Test-Retest Reliability of a 6DoF Marker Set for Gait Analysis in Cerebral Palsy Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants Selection
2.3. Gait Protocol
2.4. Data Processing
2.5. Statistical Methods
3. Results
3.1. Reliability of Anthropometric Parameters
3.2. Reliability of Time-Distance Parameters
3.3. Reliability of Kinematic Parameters
3.4. Reliability of Kinetic Parameters
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenbaum, P. Definition and Clinical Classification. In Cerebral Palsy: Science and Clinical Practice, 1st ed.; Dan, B., Mayston, M., Paneth, N., Rosenbloom, L., Eds.; Mac Keith Press: London, UK, 2014; pp. 17–26. [Google Scholar]
- Graham, K.K.H.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.-P.; Damiano, D.L.; Becher, J.G.J.; Gaebler-Spira, D.; Colver, A.A.; Reddihough, D.S.D.; et al. Cerebral palsy. Nat. Rev. Dis. Prim. 2016, 2, 15082. [Google Scholar] [CrossRef]
- Eunson, P. Aetiology and epidemiology of cerebral palsy. Paediatr. Child Health 2016, 26, 367–372. [Google Scholar] [CrossRef]
- Surveillance of cerebral palsy in Europe: A collaboration of cerebral palsy surveys and registers. Dev. Med. Child Neurol. 2000, 42, 816–824. [CrossRef]
- Cerebral Palsy. In Cerebral Palsy; Springer: Berlin/Heidelberg, Germany, 2018; pp. 19–28.
- Colver, A.; Fairhurst, C.; Pharoah, P. Cerebral palsy. Lancet 2014, 383, 1240–1249. [Google Scholar] [CrossRef]
- Maenner, M.J.; Blumberg, S.J.; Kogan, M.D.; Christensen, D.; Yeargin-Allsopp, M.; Schieve, L.A. Prevalence of cerebral palsy and intellectual disability among children identified in two U.S. National Surveys, 2011–2013. Ann. Epidemiol. 2016, 26, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Hoffer, M.M.; Perry, J. Pathodynamics of Gait Alterations in Cerebral Palsy and the Significance of Kinetic Electromyography in Evaluating Foot and Ankle Problems. Foot Ankle 1983, 4, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M. The Definition and Classification of Cerebral Palsy. Dev. Med. Child Neurol. 2007, 49, 1–44. [Google Scholar]
- Wright, E.; Dibello, S.A. Principles of Ankle-Foot Orthosis Prescription in Ambulatory Bilateral Cerebral Palsy. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 69–89. [Google Scholar] [CrossRef] [PubMed]
- McGinley, J.L.; Baker, R.; Wolfe, R.; Morris, M.E. The reliability of three-dimensional kinematic gait measurements: A systematic review. Gait Posture 2009, 29, 360–369. [Google Scholar] [CrossRef]
- Altman, D. Practical Statistics for Medical Research, 2nd ed.; Chapman & Hall/CRC: London, UK, 2020. [Google Scholar]
- Molina-Rueda, F.; Fernández-González, P.; Cuesta-Gómez, A.; Koutsou, A.; Carratalá-Tejada, M.; Miangolarra-Page, J. Test-Retest Reliability of a Conventional Gait Model for Registering Joint Angles during Initial Contact and Toe-Off in Healthy Subjects. Int. J. Environ. Res. Public Health 2021, 18, 1343. [Google Scholar] [CrossRef]
- Horsak, B.; Pobatschnig, B.; Baca, A.; Greber-Platzer, S.; Kreissl, A.; Nehrer, S.; Wondrasch, B.; Crevenna, R.; Keilani, M.; Kranzl, A. Within-assessor reliability and minimal detectable change of gait kinematics in a young obese demographic. Gait Posture 2017, 54, 112–118. [Google Scholar] [CrossRef]
- Stief, F. Variations of marker sets and models for standard gait analysis. In Handbook of Human Motion, 1st ed.; Müller, B., Wolf, S., Eds.; Springer AG: Cham, Switzerland, 2018; pp. 509–523. [Google Scholar]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E.; Gainey, J.; Gorton, G.; Cochran, G.V.B. Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. J. Orthop. Res. 1989, 7, 849–860. [Google Scholar] [CrossRef]
- Ferrari, A.; Benedetti, M.G.; Pavan, E.E.; Frigo, C.; Bettinelli, D.; Rabuffetti, M.; Crenna, P.; Leardini, A. Quantitative comparison of five current protocols in gait analysis. Gait Posture 2008, 28, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Leboeuf, F.; Reay, J.; Sageux, M. The Conventional Gait Model—Success and Limitations. In Handbook of Human Motion, 1st ed.; Müller, B., Wolf, S., Eds.; Springer AG: Cham, Switzerland, 2018; pp. 490–505. [Google Scholar]
- Collins, T.D.; Ghoussayni, S.N.; Ewins, D.J.; Kent, J.A. A six degrees-of-freedom marker set for gait analysis: Repeatability and comparison with a modified Helen Hayes set. Gait Posture 2009, 30, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, D.; Simms, C. Reliability and measurement error of multi-segment trunk kinematics and kinetics during cerebral palsy gait. Med. Eng. Phys. 2020, 75, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Armadadasilva, P.A.S.; Pool-Goudzwaard, A.L.; Moniz-Pereira, V.; Veloso, A.P. Test–retest reliability and minimal detectable change of three-dimensional gait analysis in chronic low back pain patients. Gait Posture 2015, 42, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Barré, A.; Thiran, J.-P.; Jolles, B.M.; Theumann, N.; Aminian, K. Soft Tissue Artifact Assessment during Treadmill Walking in Subjects with Total Knee Arthroplasty. IEEE Trans. Biomed. Eng. 2013, 60, 3131–3140. [Google Scholar] [CrossRef]
- Steinwender, G.; Saraph, V.; Scheiber, S.; Zwick, E.B.; Uitz, C.; Hackl, K. Intrasubject repeatability of gait analysis data in normal and spastic children. Clin. Biomech. 2000, 15, 134–139. [Google Scholar] [CrossRef]
- Miller, F.; Castagno, P.; Richards, J.; Lennon, N.; Quigley, E.; Niiler, T. Reliablity of kinematics during clinical gait analysis: A comparison between normal and children with cerebal palsy. Gait Posture 1996, 4, 169–170. [Google Scholar] [CrossRef]
- Noonan, K.J.; Halliday, S.; Browne, R.; O’Brien, S.; Kayes, K.; Feinberg, J. Interobserver Variability of Gait Analysis in Patients with Cerebral Palsy. J. Pediatr. Orthop. 2003, 23, 279–287. [Google Scholar] [CrossRef]
- Mackey, A.; Walt, S.E.; Lobb, G.A.; Stott, N.S. Reliability of upper and lower limb three-dimensional kinematics in children with hemiplegia. Gait Posture 2005, 22, 1–9. [Google Scholar] [CrossRef]
- Røislien, J.; Skare, Ø.; Opheim, A.; Rennie, L. Evaluating the properties of the coefficient of multiple correlation (CMC) for kinematic gait data. J. Biomech. 2012, 45, 2014–2018. [Google Scholar] [CrossRef]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Gibson, J. Gibson Theory of Affordances, 1st ed.; Lawrence Erlbaum Associates, Inc.: Mahwah, NJ, USA, 1986. [Google Scholar]
- Chiari, L.; Della Croce, U.; Leardini, A.; Cappozzo, A. Human movement analysis using stereophotogrammetry: Part 2: Instrumental errors. Gait Posture 2005, 21, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Moniz-Pereira, V.; Cabral, S.; Carnide, F.; Veloso, A.P. Sensitivity of Joint Kinematics and Kinetics to Different Pose Estimation Algorithms and Joint Constraints in the Elderly. J. Appl. Biomech. 2013, 30, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Diss, C. The reliability of kinetic and kinematic variables used to analyse normal running gait. Gait Posture 2001, 14, 98–103. [Google Scholar] [CrossRef]
- Schmid, S.; Romkes, J.; Taylor, W.R.; Lorenzetti, S.; Brunner, R. Orthotic correction of lower limb function during gait does not immediately influence spinal kinematics in spastic hemiplegic cerebral palsy. Gait Posture 2016, 49, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, K.; Delahunt, E.; Caulfield, B. Increasing the number of gait trial recordings maximises intra-rater reliability of the CODA motion analysis system. Gait Posture 2007, 25, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, K.; Miller, E.; Kingsbury, T.; Esposito, E.R.; Wolf, E.; Wilken, J.; Wyatt, M. Reliability of 3D gait data across multiple laboratories. Gait Posture 2016, 49, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Cappozzo, A.; Catani, F.; Croce, U.D.; Leardini, A. Position and orientation in space of bones during movement: Anatomical frame definition and determination. Clin. Biomech. 1995, 10, 171–178. [Google Scholar] [CrossRef]
- Bell, L.; Pedersen, R.; Brand, A. A comparison of the accuracy of several hip center location prediction methods. J. Biomech. 1990, 23, 617–621. [Google Scholar] [CrossRef]
- Cappello, A.; Francesco, P.; Palombara, L.; Leardini, A. Optimization and smoothing techniques in movement analysis. Int. J. Biomed. Comput. 1996, 41, 137–151. [Google Scholar] [CrossRef]
- Dempster, W.T. Space Requirements of the Seated Operator, Geometrical, Kinematic, and Mechanical Aspects of the Body with Special Reference to the Limbs; Wright-Patterson Air Force Base: Dayton, OH, USA, 1955. [Google Scholar]
- Hanavan, E.P. A Mathematical Model of the Human Body; Office of Technical Services: Dayton, OH, USA, 1964.
- Wu, G.; Siegler, S.; Allard, P.; Kirtley, C.; Leardini, A.; Rosenbaum, D.; Whittle, M.; D’Lima, D.D.; Cristofolini, L.; Witte, H.; et al. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion—Part I: Ankle, hip, and spine. J. Biomech. 2002, 35, 543–548. [Google Scholar] [CrossRef]
- Robertson, D.G.E.; Caldwell, G.E.; Hamill, J.; Kamen, G.; Whittlesey, S.N. Research Methods in Biomechanics, 2nd ed.; Human Kinetics Publisher: Champaign, IL, USA, 2014. [Google Scholar]
- Grood, E.S.; Suntay, W.J. A Joint Coordinate System for the Clinical Description of Three-Dimensional Motions: Application to the Knee. J. Biomech. Eng. 1983, 105, 136–144. [Google Scholar] [CrossRef]
- Quigley, E.; Miller, F.; Castagno, P.; Richards, J.; Lennon, N. Variability of gait measurements for typically developing children and children with cerebral palsy. Gait Posture 1999, 10, 58. [Google Scholar] [CrossRef]
- Mcgraw, K.; Wong, S. Forming Inferences about Some Intraclass Correlation Coefficients. Psychol. Methods 1996, 1, 30–46. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zeng, L.; Lin, Z.-J.; Cazzell, M.; Liu, H. Tutorial on use of intraclass correlation coefficients for assessing intertest reliability and its application in functional near-infrared spectroscopy–based brain imaging. J. Biomed. Opt. 2015, 20, 050801. [Google Scholar] [CrossRef]
- Rodda, J.M.; Graham, H.K.; Carson, L.; Galea, M.P.; Wolfe, R. Sagittal gait patterns in spastic diplegia. J. Bone Jt. Surg. 2004, 86, 251–258. [Google Scholar] [CrossRef]
- Winters, T.F., Jr.; Gage, J.R.; Hicks, R. Gait patterns in spastic hemiplegia in children and young adults. J. Bone Jt. Surg. 1987, 69, 437–441. [Google Scholar]
- Leardini, A.; Chiari, L.; Croce, U.D.; Cappozzo, A. Human movement analysis using stereophotogrammetry: Part 3. Soft tissue artifact assessment and compensation. Gait Posture 2005, 21, 212–225. [Google Scholar] [CrossRef]
- Akbarshahi, M.; Schache, A.G.; Fernandez, J.W.; Baker, R.; Banks, S.; Pandy, M.G. Non-invasive assessment of soft-tissue artifact and its effect on knee joint kinematics during functional activity. J. Biomech. 2010, 43, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Benoit, D.; Damsgaard, M.; Andersen, M. Surface marker cluster translation, rotation, scaling and deformation: Their contribution to soft tissue artefact and impact on knee joint kinematics. J. Biomech. 2015, 48, 2124–2129. [Google Scholar] [CrossRef]
- Camomilla, V.; Bonci, T.; Dumas, R.; Cheze, L.; Cappozzo, A. A model of the soft tissue artefact rigid component. J. Biomech. 2015, 48, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Reinschmidt, C.; Bogert, A.; Lundberg, A.; Nigg, B.M.; Murphy, N.; Alta, T. Tibiofemoral and tibiocalcaneal motion during walking: External vs. skeletal markers. Gait Posture 1997, 6, 98–109. [Google Scholar] [CrossRef]
- Schlough, K.; Andre, K.; Owen, M.; Adelstein, L.; Hartford, M.C.; Javier, B.; Kern, R. Differentiating between Idiopathic Toe Walking and Cerebral Palsy: A Systematic Review. Pediatr. Phys. Ther. 2020, 32, 2–10. [Google Scholar] [CrossRef]
- Hicks, R.; Durinick, N.; Gage, J. Differentiation of idiopathic toe-walking and cerebral palsy. J. Pediatr. Orthop. 1988, 8, 160–163. [Google Scholar] [CrossRef]
- Winter, D.A. Kinematic and kinetic patterns in human gait: Variability and compensating effects. Hum. Mov. Sci. 1984, 3, 51–76. [Google Scholar] [CrossRef]
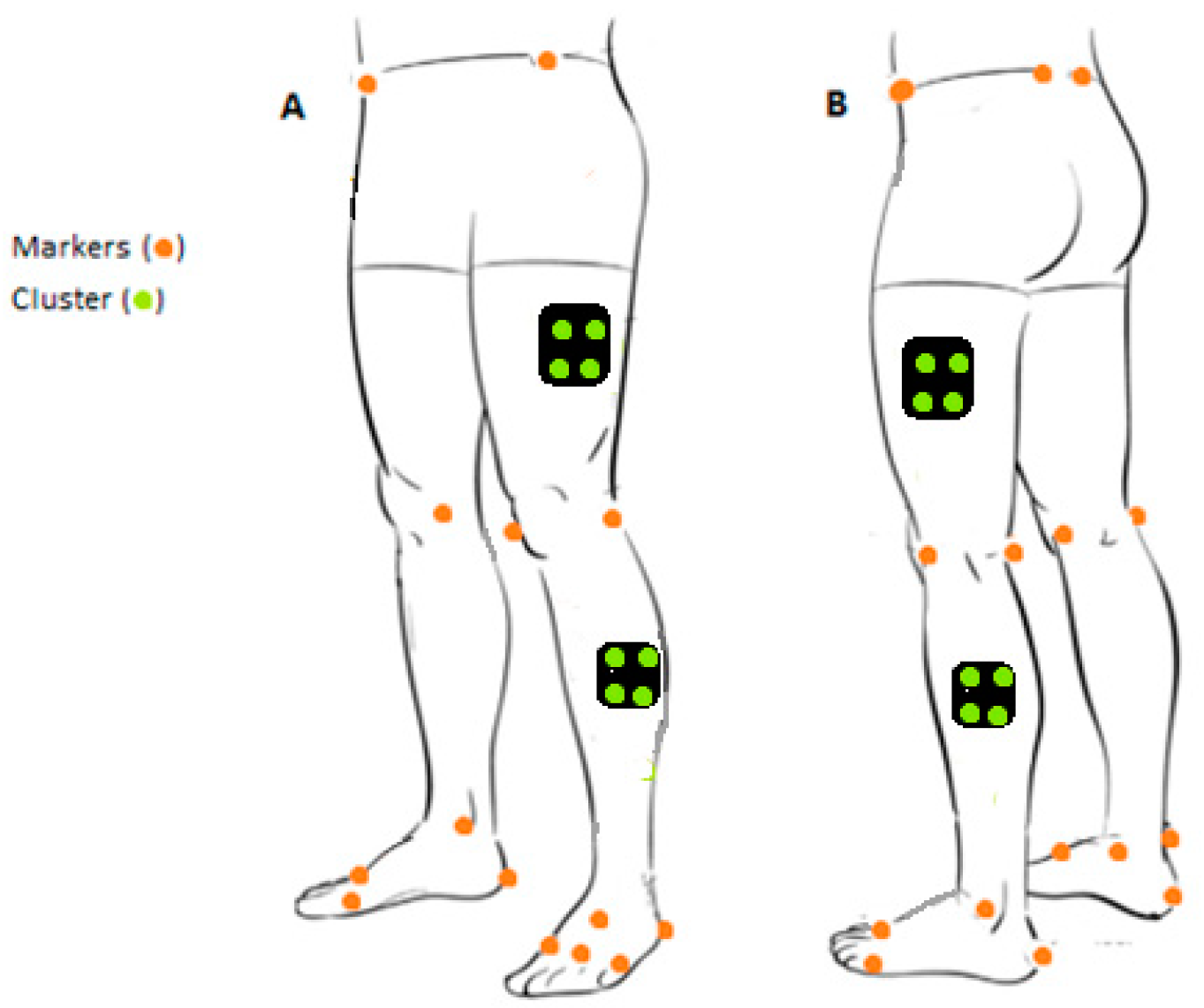
| Left Lower Limb | Right Lower Limb | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | Affected Side | Height (m) | Mass (Kg) | True Leg Length (cm) | Sagittal Gait Pattern | Gastrocnemius Spasticity (Modified Ashworth Scale) | True Leg Length (cm) | Sagittal Gait Pattern | Gastrocnemius Spasticity (Modified Ashworth Scale) |
| 001 | Bilateral | 1.09 | 19.5 | 52.5 | True equinus [48] | 1+ | 54.5 | True equinus [48] | 2 |
| 002 | Unilateral | 1.14 | 26 | 54.6 | Normal | 0 | 54.3 | True equinus [49] | 2 |
| 003 | Bilateral | 1.32 | 26 | 66 | Apparent equinus [48] | 1+ | 66 | Apparent equinus [48] | 1+ |
| 004 | Unilateral | 0.98 | 13.5 | 46 | True equinus [48] | 1+ | 45 | Normal | 0 |
| 005 | Bilateral | 1.37 | 34 | 71 | Apparent equinus [48] | 2 | 70.5 | Apparent equinus [48] | 2 |
| 006 | Unilateral | 1.32 | 37 | 70.2 | Normal | 0 | 70.1 | True equinus with recurvatum knee [49] | 1+ |
| 007 | Bilateral | 1.06 | 15.5 | 52 | True equinus [48] | 3 | 52.7 | True equinus [48] | 3 |
| 008 | Bilateral | 1.10 | 18 | 54 | Jump gait [48] | 2 | 54.5 | Jump gait [48] | 2 |
| Anthropometric Parameters | ICC | ICC 95% CI | Mean | Mean Diff | Mean Diff 95% CI | 95% LOA | SEM | |
|---|---|---|---|---|---|---|---|---|
| Pelvis Segment Depth (cm) | 0.98 | (0.93, 0.99) | 13.2 | 0.2 | (−0.2, 0.7) | 0.6 | (−0.97, 1.40) | 0.4 |
| Inter ASIS Distance (cm) | 0.98 | (0.94, 0.99) | 17.3 | −0.1 | (−0.7, 0.3) | 0.6 | (−1.50, 1.13) | 0.4 |
| Right Tight Segment Length (cm) | 0.99 | (0.97, 0.99) | 26.6 | −0.1 | (−0.7, 0.4) | 0.7 | (−1.55, 1.20) | 0.5 |
| Left Tight Segment Length (cm) | 0.99 | (0.89, 0.99) | 26.7 | −0.5 | (−0.9, 0.1) | 0.4 | (−1.50, 0.42) | 0.3 |
| Right Leg Segment Length (cm) | 0.99 | (0.95, 0.99) | 25.8 | 0.1 | (−0.7, 0.8) | 0.9 | (−1.68, 1.85) | 0.6 |
| Left Leg Segment Length (cm) | 0.99 | (0.97, 0.99) | 25.9 | 0.3 | (−0.0, 0.7) | 0.4 | (−0.53, 1.23) | 0.3 |
| Right Foot Segment Length (cm) | 0.97 | (0.86, 0.99) | 8.8 | 0.1 | (−0.2, 0.4) | 0.4 | (−0.76, 0.96) | 0.3 |
| Left Foot Segment Length (cm) | 0.96 | (0.83, 0.99) | 9.0 | 0.1 | (−0.3, 0.5) | 0.5 | (−1.01, 1.21) | 0.4 |
| Average | 0.98 | 0.4 |
| Time-Distance Parameters | ICC | ICC 95% CI | Mean | Mean Diff | Mean Diff 95% CI | 95% LOA | SEM | |
|---|---|---|---|---|---|---|---|---|
| Speed (m/s) | 0.78 | (0.08, 0.99) | 0.82 | −0.08 | (−0.21, 0.06) | 0.16 | (−0.40, 0.24) | 0.12 |
| Cycle Time (s) | 0.86 | (0.34, 0.97) | 0.92 | 0.04 | (−0.06, 0.13) | 0.11 | (−0.19, 0.26) | 0.08 |
| Double Limb Support Time (s) | 0.84 | (0.01, 0.97) | 0.2 | 0.05 | (0.01, 0.09) | 0.05 | (−0.05, 0.15) | 0.03 |
| Stride Length (m) | 0.94 | (0.65, 0.99) | 0.74 | −0.04 | (−0.08, 0.01) | 0.05 | (−0.14, 0.07) | 0.04 |
| Stride Width (m) | 0.94 | (0.73, 0.99) | 0.12 | 0.01 | (0.00, 0.02) | 0.02 | (−0.02, 0.04) | 0.01 |
| Average | 0.87 | 0.06 | ||||||
| Left lower Limb | ||||||||
| Cycle Time (s) | 0.84 | (0.31, 0.97) | 0.92 | 0.06 | (−0.05, 0.16) | 0.12 | (−0.19, 0.30) | 0.09 |
| Stance Time (s) | 0.85 | (0.33, 0.97) | 0.58 | 0.05 | (−0.03, 0.13) | 0.10 | (−0.15, 0.25) | 0.07 |
| Swing Time(s) | 0.76 | (0.00, 0.95) | 0.35 | 0.01 | (−0.03, 0.04) | 0.04 | (−0.07, 0.08) | 0.03 |
| Step Time (s) | 0.79 | (0.00, 0.96) | 0.45 | 0.01 | (−0.04, 0.06) | 0.06 | (−0.11, 0.13) | 0.04 |
| Step Length (m) | 0.93 | (0.63, 0.99) | 0.38 | 0.00 | (−0.03, 0.03) | 0.04 | (−0.08, 0.08) | 0.03 |
| Stride Length (m) | 0.93 | (0.63, 0.99) | 0.75 | 0.00 | (−0.07, 0.07) | 0.08 | (−0.16, 0.16) | 0.06 |
| Average | 0.85 | 0.05 | ||||||
| Right lower Limb | ||||||||
| Cycle Time (s) | 0.86 | (0.30, 0.97) | 0.93 | 0.02 | (−0.08, 0.12) | 0.12 | (−0.21, 0.25) | 0.08 |
| Stance Time (s) | 0.87 | (0.44, 0.97) | 0.57 | 0.04 | (−0.03, 0.10) | 0.08 | (−0.12, 0.19) | 0.05 |
| Swing Time(s) | 0.84 | (0.24, 0.97) | 0.36 | −0.02 | (−0.06, 0.02) | 0.05 | (−0.11, 0.08) | 0.03 |
| Step Time (s) | 0.79 | (0.00, 0.96) | 0.46 | 0.00 | (−0.07, 0.07) | 0.09 | (−0.16, 0.17) | 0.06 |
| Step Length (m) | 0.64 | (0.00, 0.93) | 0.36 | −0.05 | (−0.12, 0.02) | 0.08 | (−0.21, 0.11) | 0.06 |
| Stride Length (m) | 0.64 | (0.00, 0.93) | 0.72 | −0.11 | (−0.24, 0.03) | 0.16 | (−0.42, 0.21) | 0.11 |
| Average | 0.73 | 0.07 | ||||||
| Kinematic Parameters | ICC | ICC 95% CI | Mean | Mean Diff | Mean Diff 95% CI | 95% LOA | SEM | |
|---|---|---|---|---|---|---|---|---|
| Pelvic joint angle (°) | ||||||||
| Left lower Limb | ||||||||
| Anterior Tilt + | 0.40 | (0.00, 0.88) | 16.0 | −0.1 | (−5.2, 5.0) | 6.1 | (−12.24, 12.02) | 4.3 |
| Posterior Tilt − | 0.83 | (0.20, 0.97) | 10.4 | −1.2 | (−5.2, 2.8) | 4.7 | (−10.58, 8.19) | 3.3 |
| Obliquity Up + | 0.84 | (0.20, 0.97) | 2.7 | 0.5 | (−1.7, 2.7) | 2.6 | (−4.69, 5.69) | 1.8 |
| Obliquity Down − | 0.75 | (0.00, 0.95) | −4.5 | 0.2 | (−1.9, 2.3) | 2.5 | (−4.87, 5.28) | 1.8 |
| External Rotation − | 0.44 | (0.00, 0.89) | −6.6 | 0.2 | (−6.4, 7.0) | 8.0 | (−15.55, 16.10) | 5.3 |
| Internal Rotation + | 0.76 | (0.00, 0.95) | 13.7 | 1.1 | (−5.0, 7.2) | 7.3 | (−13.32, 15.55) | 5.2 |
| Average | 0.67 | 3.6 | ||||||
| Right lower Limb | ||||||||
| Anterior Tilt + | 0.51 | (0.00, 0.91) | 16.1 | −0.8 | (−6.1, 4.3) | 6.2 | (−13.15, 11.37) | 4.4 |
| Posterior Tilt − | 0.84 | (0.31, 0.97) | 10.3 | −2.2 | (−6.1, 1.6) | 4.6 | (−11.30, 6.82) | 3.2 |
| Obliquity Up + | 0.67 | (0.00, 0.94) | 3.8 | 0.1 | (−2.2, 2.5) | 2.8 | (−5.44, 5.81) | 2.0 |
| Obliquity Down − | 0.85 | (0.31, 0.97) | −2.7 | −0.7 | (−2.9, 1.3) | 2.5 | (−5.78, 4.20) | 1.8 |
| External Rotation − | 0.88 | (0.44, 0.98) | −12.0 | −1.8 | (−6.2, 2.4) | 5.2 | (−12.06, 8.32) | 3.6 |
| Internal Rotation + | 0.85 | (0.21, 0.97) | 7.5 | −4.2 | (−8.7, 0.2) | 5.4 | (−14.86, 6.30) | 3.8 |
| Average | 0.77 | 3.1 | ||||||
| Hip Joint angle (°) | ||||||||
| Left lower Limb | ||||||||
| Flexion + | 0.79 | (0.00, 0.96) | 45.0 | −1.4 | (−6.2, 3.5) | 5.8 | (−12.78, 9.98) | 4.1 |
| Extension − | 0.78 | (0.00, 0.96) | 1.3 | −0.7 | (5.8, 4.3) | 6.1 | (−12.72, 11.24) | 4.3 |
| Abduction − | 0.60 | (0.00, 0.92) | −10.4 | 0.3 | (−4.2, 4.9) | 5.5 | (−10.41, 11.15) | 3.9 |
| Adduction + | 0.76 | (0.00, 0.95) | 4.8 | 0.8 | (−2.7, 4.4) | 4.3 | (−7.62, 9.27) | 3.0 |
| External Rotation − | 0.58 | (0.00, 0.90) | −8.9 | 4.3 | (−7.3, 18.0) | 15.1 | (−24.37, 35.08) | 9.7 |
| Internal Rotation + | 0.67 | (0.00, 0.92) | 3.9 | 4.9 | (−4.1, 16.0) | 12.0 | (−17.69, 29.66) | 8.5 |
| Average | 0.70 | 5.6 | ||||||
| Right lower Limb | ||||||||
| Flexion + | 0.14 | (0.00, 0.85) | 45.5 | −0.9 | (−9.1, 7.1) | 9.7 | (−20.10, 18.11) | 6.9 |
| Extension − | 0.82 | (0.12, 0.96) | 1.5 | −1.8 | (−7.6, 3.9) | 6.9 | (−15.46, 11.80) | 4.9 |
| Abduction − | 0.75 | (0.00, 0.95) | −9.9 | 0.2 | (−3.5, 4.1) | 4.6 | (−8.78, 9.37) | 3.2 |
| Adduction + | 0.79 | (0.00, 0.96) | 6.9 | −0.4 | (−3.9, 3.0) | 4.1 | (−8.62, 7.71) | 2.9 |
| External Rotation − | 0.67 | (0.00, 0.93) | −10.7 | −6.1 | (−16.8, 4.4) | 12.7 | (−31.10, 18.77) | 9.0 |
| Internal Rotation + | 0.73 | (0.00, 0.95) | 1.0 | −4.0 | (−14.5, 6.3) | 12.4 | (−28.54, 20.40) | 8.8 |
| Average | 0.65 | 5.9 | ||||||
| Knee Joint angle (°) | ||||||||
| Left lower Limb | ||||||||
| Flexion + | 0.75 | (0.00, 0.95) | 70.6 | 0.2 | (−6.5, 7.0) | 8.1 | (−15.71, 16.17) | 5.7 |
| Extension − | 0.85 | (0.17, 0.97) | 8.6 | 0.4 | (−3.6, 4.5) | 4.9 | (−9.15, 10.04) | 3.4 |
| Abduction − | 0.48 | (0.00, 0.90) | −7.4 | 0.5 | (−5.1, 6.1) | 6.7 | (−12.68, 13.74) | 4.7 |
| Adduction + | 0.46 | (0.00, 0.90) | 5.8 | 1.5 | (−7.7, 10.9) | 11.2 | (−20.27, 23.42) | 6.8 |
| External Rotation − | 0.75 | (0.00, 0.95) | −8.4 | −0.6 | (−7.9, 6.6) | 8.7 | (−17.73, 16.45) | 6.1 |
| Internal Rotation + | 0.62 | (0.00, 0.92) | 4.7 | 3.0 | (−5.0, 11.0) | 9.7 | (−15.91, 21.94) | 6.8 |
| Average | 0.65 | 5.6 | ||||||
| Right lower Limb | ||||||||
| Flexion + | 0.86 | (0.25, 0.97) | 68.5 | −0.1 | (−8.3, 8.0) | 9.8 | (−19.38, 19.13) | 5.9 |
| Extension − | 0.98 | (0.88, 0.99) | 6.4 | 1.5 | (−0.6, 3.6) | 2.5 | (−3.50, 6.50) | 1.8 |
| Abduction − | 0.37 | (0.00, 0.88) | −6.9 | −2.0 | (−10.2, 6.1) | 9.8 | (−21.31, 17.13) | 6.9 |
| Adduction + | 0.33 | (0.00, 0.87) | 4.7 | −3.8 | (−14.2, 6.6) | 12.4 | (−28.21, 20.54) | 8.7 |
| External Rotation − | 0.76 | (0.00, 0.95) | −7.5 | 3.5 | (−4.9, 12.1) | 10.2 | (−16.43, 23.61) | 7.2 |
| Internal Rotation + | 0.00 | (0.00, 0.69) | 5.4 | 0.8 | (−11.4, 13.0) | 14.6 | (−27.87, 29.49) | 9.3 |
| Average | 0.55 | 6.6 | ||||||
| Ankle Joint angle (°) | ||||||||
| Left lower Limb | ||||||||
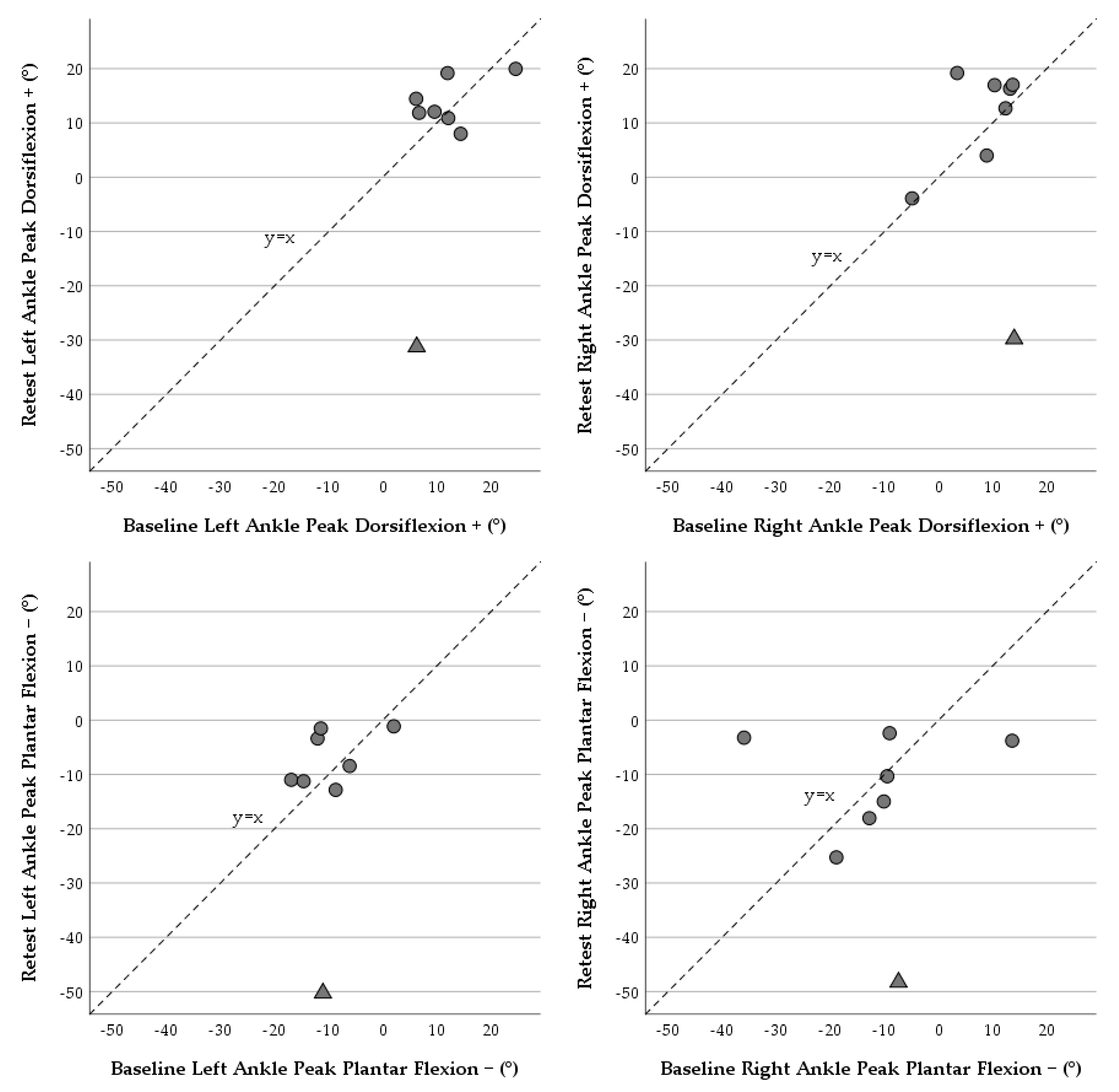
| Dorsiflexion + | 0.46 | (0.00, 0.90) | 9.8 | 3.3 | (−9.0, 15.7) | 14.8 | (−25.69, 32.37) | 10.4 |
| Plantar Flexion − | 0.27 | (0.00, 0.86) | −11.1 | 2.6 | (−10.5, 15.7) | 15.7 | (−28.22, 33.48) | 11.1 |
| Eversion − | 0.60 | (0.00, 0.91) | 1.2 | 2.4 | (−2.0, 7.0) | 5.4 | (−8.13, 13.11) | 3.8 |
| Inversion + | 0.75 | (0.00, 0.94) | 13.0 | 1.6 | (−3.1, 6.3) | 5.6 | (−9.44, 12.68) | 3.9 |
| Foot Internal Progression + | 0.95 | (0.75, 0.99) | 3.8 | −0.4 | (−4.1, 3.1) | 4.4 | (−9.13, 8.14) | 3.1 |
| Foot External Progression − | 0.87 | (0.34, 0.97) | −14.3 | 1.4 | (−7.5, 10.3) | 10.6 | (−19.40, 22.29) | 6.5 |
| Average | 0.65 | 6.5 | ||||||
| Right lower Limb | ||||||||
| Dorsiflexion + | 0.40 | (0.00, 0.82) | 7.7 | 2.3 | (−12.5, 17.1) | 17.7 | (−32.48, 37.14) | 12.6 |
| Plantar Flexion − | 0.00 | (0.00, 0.81) | −13.5 | 4.5 | (12.8, 21.9) | 20.7 | (−36.15, 45.23) | 14.6 |
| Eversion − | 0.43 | (0.00, 0.76) | 1.1 | 0.0 | (−5.7, 5.7) | 6.9 | (−13.48, 13.52) | 4.8 |
| Inversion + | 0.00 | (0.00, 0.80) | 14.1 | 0.0 | (−3.9, 3.8) | 4.6 | (−9.11, 8.99) | 3.2 |
| Foot Internal Progression + | 0.95 | (0.78, 0.99) | −11.7 | −3.1 | (−9.0, 2.7) | 7.0 | (−16.97, 10.67) | 4.9 |
| Foot External Progression − | 0.94 | (0.72, 0.99) | 29.3 | −4.6 | (−13.9, 4.6) | 11.0 | (−26.37, 17.05) | 6.8 |
| Average | 0.45 | 7.8 | ||||||
| Kinetic Parameters | ICC | ICC 95% CI | Mean | Mean Diff | Mean Diff 95% CI | 95% LOA | SEM | |
|---|---|---|---|---|---|---|---|---|
| Hip Joint Moment (N m/Kg) | ||||||||
| Left lower Limb | ||||||||
| Flexion − | 0.95 | (0.76, 0.99) | −0.46 | −0.02 | (−0.11, 0.06) | 0.10 | (−0.22, 0.17) | 0.07 |
| Extension + | 0.67 | (0.00, 0.94) | 0.50 | 0.02 | (−0.12, 0.16) | 0.17 | (−0.31, 0.34) | 0.12 |
| Abduction + | 0.79 | (0.00, 0.96) | 0.43 | 0.01 | (−0.08, 0.10) | 0.11 | (−0.20, 0.22) | 0.08 |
| Adduction − | 0.00 | (0.00, 0.75) | −0.21 | −0.05 | (−0.28, 0.18) | 0.28 | (−0.60, 0.50) | 0.20 |
| Average | 0.61 | 0.12 | ||||||
| Right lower Limb | ||||||||
| Flexion − | 0.84 | (0.11, 0.97) | −0.37 | 0.01 | (−0.12, 0.13) | 0.15 | (−0.29, 0.30) | 0.11 |
| Extension + | 0.40 | (0.00, 0.86) | 0.47 | 0.08 | (−0.13, 0.30) | 0.26 | (−0.43, 0.59) | 0.18 |
| Abduction + | 0.73 | (0.00, 0.95) | 0.48 | 0.00 | (−0.13, 0.12) | 0.15 | (−0.29, 0.29) | 0.11 |
| Adduction − | 0.79 | (0.16, 0.96) | −0.12 | −0.04 | (−0.11, 0.02) | 0.08 | (−0.20, 0.11) | 0.06 |
| Average | 0.69 | 0.12 | ||||||
| Knee Joint Moment (N m/Kg) | ||||||||
| Left lower Limb | ||||||||
| Flexion − | 0.69 | (0.00, 0.94) | −0.27 | 0.03 | (−0.04, 0.11) | 0.09 | (−0.15, 0.21) | 0.07 |
| Extension + | 0.79 | (0.00, 0.96) | 0.41 | −0.02 | (−0.19, 0.15) | 0.21 | (−0.42, 0.38) | 0.15 |
| Valgus + | 0.72 | (0.00, 0.95) | 0.17 | 0.01 | (−0.09, 0.11) | 0.12 | (−0.23, 0.25) | 0.09 |
| Varus − | 0.76 | (0.00, 0.95) | −0.16 | 0.06 | (0.00, 0.12) | 0.07 | (−0.07, 0.20) | 0.05 |
| Average | 0.74 | 0.09 | ||||||
| Right lower Limb | ||||||||
| Flexion − | 0.49 | (0.00, 0.90) | −0.26 | 0.13 | (−0.06, 0.32) | 0.23 | (−0.32, 0.58) | 0.16 |
| Extension + | 0.92 | (0.63, 0.98) | 0.31 | −0.06 | (−0.19, 0.07) | 0.16 | (−0.36, 0.24) | 0.11 |
| Valgus + | 0.00 | (0.00, 0.78) | 0.27 | −0.13 | (−0.39, 0.13) | 0.31 | (−0.74, 0.48) | 0.22 |
| Varus − | 0.61 | (0.00, 0.92) | −0.14 | −0.04 | (−0.12, 0.03) | 0.09 | (−0.23, 0.14) | 0.07 |
| Average | 0.51 | 0.14 | ||||||
| Ankle Joint Moment (N m/Kg) | ||||||||
| Left lower Limb | ||||||||
| Dorsiflexion − | 0.72 | (0.00, 0.95) | −0.02 | 0.01 | (−0.01, 0.04) | 0.03 | (−0.05, 0.08) | 0.02 |
| Plantar Flexion + | 0.93 | (0.61, 0.99) | 0.85 | 0.00 | (−0.12, 0.11) | 0.14 | (−0.27, 0.26) | 0.10 |
| Eversion + | 0.57 | (0.00, 0.92) | 0.07 | 0.02 | (−0.05, 0.08) | 0.08 | (−0.14, 0.17) | 0.06 |
| Inversion − | 0.75 | (0.00, 0.95) | −0.13 | 0.02 | (−0.06, 0.09) | 0.09 | (−0.16, 0.19) | 0.06 |
| Average | 0.74 | 0.06 | ||||||
| Right lower Limb | ||||||||
| Dorsiflexion − | 0.00 | (0.00, 0.77) | −0.02 | −0.02 | (−0.05, 0.02) | 0.04 | (−0.10, 0.06) | 0.03 |
| Plantar Flexion + | 0.78 | (0.00, 0.96) | 0.75 | −0.01 | (−0.15, 0.13) | 0.17 | (−0.34, 0.32) | 0.12 |
| Eversion + | 0.85 | (0.21, 0.97) | 0.04 | 0.00 | (−0.03, 0.03) | 0.04 | (−0.07, 0.07) | 0.03 |
| Inversion − | 0.55 | (0.00, 0.91) | −0.16 | −0.03 | (−0.18, 0.13) | 0.18 | (−0.39, 0.33) | 0.13 |
| Average | 0.55 | 0.08 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricardo, D.; Teles, J.; Raposo, M.R.; Veloso, A.P.; João, F. Test-Retest Reliability of a 6DoF Marker Set for Gait Analysis in Cerebral Palsy Children. Appl. Sci. 2021, 11, 6515. https://doi.org/10.3390/app11146515
Ricardo D, Teles J, Raposo MR, Veloso AP, João F. Test-Retest Reliability of a 6DoF Marker Set for Gait Analysis in Cerebral Palsy Children. Applied Sciences. 2021; 11(14):6515. https://doi.org/10.3390/app11146515
Chicago/Turabian StyleRicardo, Diogo, Júlia Teles, Maria Raquel Raposo, António Prieto Veloso, and Filipa João. 2021. "Test-Retest Reliability of a 6DoF Marker Set for Gait Analysis in Cerebral Palsy Children" Applied Sciences 11, no. 14: 6515. https://doi.org/10.3390/app11146515
APA StyleRicardo, D., Teles, J., Raposo, M. R., Veloso, A. P., & João, F. (2021). Test-Retest Reliability of a 6DoF Marker Set for Gait Analysis in Cerebral Palsy Children. Applied Sciences, 11(14), 6515. https://doi.org/10.3390/app11146515






