Limbic Encephalitis with HU-Antibodies in T-cell Anaplastic Lymphoma. A Case Report
Abstract
:Featured Application
Abstract
1. Introduction
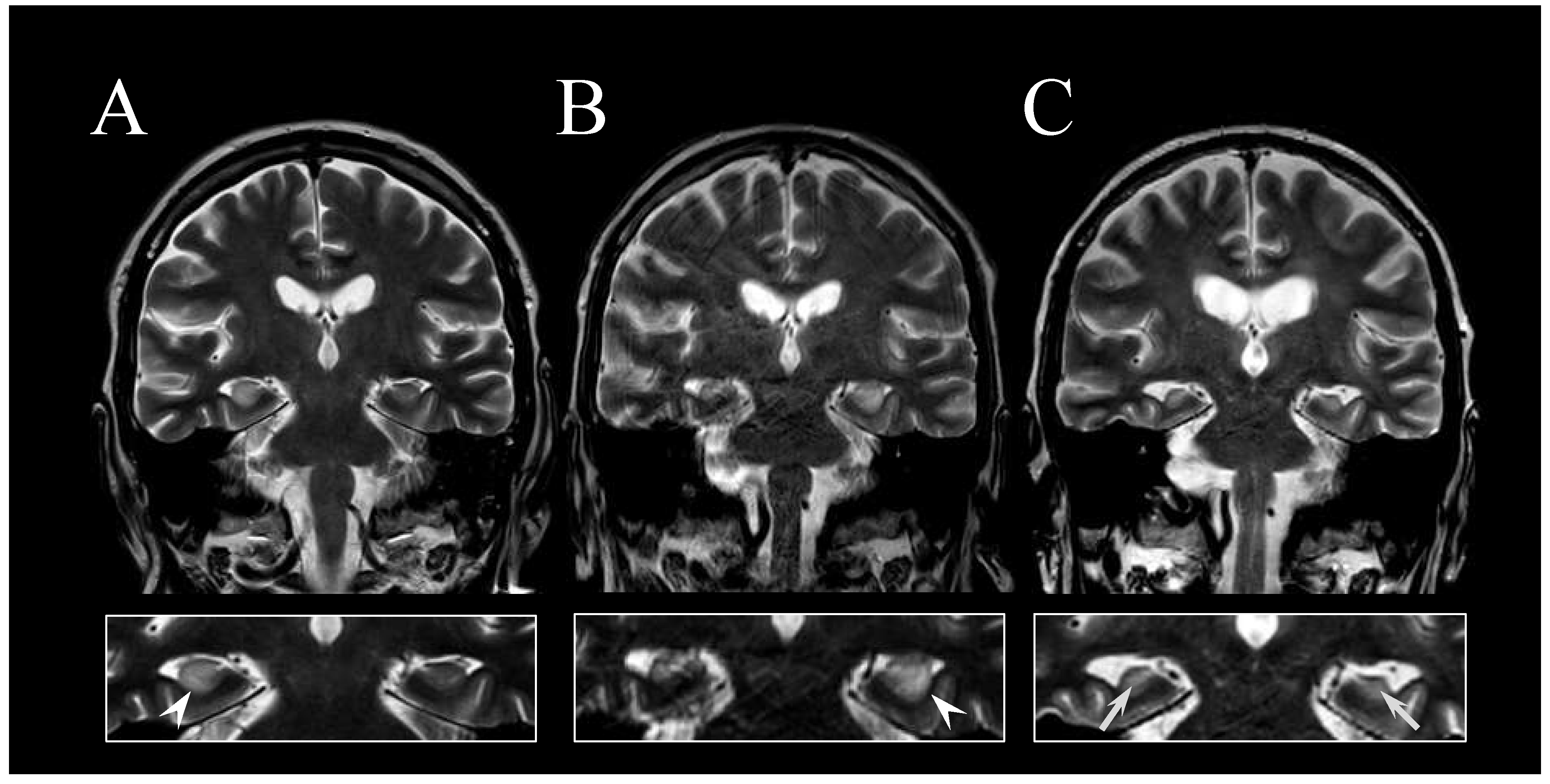
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darnell, R.B.; Posner, J.B. Paraneoplastic syndromes involving the nervous system. N. Engl. J. Med. 2003, 349, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Delattre, J.Y.; Antoine, J.E.; Dalmau, J.; Giometto, B.; Grisold, W.; Honnorat, J.; Smitt, P.S.; Vedeler, C.; Verschuuren, J.J.G.M.; et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1135–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briani, C.; Vitaliani, R.; Grisold, W.; Honnorat, J.; Graus, F.; Antoine, J.C.; Bertolini, G.; Giometto, B. PNS Euronetwork. Spectrum of paraneoplastic disease associated with lymphoma. Neurology 2011, 76, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Briani, C.; Visentin, A.; Campagnolo, M.; Salvalaggio, A.; Ferrari, S.; Cavallaro, T.; Manara, R.; Gasparotti, R.; Piazza, F. Peripheral nervous system involvement in lymphomas. J. Peripher. Nerv. Syst. 2019, 24, 5–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancaster, E.; Martinez-Hernandez, E.; Titulaer, M.J.; Boulos, M.; Weaver, S.; Antoine, J.C.; Liebers, E.; Kornblum, C.; Bien, C.G.; Honnorat, J.; et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology 2011, 77, 1698–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreri, A.J.M.; Doorduijn, J.K.; Re, A.; Cabras, M.G.; Smith, J.; Ilariucci, F.; Luppi, M.; Calimeri, T.; Cattaneo, C.; Khwaja, J.; et al. International Extranodal Lymphoma Study Group (IELSG). MATRix-RICE therapy and autologous haematopoietic stem-cell transplantation in diffuse large B-cell lymphoma with secondary CNS involvement (MARIETTA): An international, single-arm, phase 2 trial. Lancet Haematol. 2021, 8, e110–e121. [Google Scholar] [CrossRef]
- Carr, I. The Ophelia syndrome: Memory loss in Hodgkin’s disease. Lancet 1982, 319, 844–845. [Google Scholar] [CrossRef]
- Hentschke, S.; Malzfeldt, E.; Salwender, H.J.; Braumann, D.; Stang Aand Hentschke, M. Hu-antibody positive limbic encephalitis in a patient with Hodgkin lymphoma. Leuk. Lymphoma. 2008, 49, 2374–2376. [Google Scholar] [CrossRef] [PubMed]
- Laffon, M.; Giordana, C.; Almairac, F.; Benchetrit, M.; Thomas, P. Anti-Hu-associated paraneoplastic limbic encephalitis in Hodgkin lymphoma. Leuk. Lymphoma. 2012, 53, 1433–1434. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Ariño, H.; Dalmau, J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood 2014, 123, 3230–3238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Markers | Normal Value | Patient’s Data | Markers | Normal Value | Patient’s Data |
|---|---|---|---|---|---|
| Blood analysis | |||||
| WBC | 4.0–11 × 109/L | 3.5 × 109/L | LDH | 135–225 U/L | 173 U/L |
| Lymphocyte | 1.1–4.8 × 109/L | 0.7 × 109/L | Creatinine | 59–100 μmol/L | 43 mol/L |
| Neutrophils | 1.8–7.8 × 109/L | 2.1 × 109/L | AST | 10–45 U/L | 17 U/L |
| Hemoglobin | 140–170 g/L | 87 g/L | ALT | 10–50 U/L | 32 U/L |
| Platelets | 150–450 × 109/L | 157 × 109/L | Protein | 64–83 g/L | 48 g/L |
| ESR | 2–37 mm/h | 5 mm/h | IgG | 7–16 g/L | 6.05 g/L |
| CRP | <6 mg/L | 7 mg/L | Presepsin | 57–37 g/L | 148 ng/L |
| Cerebrospinal fluid tests | |||||
| Protein | 0.15–0.45 | 0.6 g/L | Glucose | 2.2–3.9 mmol/L | 2.7 mmol/L |
| Lactate | 1.1–2.4 mmol/L | 3.9 mmol/L | Red cells | 0/μL | 0/μL |
| anti-HU | negative | positive | CASPR2 | negative | negative |
| anti-AMPA | negative | negative | anti-LG1 | negative | negative |
| anti-GABA | negative | negative | anti-YO | negative | negative |
| anti-NMDAR | negative | negative | anti-RI | negative | negative |
| anti-amphiphysin | negative | negative | oligoclonal bands | negative | negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurrieri, C.; Visentin, A.; Bussè, C.; Piazza, F.; Manara, R.; Trentin, L.; Briani, C. Limbic Encephalitis with HU-Antibodies in T-cell Anaplastic Lymphoma. A Case Report. Appl. Sci. 2021, 11, 6548. https://doi.org/10.3390/app11146548
Gurrieri C, Visentin A, Bussè C, Piazza F, Manara R, Trentin L, Briani C. Limbic Encephalitis with HU-Antibodies in T-cell Anaplastic Lymphoma. A Case Report. Applied Sciences. 2021; 11(14):6548. https://doi.org/10.3390/app11146548
Chicago/Turabian StyleGurrieri, Carmela, Andrea Visentin, Cinzia Bussè, Francesco Piazza, Renzo Manara, Livio Trentin, and Chiara Briani. 2021. "Limbic Encephalitis with HU-Antibodies in T-cell Anaplastic Lymphoma. A Case Report" Applied Sciences 11, no. 14: 6548. https://doi.org/10.3390/app11146548
APA StyleGurrieri, C., Visentin, A., Bussè, C., Piazza, F., Manara, R., Trentin, L., & Briani, C. (2021). Limbic Encephalitis with HU-Antibodies in T-cell Anaplastic Lymphoma. A Case Report. Applied Sciences, 11(14), 6548. https://doi.org/10.3390/app11146548







