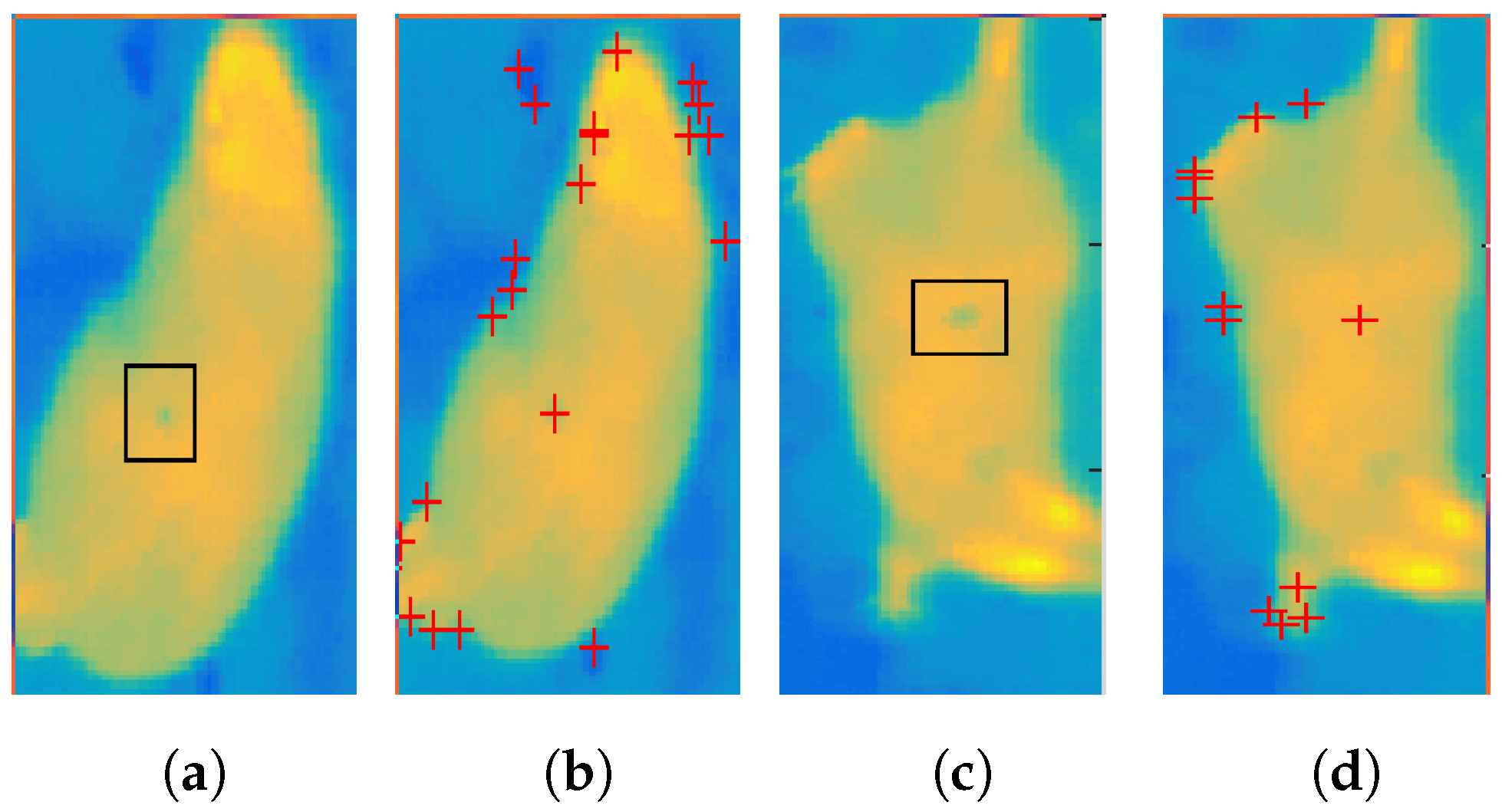
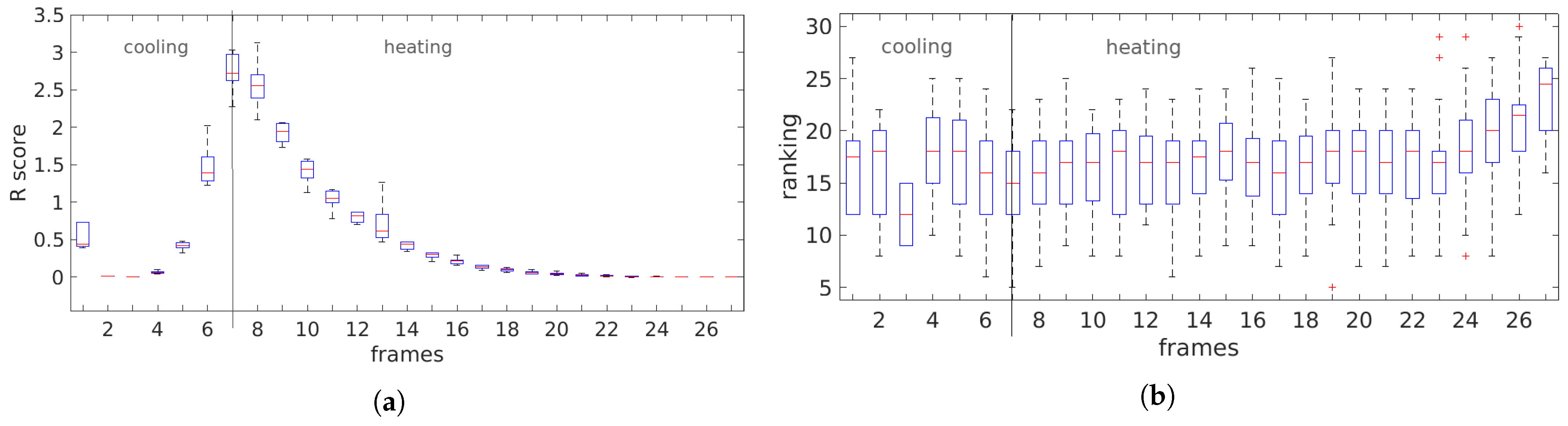
3.3. Temporal Analysis
- computing the significance of corners using a specific measure, e.g., R score or ranking—rejection of irrelevant points,
- elimination of corners that do not meet certain conditions: e.g., temperature value within a certain range, location on the animal’s body, and
- prediction of trace localization based on the animal’s displacement vectors from previous frames.
4. Results
4.1. Static Analysis
4.2. Temporal Analysis
5. Discussion
5.1. Static Analysis
5.2. Temporal Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Martin, P.; Bateson, P. Measuring Behavior: An Introductory Guide; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Burgos-Artizzu, X.P.; Dollar, P.; Lin, D.; Anderson, D.J.; Perona, P. Social behavior recognition in continuous video. In Proceedings of the Conference Computer Vision and Pattern Recognition (CVPR), Providence, RI, USA, 16–21 June 2012; pp. 1322–1329. [Google Scholar] [CrossRef]
- Giancardo, L.; Sona, D.; Huang, H.; Sannino, S.; Manago, F.; Scheggia, D.; Papaleo, F.; Murino, V. Automatic Visual Tracking and Social Behaviour Analysis with Multiple Mice. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noldus, L.P.J.J.; Spink, A.J.; Tegelenbosch, R.A.J. EthoVision: A versatile video tracking system for automation of behavioral experiments. Behav. Res. Methods Instruments Comput. 2001, 33, 398–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drai, D.; Benjamini, Y.; Golani, I. Statistical discrimination of natural modes of motion in rat exploratory behavior. J. Neurosci. Methods 2000, 96, 119–131. [Google Scholar] [CrossRef]
- Drai, D.; Golani, I. SEE: A tool for the visualization and analysis of rodent exploratory behavior. Neurosci. Biobehav. Rev. 2001, 25, 409–426. [Google Scholar] [CrossRef]
- Peters, S.M.; Pinter, I.J.; Pothuizen, H.H.; de Heer, R.C.; van der Harst, J.E.; Spruijt, B.M. Novel approach to automatically classify rat social behavior using a video tracking system. J. Neurosci. Methods 2016, 268. [Google Scholar] [CrossRef] [PubMed]
- Casarrubea, M.; Jonsson, G.K.; Faulisi, F.; Sorbera, F.; Di Giovanni, G.; Benigno, A.; Crescimanno, G.; Magnusson, M.S. T-pattern analysis for the study of temporal structure of animal and human behavior: A comprehensive review. J. Neurosci. Methods 2015, 239, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, E.A.; Noldus, L.P.; van Gerven, M.A. Deep learning improves automated rodent behavior recognition within a specific experimental setup. J. Neurosci. Methods 2020, 332, 108536. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Duan, F. Rat Behavior Observation System Based on Transfer Learning. IEEE Access 2019, 7, 62152–62162. [Google Scholar] [CrossRef]
- Lee, S.; Hayakawa, T.; Nishimura, C.; Yawata, S.; Yagi, H.; Watanabe, D.; Ishikawa, M. Comparison of Deep Learning and Image Processing for Tracking the Cognitive Motion of a Laboratory Mouse. In Proceedings of the 2019 IEEE Biomedical Circuits and Systems Conference (BioCAS), Nara, Japan, 17–19 October 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Lorbach, M.; Kyriakou, E.I.; Poppe, R.; van Dam, E.A.; Noldus, L.P.J.J.; Veltkamp, V.R. Learning to recognize rat social behavior: Novel dataset and cross-dataset application. J. Neurosci. Methods 2018, 300, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Kennedy, A.; Burgos-Artizzu, X.P.; Zelikowsky, M.; Navonne, S.G.; Perona, P.; Anderson, D.J. Automated measurement of mouse social behaviors using depth sensing, video tracking, and machine learning. Proc. Natl. Acad. Sci. USA 2015, 112, E5351–E5360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.; Annie, A.N.; Ciernia, V.; Lee, Y.J. Who Moved My Cheese? In Automatic Annotation of Rodent Behaviors with Convolutional Neural Networks. In Proceedings of the 2017 IEEE Winter Conference on Applications of Computer Vision (WACV), Santa Rosa, CA, USA, 24–31 March 2017; pp. 1277–1286. [Google Scholar] [CrossRef]
- Crispim-Junior, C.F.; Mendes de Azevedo, F.; Marino-Neto, J. What is my rat doing? Behavior understanding of laboratory animals. Pattern Recognit. Lett. 2017, 94, 134–143. [Google Scholar] [CrossRef]
- Wang, Z.; Mirbozorgi, S.A.; Ghovanloo, M. An automated behavior analysis system for freely moving rodents using depth image. Med. Biol. Eng. Comput. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mufford, J.T.; Paetkau, M.J.; Flood, N.J.; Regev-Shoshani, G.; Miller, C.C.; Church, J.S. The development of a non-invasive behavioral model of thermal heat stress in laboratory mice (Mus musculus). J. Neurosci. Methods 2016, 268, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Milecka, M.; Ruminski, J. Automatic analysis of the aggressive behavior of laboratory animals using thermal video processing. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Korea, 11–15 July 2017; pp. 3827–3830. [Google Scholar] [CrossRef]
- Vianna, D.M.; Carrive, P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur. J. Neurosci. 2005, 21, 2505–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marks, A.; Vianna, D.M.L.; Carrive, P. Nonshivering thermogenesis without interscapular brown adipose tissue involvement during conditioned fear in the rat. Am. J.-Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R1239–R1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giancardo, L.; Sona, D.; Scheggia, D.; Papaleo, F.; Murino, V. Segmentation and Tracking of Multiple Interacting Mice by Temperature and Shape Information. In Proceedings of the 21st Internationl Conference on Pattern Recognition, Tsukuba, Japan, 11–15 November 2012; pp. 2520–2523. [Google Scholar]
- Decker, C.; Hamprecht, F.A. Detecting individual body parts improves mouse behavior classification. In Proceedings of the Workshop on Visual Observation and Analysis of Vertebrate and Insect Behavior, Stockholm, Sweden, 24 August 2014. [Google Scholar]
- Lorbach, M.; Poppe, R.; van Dam, E.A.; Noldus, L.P.J.J.; Veltkamp, R.C. Automated Recognition of Social Behavior in Rats: The Role of Feature Quality. In Proceedings of the Image Analysis and Processing—ICIAP 2015, Genoa, Italy, 7–11 September 2015; Murino, V., Puppo, E., Eds.; Springer International Publishing: Cham, Switerland, 2015; pp. 565–574. [Google Scholar] [CrossRef] [Green Version]
- Albonetti, M.E.; Farabollini, F. Social stress by repeated defeat: Effects on social behavior and emotionality. Behav. Brain Res. 1994, 62, 187–193. [Google Scholar] [CrossRef]
- Mazur-Milecka, M.; Ruminski, J. The analysis of temperature changes of the saliva traces left on the fur during laboratory rats social contacts. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–22 July 2018; pp. 2607–2610. [Google Scholar] [CrossRef]
- Lam, L.; Lee, S.; Suen, C.Y. Thinning methodologies—A comprehensive survey. IEEE Trans. Pattern Anal. Mach. Intell. 1992, 14, 869–885. [Google Scholar] [CrossRef] [Green Version]
- Schuldt, C.; Laptev, I.; Caputo, B. Recognizing human actions: A local SVM approach. In Proceedings of the ICPR, Cambridge UK, 26–26 August 2004; pp. 32–36. [Google Scholar] [CrossRef]
- Schmidt, C.; Mohr, R.; Bauckhage, C. Evaluation of interest point detectors. IJCV 2000, 37, 151–172. [Google Scholar] [CrossRef] [Green Version]
- Harris, C.; Stephens, M. A combined corner and edge detector. In Proceedings of the Alvey Vision Conferen, Manchester, UK, 31 August–2 September 1988; pp. 147–152. [Google Scholar] [CrossRef]
- Shi, J.; Tomasi, C. Good features to track. In Proceedings of the CVPR, Seattle, WA, USA, 21–23 June 1994. [Google Scholar] [CrossRef]
- Rosten, E.; Drummond, T. Fusing Points and Lines for High Performance Tracking. In Proceedings of the IEEE International Conference on Computer Vision, Beijing, China, 17–21 October 2005; pp. 1508–1511. [Google Scholar] [CrossRef]
- Mazur-Milecka, M. Thermal imaging in automatic rodent’s social behaviour analysis. In Proceedings of the QIRT, Gdansk, Poland, 4–8 July 2016; pp. 563–569. [Google Scholar] [CrossRef]
- Kwasniewska, A.; Ruminski, J.; Szankin, M.; Kaczmarek, M. Super-resolved thermal imagery for high-accuracy facial areas detection and analysis. Eng. Appl. Artif. Intell. 2020, 87, 103263. [Google Scholar] [CrossRef]



| Harris | FAST | |||
|---|---|---|---|---|
| R Score | Rank | R Score | Rank | |
| temperature | 0 | 0 | 0 | 0 |
| size of a trace | 0 | 0 | 0 | 0 |
| filter size | 0 | 0.1241 | - | - |
| temperature ∗ size of a trace | 0 | 0 | 0 | 0 |
| temperature ∗ filter size | 0 | 0.0023 | - | - |
| size of a trace ∗ filter size | 0 | 0.0165 | - | - |
| temperature ∗ size of a trace ∗ filter size | 0 | 0.3671 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur-Milecka, M.; Ruminski, J.; Glac, W.; Glowacka, N. Detection and Model of Thermal Traces Left after Aggressive Behavior of Laboratory Rodents. Appl. Sci. 2021, 11, 6644. https://doi.org/10.3390/app11146644
Mazur-Milecka M, Ruminski J, Glac W, Glowacka N. Detection and Model of Thermal Traces Left after Aggressive Behavior of Laboratory Rodents. Applied Sciences. 2021; 11(14):6644. https://doi.org/10.3390/app11146644
Chicago/Turabian StyleMazur-Milecka, Magdalena, Jacek Ruminski, Wojciech Glac, and Natalia Glowacka. 2021. "Detection and Model of Thermal Traces Left after Aggressive Behavior of Laboratory Rodents" Applied Sciences 11, no. 14: 6644. https://doi.org/10.3390/app11146644
APA StyleMazur-Milecka, M., Ruminski, J., Glac, W., & Glowacka, N. (2021). Detection and Model of Thermal Traces Left after Aggressive Behavior of Laboratory Rodents. Applied Sciences, 11(14), 6644. https://doi.org/10.3390/app11146644






