Abstract
The ideal biomaterial used in endodontics in the process of sealing the radicular canals should possess a group of qualities for a predictable outcome: biocompatibility, initiation of ontogenesis and cementogenesis, ease of handling, sufficient manipulation time, and convenient price. For a perfect sealing, the root canal treatment can be followed by prosthetic restoration. This study of biocompatibility aims to determine the quantification of the local reaction following the implantation of three biomaterials in the rabbit subcutaneous connective tissue. The used biomaterials with particular reparative properties are: MTA (Mineral Trioxide Aggregate, Dentsply, Tulsa Dental, Johnson City, TN, USA), Sealapex (Kerr, Switzerland), and DiaRoot BioAggregate (Innovative BioCaramix Inc, Vancouver, BC, Canada). The first two biomaterials (MTA, Sealapex) are already being used in endodontic treatments, and the latter was newly introduced during the concrete development of the study. This is an experimental study focused on qualitative and quantitative analysis based on histopathological examination and underlined by the positive result of the study undertaken of the applicability of oral rehabilitation treatments, increasing patients’ quality of life by a significant proportion of 95%, and generating optimal functionality of the stomatognathic system with prosthetic devices as well as accomplishing the objectives of homeostasis.
1. Introduction
An important objective in endodontic therapy is to induce periapical bone repair and to stimulate cementogenesis. The biomaterials used are placed in close contact with both the soft and hard periodontal tissues [1]. The biomaterial may cause local or systemic side effects due to direct contact or through the leakage of the substances released from the material into the periodontal tissue or alveolar bone [2,3].
The biological compatibility of the root sealant is of key importance, because in clinical conditions these materials are put in direct contact with vital tissues, and the tissue response to these materials can influence the outcome of endodontic treatment [3,4].
Considering the above-mentioned aspects, the ideal endodontic repair and sealing materials should possess some of these properties: adherent to the canal walls, in order to ensure a tight seal of the root canal system; nontoxic; easy to manipulate; well tolerated by the periradicular tissues; promoting bone healing, non-resorbable; radiopaque; unaffected by the presence of moisture; and not staining the surrounding tissues [5,6].
Several methods have been used to evaluate the biocompatibility of endodontic cements. One of the most practical and widely used methods is the implantation of the material in the subcutaneous connective tissue in rats [7,8]. The irritating effect of the materials can be evaluated by histopathological examination of the tissue response around the implants.
Ongoing studies aim to create a well-adapted dental structure as a support for further biocompatible restorations in the context of complex oral rehabilitation treatments, so as to reduce the occlusal forces that can be exerted through these materials to keep the oral homeostasis in normal parameters [9].
Aim of Study
Our biocompatibility experimental study aims to evaluate the local reaction following the implantation of three biomaterials in the rabbit subcutaneous connective tissue.
The main objective is to make a comparative assessment to understand the response of living tissues in direct contact to the biomaterials, guided by the answer suggested by the next part of the practical applicability study, by using prosthetic and complex rehabilitation means in the context of dysfunctional syndrome, which recorded a positive result followed over time by their response to applied treatments, following the principles of homeostasis.
2. Materials and Methods
The materials used were as follows:
- MTA (Mineral Trioxide Aggregate, Dentsply, Tulsa Dental, Johnson City, TN, USA) is a material with a highly efficient antibacterial effect and is alkaline, made of calcium hydroxide, bismuth oxide (Bi2O3), calcium sulfate (CaSO4), tricalcium silicate ((CaO)3·SiO2), dicalcium silicate ((CaO)2·SiO2), tricalcium aluminate ((CaO)3·Al2O3).
- Sealapex (Kerr, Switzerland)—used for root canal sealing—has the following chemical composition: barium sulfate, titanium dioxide, zinc oxide, calcium hydroxide, butilbenzen, sulfonamide, zinc stearate.
- DiaRoot BioAggregate (Innovative BioCeramix, Inc., Vancouver, BC, Canada) is a material similar in structure to MTA that additionally contains ceramic nanoparticles. It has proven antiseptic proprieties and at the same time stimulates cementogenesis. The chemical composition includes: calcium silicate, calcium hydroxide, hydroxyapatite, tantalum oxygen (Ta2O5).
The used endodontic materials were prepared according to the instructions of the producer and then were introduced in polyethylene tubes 10 mm in length and 1.5 mm in diameter.
2.1. In Vivo Experiment
Twenty-one Belgian Giant rabbits, aged 4 months and weighing 3.5 kg (±50 g), raised and fed in identical conditions (food and water ad libitum) were used.
The rabbits were divided into 4 groups:
- Group A—6 rabbits—receiving MTA implants;
- Group B—6 rabbits—receiving Sealapex implants;
- Group C—6 rabbits—receiving DiaRoot implants;
- Group D (control)—3 rabbits—receiving empty polyethylene tube implants.
The experimental period lasted 60 days, while the rabbits were kept in similar conditions and were fed identical food, except 24 h prior to the dental implant surgery when they received water but no food. All rabbits were fed a normal diet until the end of the study.
2.2. Surgical and Post-Operative Protocol
The surgical interventions were made under general anesthesia and aseptic conditions. Prior to anesthesia, rabbits received atropine premedication (0.02 mg/kg; Atropina, Pasteur Institute, Bucharest, Romania).
Anesthesia was induced with xylazine (0.1 mg/kg i.m, Xylazine Bio 2%, Bioveta, Czech Republic) and ketamine (10 mg/kg i.m., Ketaminol® 10, Intervet International GmbH, Neufahrn bei Freising, Germany).
Preoperatively, the lateral thoracic regions were shaved and disinfected with antiseptic solution 96% (Videne; Adams Healthcare Ltd., Birmingham, UK).
The rabbits were first placed in the left and then in the right lateral decubitus position.
Skin incisions were made for implanting the tubes (Figure 1).
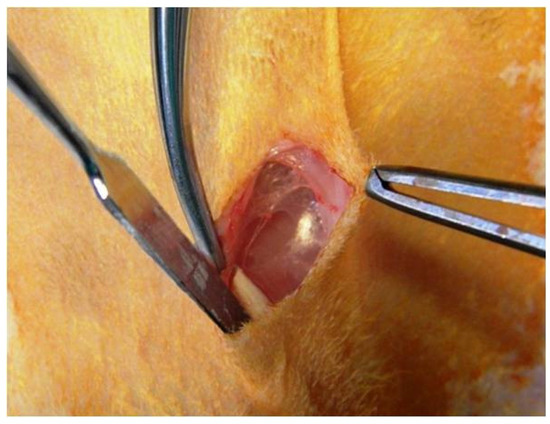
Figure 1.
Subcutaneous technique for implantation of biomaterials.
Tubes were inserted as deep as the created tissue pocket allowed. In the end, the surgical wound was sutured with non-absorbable suture thread (Figure 2).
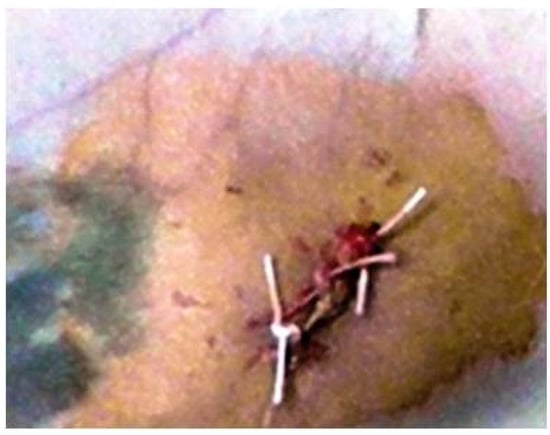
Figure 2.
Wound suture after implant.
For 3 postoperative days, each rabbit was given an analgesic (carprofen 4 mg kg−1 i.sc; Rimadyl®, Pfizer, Tadworth, UK) and prophylactic antibiotic (7.5 mg kg−1 amoxicillin; VEYX® YL LA 200, Veyx-Pharma GmbH, Schwarzenborn, Germany).
At 7, 30, and 60 days after implantation, 2 rabbits in groups A, B, and C and one rabbit in the control group (D) were killed using T61 solution (2 mL/kg, i.p., MSD Animal Health GmbH, Cuxhaven, Germany).
2.3. Histopathological Protocol
Immediately after animals were sacrificed [1], fragments of subcutaneous connective tissue were collected from biomaterial implant sites (Figure 3).
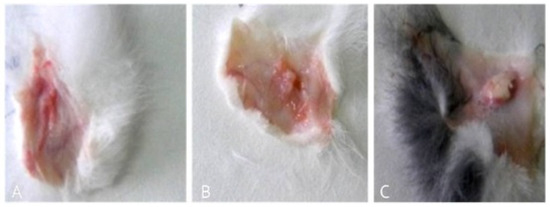
Figure 3.
Samples selected for sampling: (A–C) lax subcutaneous tissue after implantation of three biomaterials.
Briefly after sampling, all specimens were fixed in 10% buffered formalin and embedded in parafine with a tissue processor Leica TP1020 (Leica Microsystems GmbH, Wetzlar, Germany). Sections of 5μm thickness were obtained with a Microtome SLEE CUT 6062 (SLEE Medical GmbH, Mainz, Germany) and then de-paraffinized and stained by the Masson trichrome techniques. The qualitative histology was performed from stained sections using a light microscope Leica DM 750 (Leica Microsystems GmbH, Germany) with an attached digital camera Leica ICC50 HD (Leica Microsystems GmbH, Germany) Germany). The photographs were taken with Leica Application Suit Software (LAS) version 4.2.
3. Results
When the tissue sample was collected, 7 days after implantation, a well-defined fibrous capsule of large size (on average = 3.5/14.2 mm in group B; close values in group A, on average = 3.1/13.6 mm; and the lowest size in group C, on average = 1.9/10.5 mm) was formed (Figure 3A–C).
Seven days after the implantation, the implant sites of the three biomaterials in the four groups (A, B, C, and D) were histologically examined.
In group A (implanted with MTA) we noticed a well-defined area of peripheral necrosis (surrounding the biomaterial) in which incompletely resorbed MTA fragments and an intense influx of leukocytes consisting of macrophages, histiocytic cells, and neutrophils were present, together with a high number of fibroblasts and collagen fibers (Figure 4 and Figure 5).
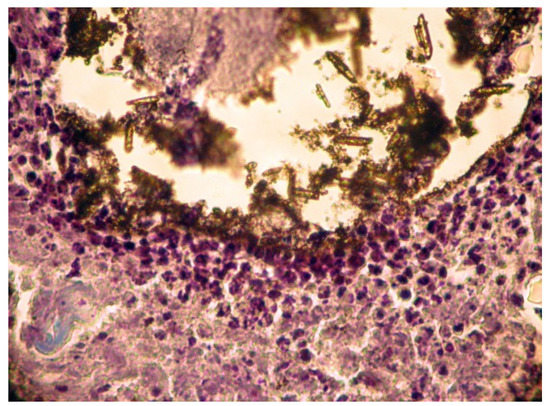
Figure 4.
Group A. Subcutaneous conjunctive tissue. Area of necrosis, intense leukocyte infiltration.
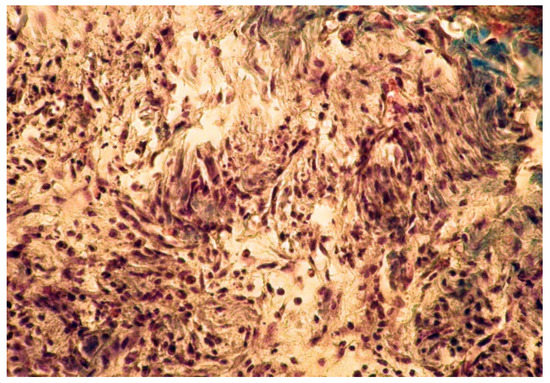
Figure 5.
Group A. Buffer zone, consisting of fibroblast proliferation and collagen fiber synthesis. Col. Trichrome Masson, ×200.
The implanted material was partially reabsorbed. Local mineralization, probably due to the diffusion of released calcium ions from the implant material, was seen in some muscle fibers of the skin (Figure 6).
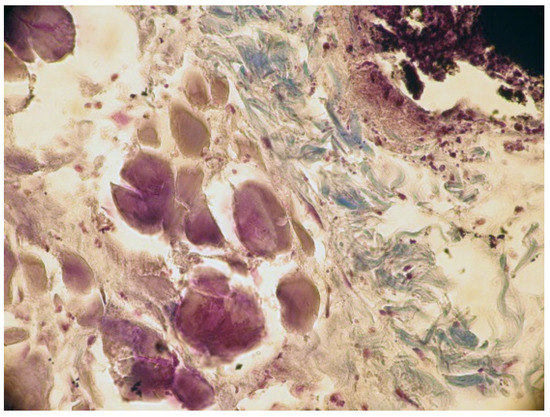
Figure 6.
Calcification of muscular fibers from the skin. Col. Trichrome Masson, ×200.
In group B, the local reaction was slightly reduced in intensity, with the necrotic area being much smaller compared to group A. The present cells, neutrophils, macrophages, and fibroblasts were fewer in number. The implanted material was partially reabsorbing, as shown by the migration of neutrophils and macrophages to the implantation site (Figure 7 and Figure 8).
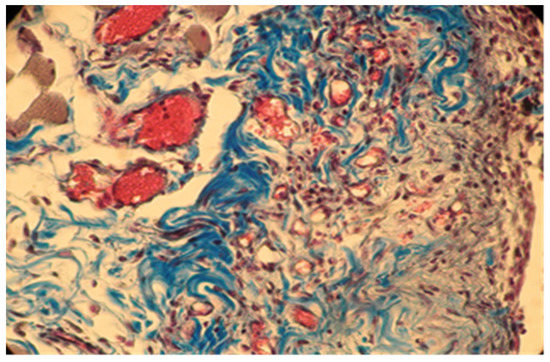
Figure 7.
Group B. Moderate inflammation and mild congestive subcutaneous connective tissue in rabbits at 7 days after implantation. Col. Trichrome Masson, ×400.
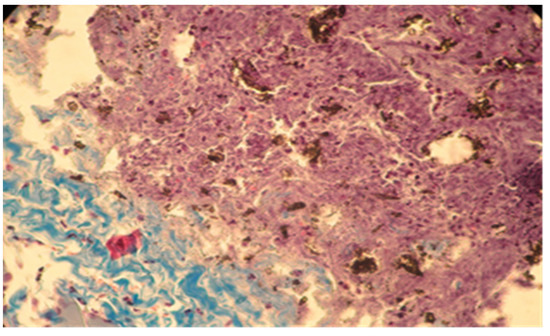
Figure 8.
Moderate necrosis area with pockets of implanted material and moderate leukocyte influx. Partial resorption of the implant material. Subcutaneous tissue, 7 days after implantation. Col. Trichrome Masson, ×200.
In group C, it was noticed that the local inflammatory reaction induced by the implantation of DiaRoot BioAggregate was much less intense than in groups A and B. The area of necrosis was a small band surrounding the implanted material, with fewer neutrophils and macrophages. Fibroblast differentiation was also reduced (Figure 9 and Figure 10).
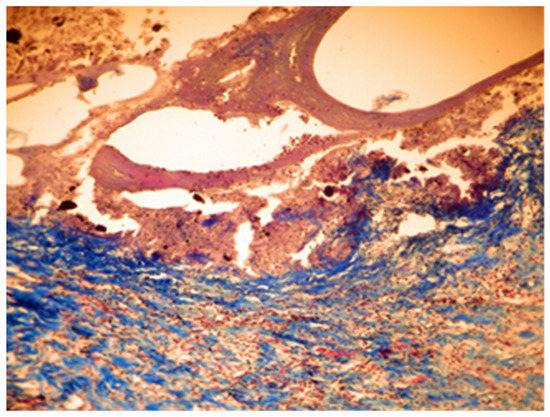
Figure 9.
Group C. Moderate necrosis area with pieces of implanted material and moderate leukocyte influx. Partial resorption of the implant material. Subcutaneous tissue—7 days after implant. Col. Trichrome Masson, ×100.
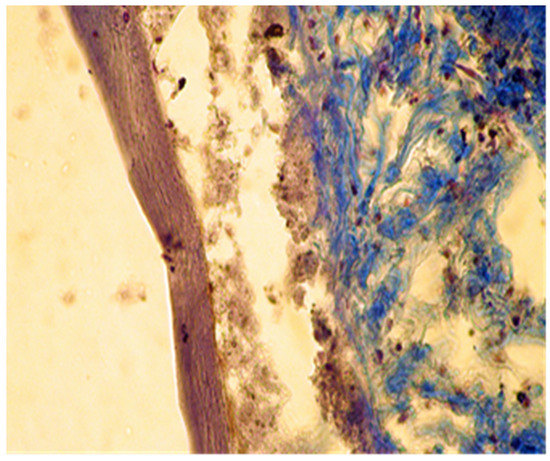
Figure 10.
Group C. Moderate necrosis area with pieces of implanted material and moderate leukocyte influx. Partial resorption of the implanted material. Subcutaneous tissue—7 days after implant. Col. Trichrome Masson, ×400.
In samples collected 30 days after implantation, there was a significant decrease in local cellular reactions in all groups (A, B, and C), and inflammation was almost nonexistent, with rare inflammatory infiltrate cells being observed only in groups B and A (Figure 11 and Figure 12).
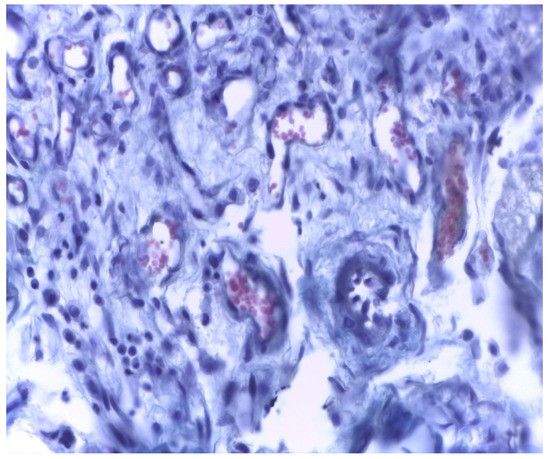
Figure 11.
Group A. Scar tissue and rare inflammatory cells at the implant site with MTA after 30 days. Rare inflammatory cells. Col. Trichrome Masson, ×400.
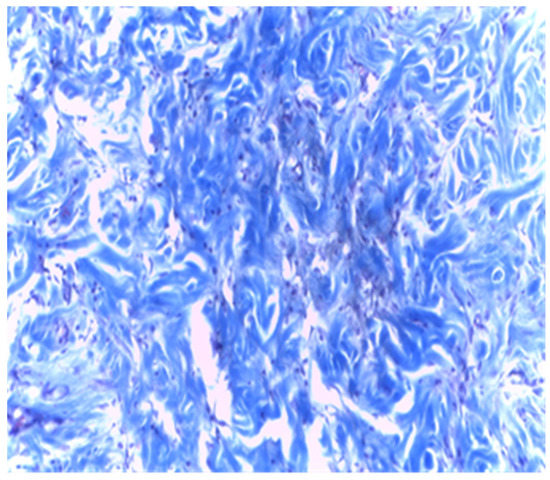
Figure 12.
Group B. Dense subcutaneous tissue over the implant with MTA after 30 days. Rare inflammatory cells. Col. Trichrome Masson, ×400.
4. Discussion
Mineral Trioxide Aggregate (MTA) was recommended in the past as a repair material for root perforations. It was developed by Loma Linda University (in 1993) and was originally introduced as a retrograde filling material [2]. It was shown to result in a much lower percolation in contact with the surrounding tissues in comparison with the most commonly used materials such as amalgam, intermediate restorative material (IRM), and Super-EBA. For this reason, MTA was considered the material of choice for the repair of root perforation [3] since it was shown to be biocompatible with the periradicular tissue (minimal inflammatory response, ability to allow regeneration of hard tissue structures such as bone and cementum) [4], thus facilitating the regeneration of the periodontal supporting apparatus [5,6]. In research on human osteoblast models, it was found that MTA stimulated the production of cytokines, such as interleukin-1α, interleukin-1β, and interleukin-6, which are involved in bone turnover [10].
Sealapex is a calcium hydroxide-based sealer commonly used in endodontics, with both its biostimulating properties and cytotoxicity in contact with the periradicular tissues being extensively discussed in the literature [10,11,12]. In general, Sealapex demonstrated reduced inflammatory reaction as compared to other endodontic sealers, showing moderate inflammation at 48 h that became mild in later periods. Other zinc oxide-eugenol-based sealers (Endoflas, Tubliseal) were toxic after 48 h and 7 days. The toxicity decreased gradually in later periods [13].
The cytotoxicity of BioAggregate (BA), the third material used in the study, was also evaluated, as compared to that of MTA, on mesenchymal cell cultures, with the results showing that there was no statistically significant difference between MTA and BA in any of the experimental time periods. DiaRoot BioAggregate displayed in vitro compatibility similar to MTA [14].
In the present study, 7 days after implantation, the presence of more pronounced inflammatory reactions and the presence of multinucleated giant cells were histologically noted in contact with the remaining enclaves of Sealapex and MTA, while in the BA samples, the inflammatory reaction was considerably reduced, as demonstrated by the absence of these cells and reduced inflammatory cell infiltrate. This indicates a biocompatibility of BA with the living subcutaneous tissue, compared to the first two materials. The inflammation [12] and the presence of thicker bands of necrotic tissue in contact with the implanted materials (thicker in MTA compared to Sealapex and BA) after the first 7 days may be accounted for by both the different chemical composition of the cements and the high alkaline pH of MTA: after manipulation, the pH of MTA was 10.2 initially and rose to 12.5, 3 h after mixing, remaining constant thereafter. The maximum pH of Sealapex is 9.1 and that of BA is 12 after setting [15].
At 30 days after implantation, histological analysis [14] of the samples showed no significant differences between the three studied materials [15], with several isolated groups of inflammation cells [16] being found only in one Sealapex sample and one MTA sample.
The fact that no inflammation reaction was histologically detected 3 months after subcutaneous implantation with any of the used materials [17] is due to the phagocytic cell activity and tissue repair capacity [18] characteristic to these animals.
All kinds of specialized research on types of biomaterials used for endodontic restorations consider in the end the benefit of the patients, ensuring good biocompatibility, integration, stability, and functionality so that the predictable prosthetic rehabilitation increases quality of life by a proportion of 95% for the patients. Unidental restoration and Richmond crown result in an increase of 45%, and double prosthetic pieces elements result in an increase of 32% in a multiple construction bridge. A special importance can be observed in the approach of patients who presented clinical signs of dysfunctional syndrome in 25% who experienced pain, 15% with clinical signs of joint cracks and crepitation, and muscle dysfunctions in 35%, all due to occlusal imbalances, which require treatment with mouth guards and balneal–physical–kinetic therapy rehabilitation complex treatment in order to accomplish the principles of homeostasis and its stability [18,19,20] (Figure 13).
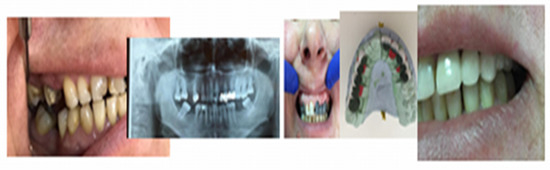
Figure 13.
Images of the initial clinical situation, paraclinical orthopantomography examination, and the occlusal rehabilitation for the necessary foundation of the stomatognathic system homeostasis.
We consider the possibility of prosthetic rehabilitation an important stage in which from the start we observe the efficiency of those biomaterials with which we experimented in our study. Functionality and integration can be ways to quantify the study’s goal.
In this case, increased efficiency and optimization of the functions of the stomatognathic system are observed resulting from the type of prosthesis and the other treatments related to the rehabilitation.
5. Conclusions
- − Based on the reaction of the three materials, it can be assumed that the most biocompatible material is DiaRoot BioAggregate, followed by Sealapex and MTA.
- − The smallest necrotic area and lowest cellularity were seen around the DiaRoot BioAggregate. Seven days after implantation, the material best preserved in tissues was BA, meaning that it was the least reabsorbing.
- − The other two implanted materials significantly induced tissue irritation, histologically reflected by a thicker necrotic area and a considerable neutrophils and macrophages influx.
- − MTA was found to have the highest local mineralization effect, based on the mineralization of adjacent muscle fibers.
- − The fact that fibroblast differentiation and synthesis of connective tissue fibers were observed around the implant site 7 days after the MTA implantation, a reaction absent in the case of the other two materials, proves a slightly lower biocompatibility of MTA compared to BioAggregate.
- − In the end, the advantage for patients is in creating better comfort under the premises of a good biocompatibility and integration in order to sustain all types of further individual prosthetic rehabilitation treatments and to ultimately obtain the attributes of homeostasis.
Author Contributions
Conceptualization, L.A. and L.E.C.; methodology, E.V.S. and A.S.P.; software, L.F.; validation, O.S. and B.P.B.; formal analysis, C.A., E.M. and Y.D.; investigation, L.I.C.; resources, E.V.S., A.S.P., O.S. and B.P.B.; data curation, L.I.C.; writing—original draft preparation, L.F.; writing—review and editing, L.A. and L.E.C.; visualization, E.V.S.; supervision, A.S.P.; project administration, C.A., E.M. and Y.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according with national and international regulations on animal welfare, identification, control andelimination of factors causing physiological and behavioral disorders: Directive EC86/609 EU; Government Ordinance no.37/2002, approved by Law no. 471/2002; Law 205/2004 on animal protection, amended and supplemented by Law no. 9/2008. The study was approved by the Ethics Committee of Faculty of Veterinary Medicine Iasi (Protocol Code No. 533 / 22.04.2020).
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Parliament, Council of the European Union. Directive 2010/63/EU of the European Parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, L276, 33–79. [Google Scholar]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An up-dated overview—part II: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef] [PubMed]
- Mathew, L.A.; Kini, S.; Acharya, S.R.; Kamath, S.; Menezes, N.D.; Rao, S. A comparative evaluation of the microleakage of blood-contaminated mineral trioxide aggregate and Biodentine as rot-end filling materials: An in vitro study. J. Interdiscip. Dent. 2016, 6, 19–24. [Google Scholar] [CrossRef]
- Asokan, S.; Samuel, A.; Priya, P.R.G.; Thomas, S. Evaluation of sealing ability of Biodentine™ and mineral trioxide aggregate in primary molars using scanning electron microscope: A randomized controlled in vitro trial. Contemp. Clin. Dent. 2016, 7, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, Y. Mineral Trioxide Aggregate Repair of Perforated Internal Resorption: A Case Report. JOHCD 2012, 6, 149–150. [Google Scholar] [CrossRef] [Green Version]
- Sinkar, R.C.; Patil, S.S.; Jogad, N.P.; Gade, V.J. Comparison of sealing ability of ProRoot MTA, RetroMTA, and Biodentine as furcation repair materials: An ultraviolet spectrophotometric analysis. J. Conserv. Dent. 2015, 18, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M. Mineral trioxide aggregate: A comprehensive literature review—Part II: Leakage and biocom-patibility investigations. J. Endod. 2010, 36, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Jafarnia, B.; Jiang, J.; He, J.; Wang, Y.-H.; Safavi, K.E.; Zhu, Q. Evaluation of cytotoxicity of MTA employing various additives. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lozano, F.J.; García-Bernal, D.; Onate-Sanchez, R.E.; Ortolani-Seltenerich, P.S.; Forner, L.; Moraleda, J.M. Evaluation of cytocompatibility of calcium silicate-based endodontic sealers and their efects on the biological responses of mesenchymal dental stem cells. Int. Endod. J. 2017, 50, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scelza, M.Z.; Linhares, A.B.; Silva, L.E.; Granjeiro, J.M.; Alves, G. A multiparametric assay to compare the cytotoxicity of endodontic sealers with primary human osteoblasts. Int. Endod. J. 2011, 45, 12–18. [Google Scholar] [CrossRef] [PubMed]
- De-Deus, G.; Canabarro, A.; Alves, G.; Linhares, A.; Senne, M.I.; Granjeiro, J.M. Optimal cyto-compatibility of a bioceramic nanoparticulate cement in primary human mesenchymal cells. J. Endod. 2009, 10, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Klasser, G.D.; Green, C.S. The changing field of Temporomandibular disorders: What dentists need to know. JCDA 2009, 5, 49–53. [Google Scholar]
- Estrela, C.; Bammann, L.L.; Estrela, C.R.D.A.; Silva, R.S.; Pécora, J.D. Antimicrobial and Chemical Study of MTA, Portland Cement, Calcium Hydroxide Paste, Sealapex and Dycal. Braz. Dent. J. 2000, 11, 3–9. [Google Scholar] [PubMed]
- Simsek, N.; Bulut, E.T.; Ahmetoğlu, F.; Alan, H. Determination of trace elements in rat organs implanted with endodontic repair materials by ICP-MS. J. Mater. Sci. Mater. Med. 2016, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Carausu, E.M.; Trandafir, V.; Ghibu, L.; Stamatin, O.; Checherita, L.E. Study of Electrolyte Serum Disturbances and Acid-base Status in Patients with Oral-maxillofacial and Dental Sepsis. Rev. Chim. 2017, 68, 1552–1556. [Google Scholar] [CrossRef]
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Cintra, L.; Tessarin, G.W.L.; Chiba, F.Y.; Mattera, M.S.D.L.C.; Scaramele, N.; Tsosura, T.V.S.; Ervolino, E.; Oliveira, S.; Sumida, D.H. Periapical Lesions Increase Macrophage Infiltration and Inflammatory Signaling in Muscle Tissue of Rats. J. Endod. 2017, 43, 982–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Totu, E.E.; Cristache, C.M.; Isildak, I.; Yildirim, R.; Burlibasa, M.; Nigde, M.; Burlbasa, L. Preliminary Studies on Citotoxicity and Genotoxicity Assessment of the PMMA-TiO2 Nanocompozites for Stereolithographic Complete Dentures Manufacturing. Rev. Chim. 2018, 69, 1160–1165. [Google Scholar] [CrossRef]
- Ogunlewe, M.O.; Agbelusi, G.A.; Gbotolorun, O.M.; James, O. A review of temporomandibular joint disorders (TMD’s) pre-senting at the Lagos University Teaching Hospital. Nig. Q. J. Hosp. Med. 2008, 18, 57–60. [Google Scholar] [PubMed]
- Chisnoiu, A.M.; Picos, A.M.; Popa, S.; Chisnoiu, P.D.; Lascu, L.; Picos, A.; Chisnoiu, R. Factors involved in the etiology of temporomandibular disorders-a literature review. Clujul Med. 2015, 88, 473. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).