Abstract
Non-ionizing millimeter-waves (MMW) interact with cells in a variety of ways. Here the inhibited cell division effect was investigated using 85–105 GHz MMW irradiation within the International Commission on Non-Ionizing Radiation Protection (ICNIRP) non-thermal 20 mW/cm2 safety standards. Irradiation using a power density of about 1.0 mW/cm2 SAR over 5–6 h on 50 cells/μL samples of Saccharomyces cerevisiae model organism resulted in 62% growth rate reduction compared to the control (sham). The effect was specific for 85–105 GHz range and was energy- and cell density-dependent. Irradiation of wild type and Δrad52 (DNA damage repair gene) deleted cells presented no differences of colony growth profiles indicating non-thermal MMW treatment does not cause permanent genetic alterations. Dose versus response relations studied using a standard horn antenna (~1.0 mW/cm2) and compared to that of a compact waveguide (17.17 mW/cm2) for increased power delivery resulted in complete termination of cell division via non-thermal processes supported by temperature rise measurements. We have shown that non-thermal MMW radiation has potential for future use in treatment of yeast related diseases and other targeted biomedical outcomes.
1. Introduction
The influence of millimeter wave (MMW) radiation on biological systems has gained prominence in recent years for two important reasons: (1) to establish safety standards for the use of MMWs in communication devices, (2) to understand the mechanisms of interaction between MMW and living systems. These investigations have opened new avenues for potential applications of MMW in the field of biomedical devices, for applications such as selective targeting of cancer cells. MMW in the range of 75–110 GHz (W-band) are classed as non-ionizing radiation because of the low 0.3–0.4 meV range of the energy of its photons.
Cancer, considered to be one of the deadliest human diseases, is challenging to diagnose at early stages [1]. Cancer is known to arise from accumulated mutations in oncogenes and tumor suppressor genes, leading to uncontrolled tumor cell growth [2,3]. Conventional radiation therapy in cancer treatment gives rise to many detrimental side effects [2] including the development of other more dangerous cancers due to the ionizing radiation (involved in such treatments) resulting in mutagenesis [3]. Thermal ablation techniques have been employed as the other alternative but with restricted application in order not to burn normal cells/tissues during treatment. Our experiments demonstrated that non-ionizing MMW (75–105 GHz) exposure with a non-thermal power density of 0.2 mW/cm2 can elicit morphological changes in H1299 human lung cancer cells [4] leading to targeted apoptosis and mortality [5] without harming normal cells under the same exposure conditions. MMWs are also reported to be helpful for detecting different types of cancers [6]. Further, such technologies involving non-ionizing radiation has shown promising applications in the treatment of other diseases [7,8] like gastrointestinal disorders, wound healing, remote monitoring of wounds, non-invasive detection of glucose levels, pain relief, diabetes, dermatitis, etc. However, the exact mechanism of the therapeutic effects on biological specimens is not well understood hindering the wide-scale application of this technology.
Being the simplest eukaryotic organism with a nucleus, Saccharomyces cerevisiae yeast cells are a standard model organism for in vitro studies of cell division applicable up to higher eukaryotes. Many essential cellular processes in yeast and humans are similar, making yeast a suitable system to study basic molecular processes [3,9,10]. About 23% of the yeast genome is conserved in human cells, including all the corresponding biological functions and biochemical pathways remaining the same [11]. Characteristics of tumor cell growth have been discovered using models of yeast cell division [3,9,10]. Yeast cells are a cheap laboratory model that are simple to grow, culture, and experiment making it easier to unravel molecular mechanisms of MMW interactions within cells. Previous studies involving irradiation of wild type Saccharomyces cerevisiae yeast culture in aqueous suspensions have been highly ambiguous [12,13]. These studies reported either no change or increased/decreased rate of growth upon microwave irradiation of 42 GHz and 50 mW power [12,13]. The authors reported the exclusion of thermal effects in such procedures by continuously monitoring temperature during the duration of exposure. On the other hand, MMW irradiation of yeast cells in the range of 41.7–41.8 GHz for 4 h and 20 mW power found frequency sensitive results with increased cell growth at some frequencies and reduced at other values [14]. Another study confirmed the increased growth rate of yeast upon irradiation with 968 MHz for 7 h at 17 dBm power [15]. Results of such studies on the interaction of millimeter waves with biological samples are often met with inconsistence and non-reproducibility due to missing investigations of deeper biological mechanisms like influence on genetic material, the involvement of free radicals in oxidative processes, other metabolic disturbances etc. to correlate with the observed effects [16]. Thus, understanding the mechanisms of action will enable better use of MMW technology for therapeutic applications.
Given the rising challenges of biochemical drug resistance and the adverse effects from ionizing electromagnetic spectra limiting the use of conventional radiation therapies [3], molecular mechanisms of non-ionizing radiation procedures can suggest novel approaches to finding effective solutions. While non-ionizing radiation has mostly been used in thermal ablation procedures, we explored the effect of MMW (85–105 GHz) irradiation on the Saccharomyces cerevisiae yeast model of eukaryotic cell division in this article within the non-thermal safety standards set by the International Commission on Non-Ionizing Radiation Protection (ICNIRP) maintained for the stringency of analyses to unravel novel mechanisms. The MMW were propagated using a standard pyramidal horn antenna in one case and a standard wave guide in another. Radiated power density and power distribution across the aperture was analyzed and the influence of MMW exposure on yeast cells manifesting in the retarded cell growth effect studied. Irradiation of cells with deletion of the RAD52 gene (Δrad52), responsible for DNA damage repair was examined for radiation related genomic perturbations. Temperature rise measurements were conducted for analysis and maintenance of non-thermal exposure conditions. Non-ionizing MMW radiation absorption by cellular water content was investigated. Dosage dependent effects to achieve complete termination of yeast cell division were studied and compared using a waveguide delivering higher energy, which presented novel avenues for microbial infection control.
2. Results
2.1. MMW Irradiation Effects Are Frequency, Energy Dose and Cell Density Dependent
Wild Type (WT) Saccharomyces cerevisiae cells cultured under standard physiological conditions were subjected to 85, 95, 105 GHz MMW exposures using a horn antenna and the effects compared with that of (sham) control. Cell densities (10,000, 1000, 100 and 50 cells/μL) measured using standard optical density (OD) measurements of liquid culture were calibrated to power dosages and treatment durations (1, 2, 3, 4, 5 and 6 h) to unravel the threshold exposure conditions (see Methods) required to elicit the inhibited cell division effect. Subsequently, six separate yeast colonies at the calibrated cellular density (50 cells/µL) and exposure duration (6 h) were irradiated corresponding to each discrete frequency regime. The growth rate and division of treated yeast cells were then examined by incubating them under physiological conditions. 85–105 GHz MMW irradiation at the range 3.48–2.08 W/kg and power densities of 0.83–1.4 mW/cm2 for 6 h of exposure affected the growth rate of (50 cells/µL). WT yeast cells for all the examined frequencies showed reduction in the rate of division by up to 62% as compared to sham (control) (Figure 1 and Table 1). Delay in cell proliferation becomes significantly noticeable over 3–5 h of physiological incubation post-irradiation treatment (Figure 1a). Interestingly, although increasing the value of frequency is correlated with a reduction in respective power densities (see Table 1); the effect on cell proliferation was similar for each discrete frequency. This suggests that anti-proliferative MMW radiation effects was not affected by the amount of power density within the 85–105 GHz range with a constant cell density (50 cells/µL) as long as a minimal threshold was attained. Indeed, a minimal power density of 0.83 ± 0.02 mW/cm2 at 105 GHz (see Table 1) was sufficient to inhibit cell growth and division for 50 cells/µL samples of WT Saccharomyces cerevisiae.
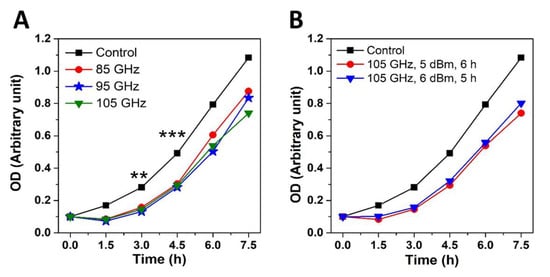
Figure 1.
(A) Growth profiles of BY4741 Saccharomyces cerevisiae (50 cells/µL) cells measured in OD units after irradiation for 6 h at 5 dBm. Plots indicate mean values of growth rate of six separate yeast colonies (n = 6) for each discrete frequency regime. Single Factor Analysis of variance (ANOVA) analysis (** indicates p value < 0.01 and *** indicates p value less than < 0.001) (B) Growth profiles of BY4741 Saccharomyces cerevisiae (50 cells/µL) cells subsequent to irradiations of different time durations and power densities for a constant frequency (and constant energy) at 105 GHz.

Table 1.
Average energy flux at the aperture of the antenna for different frequencies (see an explanation and calculation in material and methods).
Subsequently, the effect of different durations of irradiation (5 h vs. 6 h) and power densities (5 dBm vs. 6 dBm) was examined using constant frequency of 105 GHz and constant energy. Using the same number of cells, the experiment demonstrates that the inhibited cell division effect was MMW dependent (Figure 1b); being absent in the control (sham). We did not observe difference in the delay of the proliferation rate between 5 h (with 6 dBm power) and 6 h (with 5 dBm power) of irradiation. We conjecture that this is so because both exposure regimes involved the same amount of energy (i.e., product of time and power) (see Figure 1b). Single frequency at 105 GHz was used as it affects WT yeast cell growth to the same extent as for all the other examined MMW frequencies (see Figure 1a); but provides a lower bound for power densities (see Table 1) to elicit the targeted outcome. The agar layer thickness was kept constant throughout this and other repeated experiments under similar conditions. Power densities emitted by the horn antenna aperture are presented in Table 1.
2.2. Non-Thermal MMW Wave Irradiation Does Not Cause Genomic DNA Double-Strand Alterations
The effects of MMW irradiation were observed to be persistent even beyond 3–6 h after termination of MMW exposure on the irradiated cells across six separate experiments. Evidently, thermal effects can be ruled out as the same non-thermal threshold powers were involved as reported earlier [17]. Irradiations of MMW under 1 mW/cm2 are deemed not to give rise to thermal effects in living cells [14]. As our experiments involved power densities around 1 mW/cm2 placing the results in a non-thermal range (also demonstrated in experiments later in this article), it is necessary to investigate other possible mechanism(s) responsible for the decreased growth rate of MMW irradiated cells. Therefore, the next step of the investigation was to check for the possible presence of genomic alterations arising due to radiation in the treated cells. In this direction, we examined for genomic DNA perturbations using the Δrad52 deletion strain. Rad52 is a protein required to repair DNA double-strand breaks. Rad52p mediates Rad51p function in homologous recombination repair (HRR) in both yeast Saccharomyces cerevisiae and in mammalian cells of mice and humans [18]. Double-strand breaks (DSBs) are one of the most dangerous forms of DNA damage and are known to cause gross chromosomal rearrangements, which are hallmarks of human cancers [19]. Yasuhara et al. highlighted the importance of human RAD52 for maintaining genome integrity [20]. In the absence of this protein in the Δrad52 deletion strain, yeast cells reportedly die upon irradiation by DNA damaging electromagnetic spectra [21,22]. Irradiation of WT and Δrad52 at the minimal threshold powers of 5 dBm (0.83 ± 0.02 mW/cm2) and frequency (105 GHz) for 5 and 6 h respectively as indicated from the previous experiment did not yield any differences in colony growth profiles between the two. To remove the possibility that low power (corresponding to 0.83 ± 0.02 mW/cm2 surface power density) at 105 GHz (see Table 1) was unable to unravel any differences in growth profiles both WT and Δrad52 cells and were subsequently exposed to 90 GHz MMW at 5 dBm power (corresponding to ~1.0 mW/cm2 surface power density) (see Table 1) for 6 h and subsequently incubated under physiological conditions for analyses of serially diluted colony growth profiles. It was observed that both types of cells showed similar colony growth profiles (Figure 2) even after serial dilutions were made to unravel any low intensity differences, which may have been overlooked due to cell numbers; demonstrating that reduced cell growth is not accountable to genetic DNA damage of the treated cells. The absence of Rad52p didn’t show any significant effect on the cells’ growth after treatment indicating non-ionizing MMW radiation does not cause directly manifesting permanent genomic DNA double strand breaks/alterations, while other indirect DNA alterations are possible through regulatory protein networks as discussed later in the article.
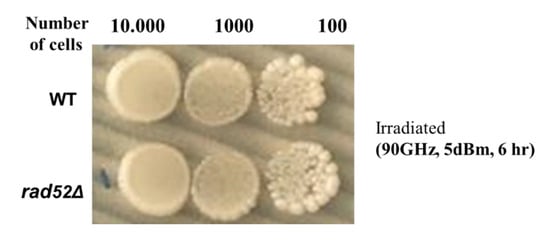
Figure 2.
Representative image of the colony growth profiles of WT and Δrad52 cells with serial dilutions after (90 GHz) MMW irradiation at 5 dBm for 6 h. The cells were grown under physiological conditions at 30 °C, over 3–4 days post-treatment period. Number of cells after serial dilutions are as indicated. Five independent experiments were performed. No statistically significant differences were found between the two examined strains.
2.3. MMW Interaction with Water as a Factor for Reduced Cell Growth
In the above section, the experiment indicated that the MMW irradiation could affect cell growth/division without causing directed DNA molecules damage. However, the radiation may elicit perturbations of DNA regulatory proteins responsible for structural or chemical modifications of the genome and proteins regulating other metabolic processes. This can happen by interaction of the radiation with water, which is well known to absorb electromagnetic radiation in the microwave and infrared spectrum [23], which indirectly can affect protein structure and function.
Thus, we examine the amount of MMW radiation, which is solely absorbed by the yeast cells and the part that is reflected. Based on the energy penetrating the cells we may be able to unravel the contributions of MMW interactions with the cytosolic part of the cells, which contains many types of macromolecules. Therefore, we measured the absorbed power over time, represented as a ratio of incident and reflected powers for yeast cells spotted on SC agar. This was compared to that of plain water and blank SC agar without cells respectively. The irradiation conditions were kept constant at 85 GHz for 5 h (5 dBm, at the maximal power level available to us corresponding to 1.39 ± 0.02 mW/cm2) for stringency of analysis.
The sample(s) absorbs a part of the incident radiation and the remaining is reflected. We find that the yeast cells spotted on SC agar absorbed more power (Figure 3) as compared to plain water and blank SC agar without cells on the same volume of media used. Interestingly, plain SC agar reflected most of the incident power. The results suggest that non-thermal exposure of MMW could affect the proteins present in the cytosolic part of the cells through interactions with water molecules without involving heat shock proteins [24]. This could account for the observed phenomenon of cell growth inhibition without genetic perturbation or by damage of other functional cytosolic protein molecules and/or structures (see Figure 2). The experiment also indicates that cells have a high absorbance of MMW irradiation by macromolecules within a cytosol made mostly of water separated from the environment within a confined volume of membrane bound structures.
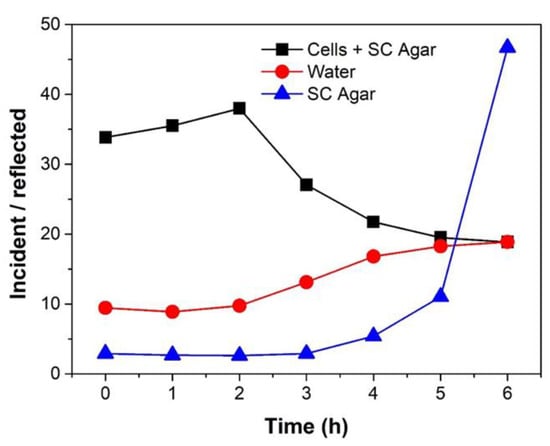
Figure 3.
Absorbed power represented as a ratio of incident and reflected power from yeast cells spotted on Synthetic Complete (SC) agar compared to that of plain water and Synthetic Complete (SC) agar without cells respectively. Experiments were performed on a 1 μL spot of 50 cells/μL concentration with a frequency of 85 GHz at an amplitude of 5 dBm over 5 h (corresponding to 1.39 ± 0.02 mW/cm2 surface power density).
Therefore, in order to ascertain any relation behind MMW absorption, temperature and volume of sample treated as indicated from the previous experiment, different volumes of water were irradiated using the horn antenna and temperature rise monitored. Irradiation conditions of frequency, power and duration of exposure were kept constant as mentioned previously to maintain empirical stringency. A 2 °C rise of temperature measured using a digital thermometer was associated with a 6 h exposure duration at 1.39 mW/cm2 from a horn antenna (Figure 4a) on a large volume of water (6500 µL). Reduction in the size of the irradiated sample to a smaller volume of water (250 µL) led to a 1 °C increase in the rate of temperature rise (Figure 4b). Interestingly, exposure involving a waveguide (delivering a higher power density of 17.17 mW/cm2) on the same small volume of water (250 µL) did not change the rate of temperature rise any further (Figure 4c). Further, contrary to the expectation of the waveguide causing thermal ablation due to higher power density, the experiment demonstrates that thermal effects were practically absent in our irradiation setup. Therefore, under conditions of constant frequency, power and exposure duration; the radiation-associated rise in temperature is inversely proportional to the volume of the sample irradiated. Finally, this experiment also confirms the hypothesis that biological cells exhibit high absorbance of MMW irradiation accountable to being constituted of water within a confined volume separated from the environment. The experiment demonstrates that MMW irradiation under the listed parameters raises temperatures to 22 °C and is therefore not expected to elicit thermal stress on yeast cells, which grow at physiological temperatures of 30 °C.
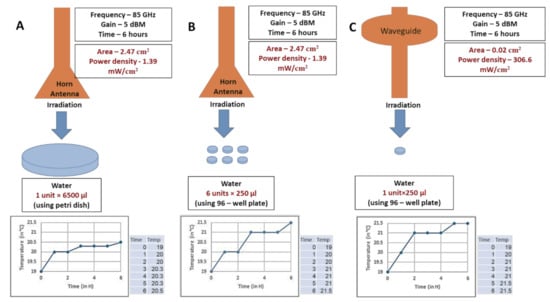
Figure 4.
Comparison of temperature rise during 85 GHz MMW exposure at constant power and treatment duration, involving horn antenna and wave-guide. Indicated volumes of water were irradiated as illustrated. (A) Temperature rise of 6500 µL water during MMW exposure at 1.39 mW/cm2. (B) Temperature rise of 250 µL water during MMW exposure at 1.39 mW/cm2. (C) Temperature rise of 250 µL water during MMW exposure at 17.17 mW/cm2. Frequency, power and duration of exposure were kept constant at the values in the specific experiments as indicated.
A wave-guide provides a focused beam of irradiation as compared to a horn antenna (see Figure 4) allowing more precise energy delivery. To investigate potential applications of this exposure regime we performed MMW irradiation at 85 GHz and 5 dBm on BY4741 Saccharomyces cerevisiae WT yeast cells using an open-ended waveguide (Figure 5). Single frequency at 85 GHz was used as it provides the maximal threshold powers and affects WT yeast cell growth to the same extent as for all the other examined MMW frequencies (see Figure 1a). The thickness of agar medium and the number of cells were kept the same (50 cells/µL) as those mentioned in the previous experiments. Cells were treated for 3–4 h at the non-thermal exposure power density of 17.17 mW/cm2 (see Figure 4). Subsequent incubation of these cells under physiological conditions (at 30 °C for 2 days over a week) did not yield any colony growth. The experiment demonstrates that MMW irradiation using an open-ended waveguide at ~12 times stronger power density than the one involving a horn antenna (see Figure 1 and Figure 4 and Table 1) completely terminates cell growth and division.
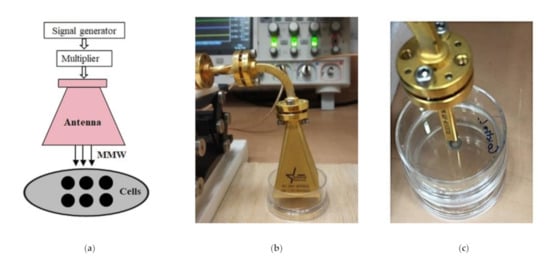
Figure 5.
Block diagram of the experimental setup for irradiation and pictures of the pyramidal horn antenna and waveguide. (a) Schematic presentation of irradiating system. (b) Picture of a pyramidal antenna. (c) Picture of a waveguide.
3. Discussion
In this work, we showed that WT Saccharomyces cerevisiae cells in response to MMW irradiation (85–105 GHz) at ~1.0 mW/cm2 for 6 h using a standard pyramidal horn antenna reduced the rate of division by up to 62% as compared to sham (control) (see Figure 1 and Table 1). The inhibition of the cell growth was performed for a constant cell density (50 cells/µL sample). We studied the frequency and energy dose dependence over the tested range. Our results have showed a strong inhibition of proliferation as compared to previous published (contradictory) results using radiation in the MMW ranges and the specified conditions, which were mentioned in the introduction [12,13,14,15]. Anti-proliferative effects of MMW radiation was elicited with a minimal power density of 0.83 ± 0.02 mW/cm2 at 105 GHz (Table 1) and was sufficient to inhibit cell growth and division (Figure 1a) on the concentration of cells used (50 cells/μL). Additionally, the effect was energy dose dependent as 5 dBm for 6 h at a constant frequency of 105 GHz showed similar cell growth reduction as 6 dBm for 5 h (Figure 1b).
Next, we examined a hypothesis, whether the MMW cell growth inhibition effect was due to permanent DNA genotoxic effects. Using the yeast strain with deletion of Rad52p, which is responsible for DNA double strand breaks repair, we found no significant effect on the cells’ growth after irradiation treatment under the specified parameters (Figure 2). Our results indicate that growth inhibitory effect on WT yeast cells by non-ionizing MMW radiation does not occur due to permanent genomic alterations, while other indirect genomic effects may be possible through protein regulatory networks.
In general, non-ionizing radiations with low photon energies of 0.3–0.4 meV are not expected to biochemically alter physiological DNA structure or function. Edwards et al. proposed a mechanism of coherent frequency-specific deposition of microwave energy on DNA in water [25]. The resonance of DNA molecules with irradiated spectra can be calculated in terms of the absorption coefficient. 2734 base pairs (bp) supercoiled circular DNA, 2734 bp linear DNA, 1786 bp linear DNA, and 948 bp linear DNA were found to resonate with 2.55–8.75 GHz, 2.75–5.60 GHz, 4.10 GHz, and 2.65 GHz respectively [25]. This indicates that DNA polymer chain length determining the structural conformation and size (globular or linear, large or small) directly correspond to their respective resonant frequencies. Illustratively, a resonance shift occurs within the frequency range of 41–52 GHz upon changing the length of the haploid genetic material in E. coli [26]. Further, relative viscosity measurements showed that the resonance frequencies decreased proportionally to the enhancement of haploid genome length. These reports suggest that resonant interactions of MMW with genetic material can arise by structure based energetic coupling without causing biochemical genomic alterations (see Figure 2) within the threshold powers of non-thermal exposure. Experimentally, irradiation of transformed Human Corneal Epithelial (HCE-T) and Human Lens Epithelial (SRA01/04) cell lines by 60 GHz at 1 mW/cm2 over 24 h of constant exposure found no statistically significant genotoxic effects on the nucleus [27].
Further, resonance absorption of MMW irradiation corresponding to different DNA polymer chain lengths [25] and found to be related to their structural conformation and size (globular or linear, large or small) as discussed above (see Results Section 2.2). Thus, certain irradiation regimes may exercise switch ON/Off control over genetic expression by affecting conformational changes, which translate into specific modifications of the proteome determining the extent of cell division effects. Experimentally, resonance shifts occurring within the frequency range of 41–52 GHz corresponded to changing lengths of the genetic material in E. coli located directly in the cytosol of the cells [26]. In eukaryotes, the genetic material in the nucleus is distinguishable into heterochromatin (condensed and inactive) and euchromatin (actively directing protein synthesis). MMW irradiation regimes may be affecting conformational changes on chromatin, which modulates the gene expression without causing genetic DNA damage. Such a mechanism can be analogous to the biochemical ON/OFF control of epigenetic regulation wherein specific biological molecules physically associate or dissociate with certain genetic sequences in the nucleus in response to external stimuli for either blocking or enhancing gene expression. In contrast, by involving a medium of radiation to enable non-biochemical control of genetic expression through conformational changes in the genome, MMW therapy can provide interesting applications of eliciting targeted biological responses for various purposes. Such effects are likely to be dose-dependent and potential applications of this technique will require characterization of the genomic conformational changes possible for different exposure conditions correspondent with their respective biological outcomes. Such a mechanism of action can also explain the ambiguous cell proliferation results after MMW treatment from the other studies described above in the introduction [12,13,14].
A biological cell is a compartmentalized structure separated from the surrounding environment by the lipid cell membrane containing transmembrane proteins. It has been reported that 65 GHz irradiation reduced the effects of factors on yeast cells due to the destabilization of intracellular water structure [28]. Under physiological conditions, yeast cells are reported to bear 65% water by composition with about 20–30% of the volume inhabited by cytosolic proteins that participate in the metabolic processes of the cells [29]. Biological functions at the cellular level are affected by proteins, and the functionality of proteins is, in turn, determined by their molecular structure. Proteins are polypeptide chains composed of sequentially joined amino acids folding into the lowest energy conformations in their physiological environment giving rise to three-dimensional structures. These structures are essential for the protein’s biochemical interactions with other molecules, which manifests in biological functions. Changes in the cytosol environment can therefore translate into changing the properties of biomolecules like proteins by affecting their charge densities in space and time.
Interactions of MMW irradiation with proteins in the cytosol could generate structural and/or functional changes, which subsequently affects cell functionality. Structurally, water is a physical participant during the folding of the polypeptide chain in protein folding through hydrophobic collapse [29]. Thus, water interacts with proteins to affect their dynamics. Conversely, changes in the chemical composition of the aqueous environment can alter the three-dimensional structure of proteins. Molecular Transfer Model (MTM) predicts conformational changes in protein structures when pH changes occur in the vicinity in solution using calculated partition functions of polypeptides [30]. Illustratively, nitrophorin 4 (NP4) is a protein that releases nitric oxide (NO) in a pH-sensitive manner. NP4 remains in a closed conformation and tightly binds NO at pH 5.5 [31]. At pH 7.5, deprotonation occurs, changing the conformation and releasing NO.
Since MMW are classed as non-ionizing radiation with low 0.3–0.4 meV range of photon energies, their direct exposure is not expected to directly generate Reactive Oxygen Species (ROS) by biochemical degradation unlike ionizing radiation therapies [2,3], which involve higher photon energies and thereby provide only safety restricted applications. We conjecture that MMW elicits therapeutic effects and limit the adversities by the phenomenon of Structure Resonant Energy Transfer (SRET). It allows membrane bound spherical core-shell charge separated bodies (from 28–100 nm diameters) to exhibit dipolar coupling with incident electric fields [32] in the range of 12–45 GHz of the electromagnetic spectrum with an inverse relation between frequency and size dimensions. Such phenomena which occur at resonant frequencies determined by the structural dimensions and charge status can lead to rupture events [33], resulting in leakage of matrix contents depending on the exposure power conditions and duration of treatment. Cells are composed of multiple subcellular organelles bound by membranes, which maintain distinct environments within the matrix separated from the cytoplasm outside. Peroxisomes and microbodies are a class of cell organelles, which function as a source of reactive oxygen species (ROS). ROS causes oxidative stress and DNA damage at high levels inhibiting cell division [34]. Generally observed to be spherical in shape, peroxisomes resemble the membrane bound core-shell charge separated structures described above [32], raising the possibility that 85–105 GHz MMW irradiation may have resulted in peroxisome rupture with subsequent ROS leakage leading to inhibited cell division. However, as per published measurements and understanding, dipolar coupling at 85–105 GHz frequencies will correspond to charge separated spherical membrane bound structures of diameters around 11–14 nm [32]; which are much below the peroxisomes size range of 200–500 nm [35,36,37], indicating that reduced cell division upon 85–105 GHz exposure is not accountable to ROS mediated mechanisms. However, other smaller vesicles with resonant dimensions covered by this bandwidth may contribute to the observed non-thermal effects by different non-ROS mediated pathways. Such processes could explain the ambiguous MMW treatment results reported from other studies [12,13,14] described above in the introduction.
As such, the apoptosis-promoting effects MMW radiation on several types of tumors could also be a potential reason for the observed effects. Sweeping frequency regimes over 75–105 GHz at 0.2 mW/cm2 resulted in apoptosis of H1299 lung cancer cells [5]. Experimentally, irradiation at 35 GHz is reported to activate the apoptosis in A375 melanoma cells in vitro [38]. The transient receptor potential vanilloid 1 (TRPV1) expression is altered in prostate, colon, bladder, pancreas and breast cancers. TRPV1 forms high conductivity channels in the cell membrane, and its prolonged activation could lead to cell apoptosis. Pre-treatment with capsaicin, TRPV1 agonist and subsequent exposure to 60 GHz radiation at 100 mW for 20 min facilitates apoptosis in Triple Negative Breast Cancer Cell Lines [39]. The combined action of TRPV1 agonist and MMW radiation demonstrates robust sensitization to cell death even at low concentrations of the chemical agonist. Our experiments have showed complete termination of the cells’ growth at 17.17 mW/cm2; possibly via strong apoptotic effect by using the higher power densities that was required for better penetration since yeast cells bear thick cell walls which were absent in the H1299 and A375 cells described above. However, further experiments are requisite to exclude intracellular thermal effects or responses of the cells when using an open-ended waveguide. We intend to investigate the complete range of effects of the W-band (85–105 GHz) MMW irradiation to unravel the mechanism(s) of action by using deleted yeast library collections of over 5100 single gene deletions.
In summary, our experiments examined the effects of MMW (85–105 GHz) on Saccharomyces cerevisiae yeast and demonstrated, that W-band (85–105 GHz) MMW irradiation at a power density of ~1.0 mW/cm2 for 5-6 h exposure duration results in a 62% reduction in the growth rate of irradiated yeast cells at a density of 50 cells/μL. MMW radiation effects were found to be frequency, energy dose and cell density dependent. Experimental exposure conditions maintained within the ICNIRP non-thermal safety standard of 20 mW/cm2 [40]. Further, measurements of temperature rise demonstrated that effects were non-thermal in nature. A comparative analysis of changes in the growth profile of irradiated wild type and Δrad52 (DNA damage repair) deletion cells revealed no directed detrimental effects on genomic stability arising from this non-ionizing radiation treatment. Analyses of dose dependent effects of MMW propagation and calibrations of cell density to exposure conditions revealed threshold parameters, which can be used for targeted inhibition of infectious microbes. Specifically, using an open-ended waveguide delivering a higher power density of 17.17 mW/cm2 for 3–4 h exposure duration achieved complete termination of cellular proliferation in 50 cells/μL samples of Saccharomyces cerevisiae yeast cells.
Our results indicate that MMW irradiation can be useful for future application in medical treatments. The advantages in using non-ionizing MMW radiation enabling non-thermal control of targeted biological responses with or without chemical and biological drugs has promising potential for innovative biomedical procedures and devices.
Invasive fungal infections remain a serious clinical problem. The current use of several classes of antifungal drugs have led to increased antimicrobial resistance of pathological species like C. albicans by accelerating the development of mutations, overexpression of multidrug efflux pumps [41], etc. Moreover, C. albicans as a pathogen depending largely on its ability to generate diversity at not only the genomic but also the morphological and physiological levels by its ability to switch from yeast to hyphal or pseudo hyphal forms [42,43]; constitutes a major challenge in the development of new anti-fungal drugs and therapeutic strategies. A direct application of the MMW radiation effect mechanisms presented in this article for non-thermal exposure regimes can be used for treating pathogenic fungal infections (via cell division inhibition effect, see Figure 1), which are common in dermatology [44] and reproductive health [45]; and cancer lesion/tissue treatments (via cancer cell mortality effect [4,5]) by appropriately scaling the exposure conditions described here to match clinical cell densities; besides various other promising purposes. Further, recent advances in endoscopic probes [46] operating in the Super High Frequency (SHF) 3–30 GHz bandwidths and similar technological trends will enable novel avenues of adapting such non-thermal techniques to treat internal body sites overcoming the burn hazard restrictions in application of conventional thermal ablation procedures. Our experiments demonstrate that non-thermal regimes of non-ionizing (85–105 GHz) MMW exposure have a promising potential not only for anti-cancer treatments [4,5], but for targeted treatment of yeast related diseases.
4. Materials and Methods
4.1. Conditions of Cell Culture
Wild type (WT) budding yeast BY4741 Saccharomyces cerevisiae (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) and its Δrad52 mutant strain BY4741 Saccharomyces cerevisiae (MATa his3∆1 leu2∆0 met15∆0 ura3∆0 RAD52:KanMX4) available through EUROSCARF (Frankfurt, Germany) were used for irradiation. Cells were grown in standard synthetic complete (SC) agar and liquid mediums at a temperature of 30 °C. The growth rate of both control and irradiated cells were measured using an absorbance plot at 600 nm measured by a standard spectrophotometer in units of optical density (OD). Cultures were adequately diluted to 0.1 OD using a standard absorbance plot at the start of experiment and incubated until they reached an OD value of 0.4 (the point at which cells initiate the logarithmic growth phase). Cultures at 0.4 OD were diluted to 10,000, 1000, 100 and 50 cells/μL (to determine the optimal concentration of cells and energy dosage). 1–2 μL volume of those solutions were dropped onto SC agar plates. Six colonies were seeded in two replicates: one for irradiation and another for comparison as control (sham) for each discrete frequency regime. After irradiation, the cells were transferred to SC liquid medium and incubated under standard conditions. Growth rate of both irradiated and control (sham) yeast cells were measured regularly at intervals of 90 min over a period of ~8 h to assay the effect of MMW exposure on physiological growth.
4.2. Conditions of Irradiation
The schematic diagram of the experimental setup for MMW irradiation is illustrated in Figure 5. Yeast cells were spotted on the Agar medium prior to irradiation. The agar layer thickness was kept constant throughout (2 mL volume) on 35 mm polystyrene plates. Cells were exposed to specific frequencies of 85 GHz, 95 GHz, and 105 GHz at 5 dBm power for 6 h respectively. The irradiation experiment for each frequency involved six distinct experiments at the same cell density. MMW (85–110 GHz) were generated using a signal generator (N5183B, 9 kHz–20 GHz, Keysight Technology, Santa Rosa, CA, USA), and 6× active frequency multiplier (QMM-311220025, Quinstar Tech Inc., Torrance, CA, USA). A standard gain pyramidal horn antenna (QWH-WPRROO, Quinstar Tech Inc.) was used for MMW emission. The power of transmitted waves from the horn was measured using an identical horn antenna and digital storage oscilloscope (DSO-X 2004A, Agilent Technology, Santa Clara, CA, USA). Distribution of relative energy across antenna aperture was measured using an open-ended waveguide (QWH-WPRROO, Quinstar Tech Inc.) in the near field (see Figure 6).
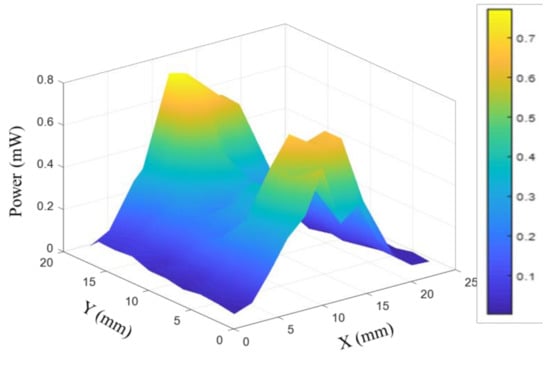
Figure 6.
Relative distribution of power across horn antenna aperture.
Specific absorption rate (SAR) was calculated (Table 1, see in results). It was in range 3.48–2.08 W/kg involving the horn antenna and 43.05 W/kg for the irradiations with 85 GHz using the waveguide, respectively:
was used to calculate the SAR values where SAR, Pa, m, , V, S, and l denote. Specific absorption rate, absorbed power, mass, density, volume, surface area and depth of medium irradiated respectively. As most of the radiation was probably absorbed by the agar rather than the cells, the total volume is more relevant than dry cell mass. Density was considered approximately equal to water (997 kg/m³) and depth l used in the experiments was 4 mm. Cells were irradiated after being immersed on the agar.
The penetration depth of 85–110 GHz MMW is very shallow, hence the exposed surface area and not the volume is considered. The appropriate exposure metric for MMW is therefore the power density, power per area (W/m2). Absorbed power Pa was calculated as:
where represents the power densities of the irradiations used (1.39 mW/cm2 for horn antenna and 17.17 mW/cm2 for the wave guide).
Author Contributions
Conceptualization, A.B. and S.L.-A.; methodology, A.B., S.R. and S.L.-A.; software, A.B., S.R., K.K. and S.L.-A.; validation, S.L.-A., K.K., J.L. and A.Y.; formal analysis, A.B., S.R., S.L.-A. and A.Y.; investigation, A.B. and S.R.; resources, S.L.-A., K.K., J.L. and A.Y.; data curation, A.B., S.R. and S.L.-A.; writing—original draft preparation, A.B.; S.R. and S.L.-A., writing—review and editing, A.B., S.R., S.L.-A., K.K. and A.Y.; visualization, A.B., S.R. and S.L.-A.; supervision, S.L.-A.; project administration, S.L.-A. and A.Y.; funding acquisition, S.L.-A., K.K., J.L. and A.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ariel Center for Applied Cancer Research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the Israeli Council for Higher Education (CHE) for fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koo, M.M.; Swann, R.; Mcphail, S.; Abel, G.A.; Elliss-Brookes, L.; Rubin, G.P.; Lyratzopoulos, G. Presenting Symptoms of Cancer and Stage at Diagnosis: Evidence from a Cross-Sectional, Population-Based Study. Lancet Oncol. 2020, 21, 73–79. [Google Scholar] [CrossRef]
- Ryan, J.L. Ionizing Radiation: The Good, the Bad, and the Ugly. J. Investig. Dermatol. 2012, 132, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Angelis, C.D.; Salvo, N.; Barnes, E.; Draanen, J.V.; Stacey, E.; Mitera, G.; Breen, D.; Giotis, A.; Czarnota, G.; Pang, J. Prophylaxis and Management of Acute Radiation-Induced Skin Reactions: A Systematic Review of the Literature. Curr. Oncol. 2010, 17. [Google Scholar] [CrossRef] [PubMed]
- Komoshvili, K.; Becker, T.; Levitan, J.; Yahalom, A.; Barbora, A.; Liberman-Aronov, S. Morphological Changes in H1299 Human Lung Cancer Cells Following W-Band Millimeter-Wave Irradiation. Appl. Sci. 2020, 10, 3187. [Google Scholar] [CrossRef]
- Komoshvili, K.; Israel, K.; Levitan, J.; Yahalom, A.; Barbora, A.; Liberman-Aronov, S. W-Band Millimeter Waves Targeted Mortality of H1299 Human Lung Cancer Cells without Affecting Non-Tumorigenic MCF-10A Human Epithelial Cells In Vitro. Appl. Sci. 2020, 10, 4813. [Google Scholar] [CrossRef]
- Mirbeik-Sabzevari, A.; Tavassolian, N. Ultrawideband, Stable Normal and Cancer Skin Tissue Phantoms for Millimeter-Wave Skin Cancer Imaging. IEEE Trans. Biomed. Eng. 2019, 66, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Coln, E.; Schoenbach, K.; Gellerman, M.; Fox, P.; Rec, L.; Beebe, S.; Liu, S. A Study on Biological Effects of Low-Intensity Millimeter Waves. IEEE Trans. Plasma Sci. 2002, 30, 1489–1496. [Google Scholar]
- Ziskin, M.C. Physiological Mechanisms Underlying Millimeter Wave Therapy. Bioelectromagnet. Curr. Concepts 2006, 241–251. [Google Scholar]
- Altmann, K.; Dürr, M.; Westermann, B. Saccharomyces cerevisiae as a Model Organism to Study Mitochondrial Biology. Methods Mol. Biol. Mitochondria 2007, 372, 81–90. [Google Scholar]
- Beck, H.; Dobritzsch, D.; Piå¡kur, J. Saccharomyces Kluyverias a Model Organism to Study Pyrimidine Degradation. FEMS Yeast Res. 2008, 8, 1209–1213. [Google Scholar] [CrossRef]
- Kachroo, A.H.; Laurent, J.M.; Yellman, C.M.; Meyer, A.G.; Wilke, C.O.; Marcotte, E.M. Systematic Humanization of Yeast Genes Reveals Conserved Functions and Genetic Modularity. Science 2015, 348, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Grundler, W. Intensity- and Frequency-Dependent Effects of Microwaves on Cell Growth Rates. J. Electroanal. Chem. 1992, 342, 361–365. [Google Scholar] [CrossRef]
- Grundler, W.; Keilmann, F.; Fröhlich, H. Resonant Growth Rate Response of Yeast Cells Irradiated by Weak Microwaves. Phys. Lett. A 1977, 62, 463–466. [Google Scholar] [CrossRef]
- Furia, L.; Hill, D.W.; Gandhi, O.P. Effect of Millimeter-Wave Irradiation on Growth of Saccharomyces cerevisiae. IEEE Trans. Biomed. Eng. 1986, BME-33, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Vojisavljevic, V.; Alsuhaim, H.S.; Pirogova, E. Low power microwave exposures at 968MHz increase the growth rate of Breanomyces bruxellensis yeast cells. In Proceedings of the 2016 IEEE International Conference on Microwave and Millimeter Wave Technology (ICMMT), Beijing, China, 5–8 June 2016; pp. 1061–1063. [Google Scholar]
- Smolyanskaya, A.Z.; Vilenskaya, R.L. Effects of Millimeter-Band Electromagnetic Radiation on the Functional Activity of Certain Genetic Elements of Bacterial Cells. Sov. Phys. Uspekhi 1974, 16, 571–572. [Google Scholar] [CrossRef]
- Komoshvili, K.; Levitan, J.; Aronov, S.; Kapilevich, B.; Yahalom, A. Millimeter waves non-thermal effect on human lung cancer cells. In Proceedings of the 2011 IEEE International Conference on Microwaves, Communications, Antennas and Electronic Systems (COMCAS 2011), Tel Aviv, Israel, 7–9 November 2011. [Google Scholar]
- Feng, Z.; Scott, S.P.; Bussen, W.; Sharma, G.; Guo, G.; Pandita, T.; Powell, S. Rad52 inactivation is synthetically lethal with BRCA2 deficiency. Proc. Natl. Acad. Sci. USA 2011, 108, 686–691. [Google Scholar] [CrossRef]
- Bhowmick, R.; Minocherhomji, S.; Hickson, I.D. RAD52 Facilitates Mitotic DNA Synthesis Following Replication Stress. Mol. Cell. 2016, 64, 1117–1126. [Google Scholar] [CrossRef]
- Yasuhara, T.; Kato, R.; Hagiwara, Y.; Shiotani, B.; Yamauchi, M.; Nakada, S.; Shibata, A.; Miyagawa, K. Human Rad52 Promotes XPG-Mediated R-loop Processing to Initiate Transcription-Associated Homologous Recombination Repair. Cell 2018, 175, 558–570.e11. [Google Scholar] [CrossRef]
- Game, J.C. Radiation-Sensitive Mutants and Repair in Yeast. Yeast Genet. 1983, 109–137. [Google Scholar]
- Game, J.C. DNA double-strand breaks and the RAD50-RAD57 genes in Saccharomyces. Semin. Cancer Biol. 1993, 4, 73–83. [Google Scholar]
- Lunkenheimer, P.; Emmert, S.; Gulich, R.; Köhler, M.; Wolf, M.; Schwab, M.; Loidl, A. Electromagnetic-Radiation Absorption by Water. Phys. Rev. E 2017, 96, 062607. [Google Scholar] [CrossRef]
- White, J. Variation in Water Content of Yeast Cells Caused BY Varying Temperatures of Growth and by Other Cultural Conditions. J. Inst. Brew. 1952, 58, 47–50. [Google Scholar] [CrossRef]
- Edwards, G.S.; Davis, C.C.; Saffer, J.D.; Swicord, M.L. Resonant Microwave Absorption of Selected DNA Molecules. Phys. Rev. Lett. 1984, 53, 2060. [Google Scholar] [CrossRef]
- Belyaev, I.Y.; Alipov, Y.D.; Polunin, V.A.; Shcheglov, V.S. Evidence for Dependence of Resonant Frequency of Millimeter Wave Interaction with Escherichia coliK12 Cells on Haploid Genome Length. Electro. Magn. 1993, 12, 39–49. [Google Scholar]
- Koyama, S.; Narita, E.; Shimizu, Y.; Suzuki, Y.; Shiina, T.; Taki, M.; Shinohara, N.; Miyakoshi, J. Effects of Long-Term Exposure to 60 GHz Millimeter-Wavelength Radiation on the Genotoxicity and Heat Shock Protein (Hsp) Expression of Cells Derived from Human Eye. Int. J. Environ. Res. Public Health 2016, 13, 802. [Google Scholar] [CrossRef] [PubMed]
- Svetlana, M.; Rogacheva, M.; Babaeva, I. The effect of millimeter waves at the yeast Saccharomyces cerevisiae during heliogeophysical disturbances. In Proceedings of the SPIE 8699 Saratov Fall Meeting and Workshop on Laser Physics and Photonics, Optical Technologies in Biophysics and Medicine XIV; and Laser Physics and Photonics XIV, 86990N, Saratov, Russian Federation, 25–28 September 2012. [Google Scholar] [CrossRef]
- Bellissent-Funel, M.-C.; Hassanali, A.; Havenith, M.; Henchman, R.; Pohl, P.; Sterpone, F.; Spoel, D.V.D.; Xu, Y.; Garcia, A.E. Water Determines the Structure and Dynamics of Proteins. Chem. Rev. 2016, 116, 7673–7697. [Google Scholar] [CrossRef]
- O’Brien, E.P.; Brooks, B.R.; Thirumalai, D. Effects of PH on Proteins: Predictions for Ensemble and Single-Molecule Pulling Experiments. J. Am. Chem. Soc. 2011, 134, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Russo, N.V.D.; Estrin, D.A.; Martí, M.A.; Roitberg, A.E. PH-Dependent Conformational Changes in Proteins and Their Effect on Experimental PKas: The Case of Nitrophorin 4. PLoS Comput. Biol. 2012, 8, e1002761. [Google Scholar] [CrossRef]
- Liu, T.-M.; Chen, H.-P.; Wang, L.-T.; Wang, J.-R.; Luo, T.-N.; Chen, Y.-J.; Liu, S.-I.; Sun, C.-K. Microwave Resonant Absorption of Viruses through Dipolar Coupling with Confined Acoustic Vibrations. Appl. Phys. Lett. 2009, 94, 043902. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, H.-C.; Liu, T.-M.; Lu, J.-T.; Hung, W.-T.; Huang, Y.-R.; Tsai, Y.-C.; Kao, C.-L.; Chen, S.-Y.; Sun, C.-K. Efficient Structure Resonance Energy Transfer from Microwaves to Confined Acoustic Vibrations in Viruses. Sci. Rep. 2015, 5, 18030. [Google Scholar] [CrossRef]
- Havens, C.; Ho, A.; Yoshioka, N.; Dowdy, S. Regulation of Late G1/S Phase Transition and APCCdh1 by Reactive Oxygen Species. Mol. Cell. Biol. 2006, 26, 4701–4711. [Google Scholar] [CrossRef]
- Veenhuis, M.; Mateblowski, M.; Kunau, W.H.; Harder, W. Proliferation of microbodies in Saccharomyces cerevisiae. Yeast 1987, 3, 77–84. [Google Scholar] [CrossRef]
- Yofe, I.; Soliman, K.; Chuartzman, S.G.; Morgan, B.; Weill, U.; Yifrach, E.; Dick, T.P.; Cooper, S.J.; Ejsing, C.S.; Schuldiner, M.; et al. Pex35 is a regulator of peroxisome abundance. J. Cell Sci. 2017, 130, 791–804. [Google Scholar] [CrossRef]
- Deng, X.; Deng, T.; Ni, Y.; Zhan, Y.; Huang, W.; Liu, J.; Liao, C. Cytochrome c modulates the mitochondrial signaling pathway and polymorphonuclear neutrophil apoptosis in bile duct-ligated rats. Exp. Ther. Med. 2016, 12, 333–342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, R.; Liu, Y.; Liu, S.; Luo, T.; Zhong, G.; Liu, A.; Zeng, Q.; Xin, X. Millimeter wave exposure induces apoptosis in human melanoma A375 cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao 2019, 39, 76–81. [Google Scholar] [PubMed]
- Sorolla, A.; Romanenko, S.; Siegel, P.H.; Wallace, V. Utilisation of MMW Radiation to Facilitate Apoptosis in Triple Negative Breast Cancer Cell Lines via TRPV1 Receptor Sensitization. In Proceedings of the 2019 44th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), Paris, France, 1–6 September 2019; pp. 1–2. [Google Scholar]
- ICNIRP. Guidelines for Limiting Exposure to Electromagnetic Fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- Cowen, L.E.; Steinbach, W.J. Stress, drugs, and evolution: The role of cellular signaling in fungal drug resistance. Eukaryot. Cell 2008, 7, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Rocha, R.; Santos, M.C.; Mateus, D.D.; Moura, G.R.; Carreto, L.; Santos, M.A. A genetic code alteration is a phenotype diversity generator in the human pathogen Candida albicans. PLoS ONE 2007, 2, e996. [Google Scholar] [CrossRef]
- Silva, R.M.; Paredes, J.A.; Moura, G.R.; Manadas, B.; Lima-Costa, T.; Rocha, R.; Miranda, I.; Gomes, A.C.; Koerkamp, M.J.; Perrot, M.; et al. Critical roles for a genetic code alteration in the evolution of the genus Candida. EMBO J. 2007, 26, 4555–4565. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-González, D.; Perusquía-Ortiz, A.M.; Hundeiker, M.; Bonifaz, A. Opportunistic yeast infections: Candidiasis, cryptococcosis, trichosporonosis and geotrichosis. J. Dtsch. Dermatol. Ges. 2013, 11, 381–394. [Google Scholar] [CrossRef]
- Aubyn, G.B.; Tagoe, D.N.A. Prevalence of vaginal infections and associated lifestyles of students in the University of Cape Coast, Ghana. Asian Pac. J. Trop. Dis. 2013, 3, 267–270. [Google Scholar] [CrossRef]
- Hancock, C. WFS-1Advanced RF, Microwave and Millimeter Wave Energy Based Systems to Address a Range of Unmet and Growing Clinical Needs. In Proceedings of the The IEEE MTT International Microwave Symposium (IMS), Honolulu, HI, USA, 4–9 June 2017; Available online: https://zenodo.org/record/1156362 (accessed on 16 July 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).