Optimization of Electric Field Assisted Mining Process Applied to Rare Earths in Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of the Real Soil
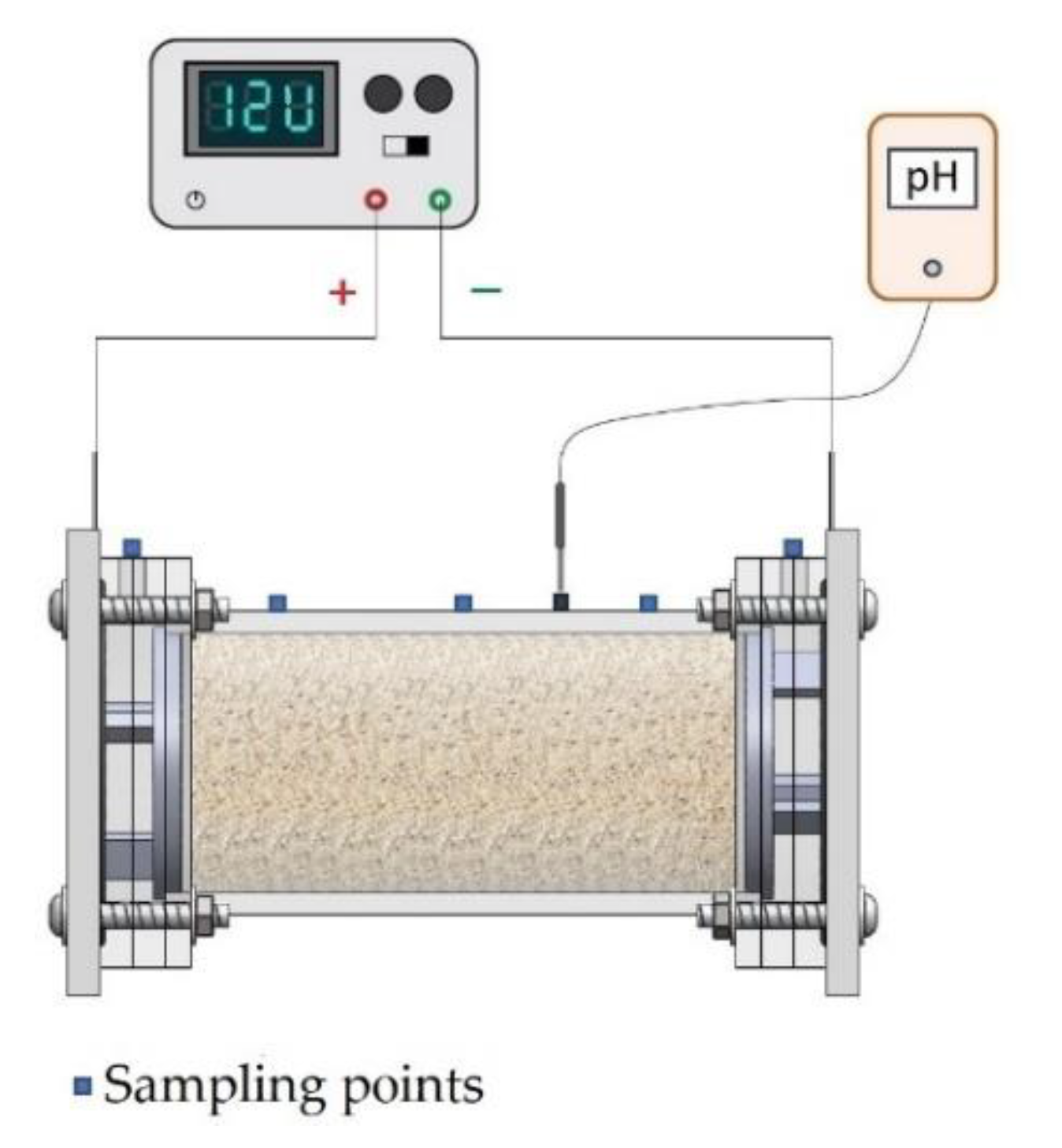
2.2. Electromining
2.3. Y3+ Electromining Process Efficiency
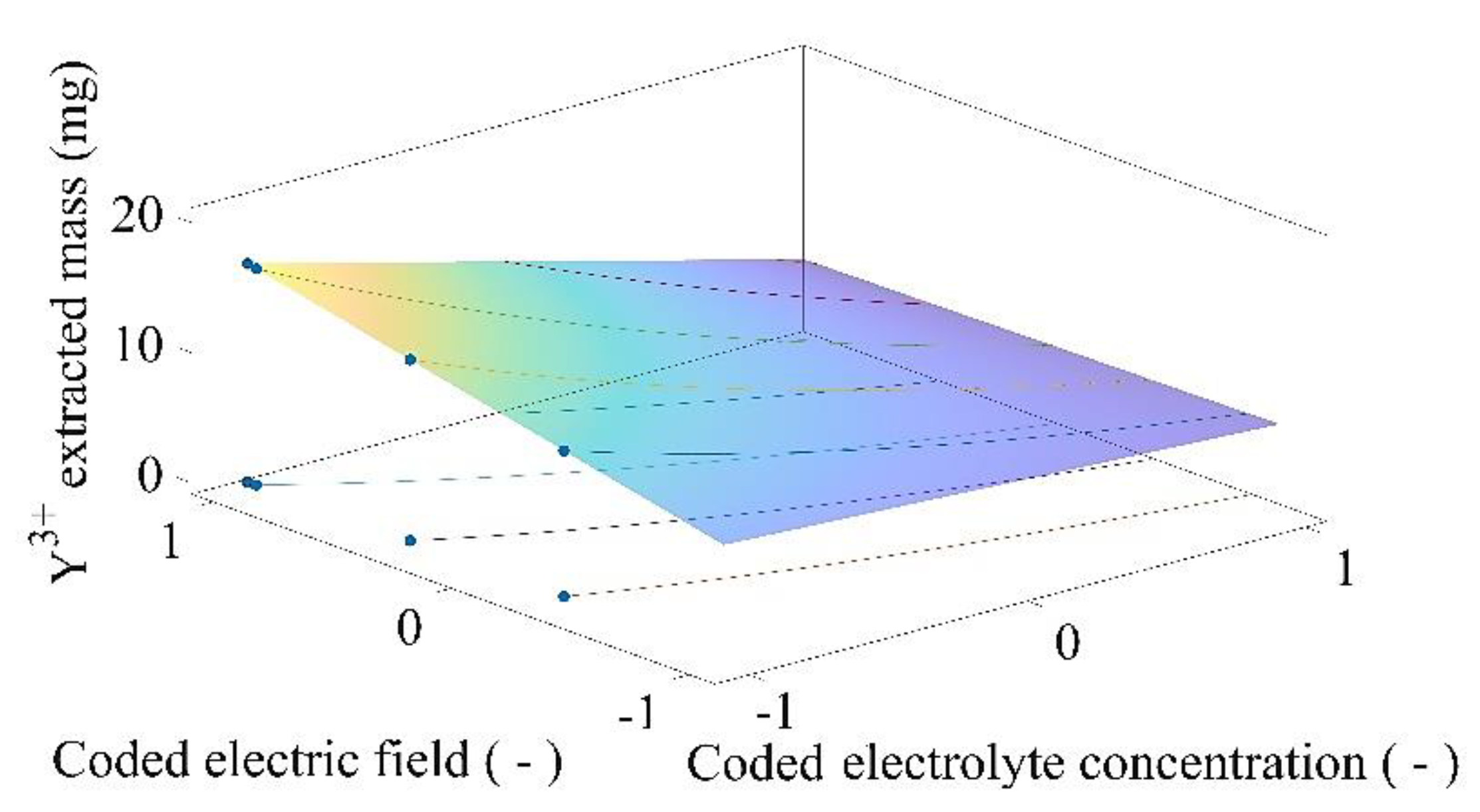
2.4. Electromining Process Optimization
3. Results and Discussion
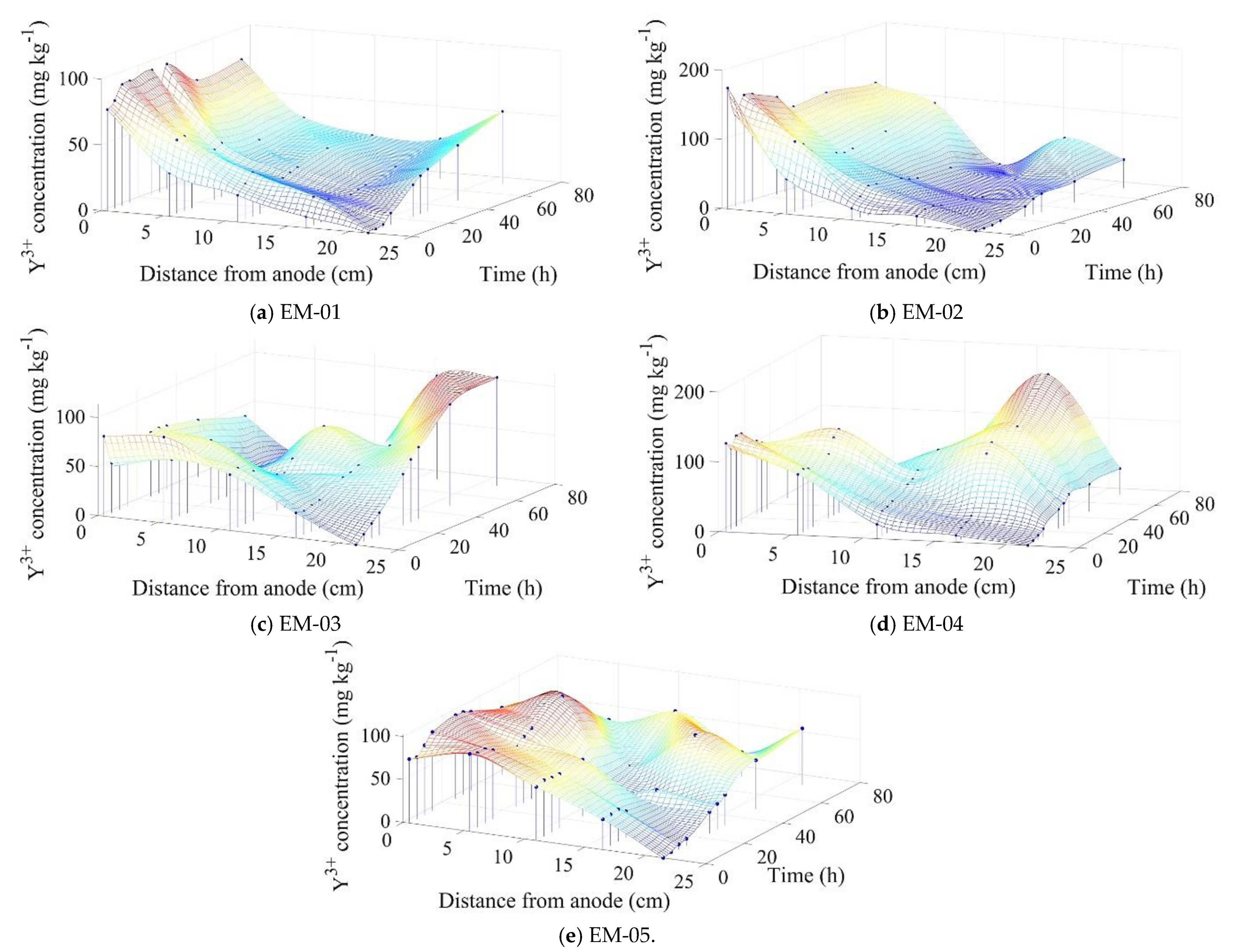
3.1. Analysis of the Migration Performance of Y3+
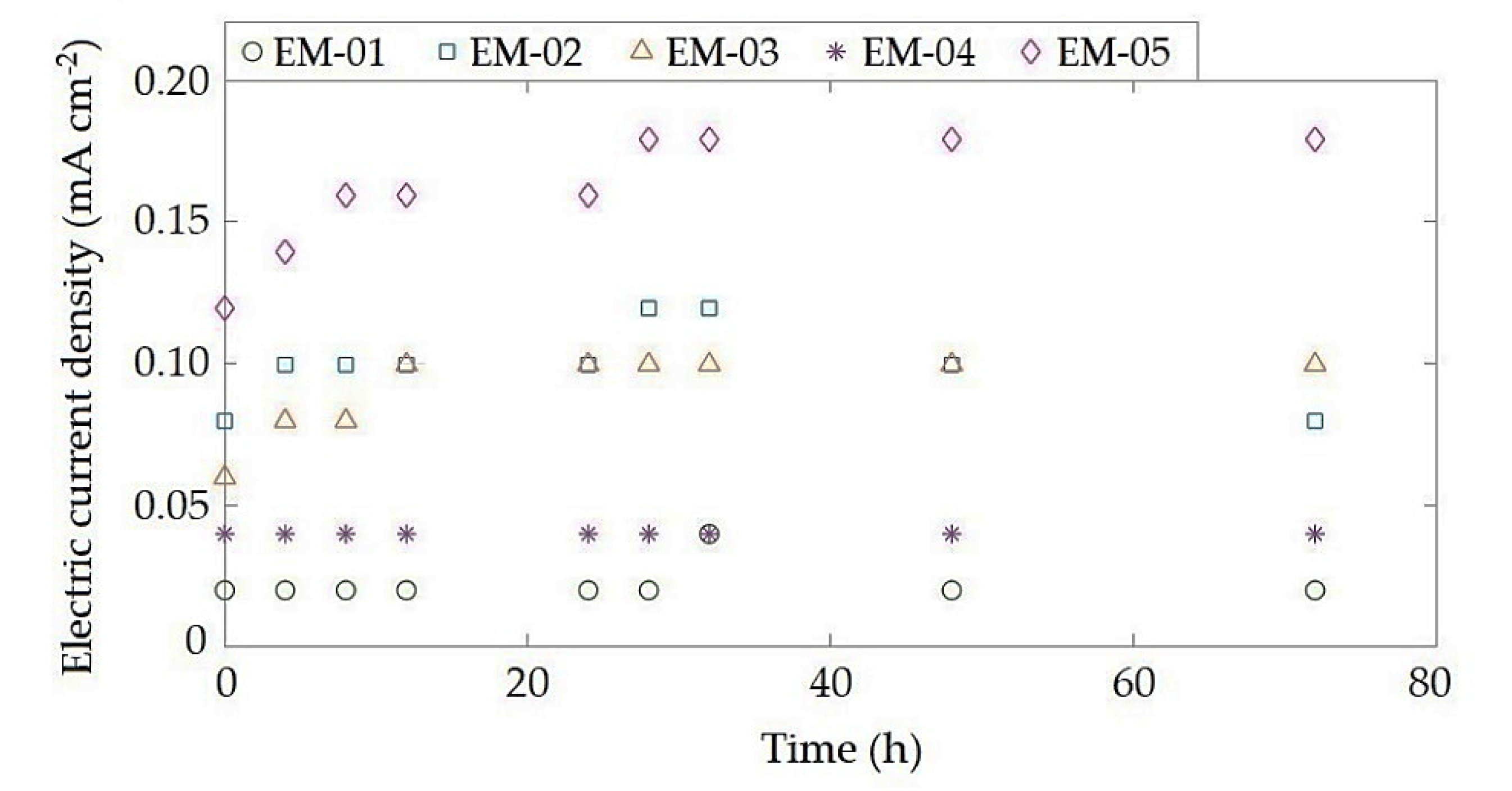
3.2. Electromining Efficiency
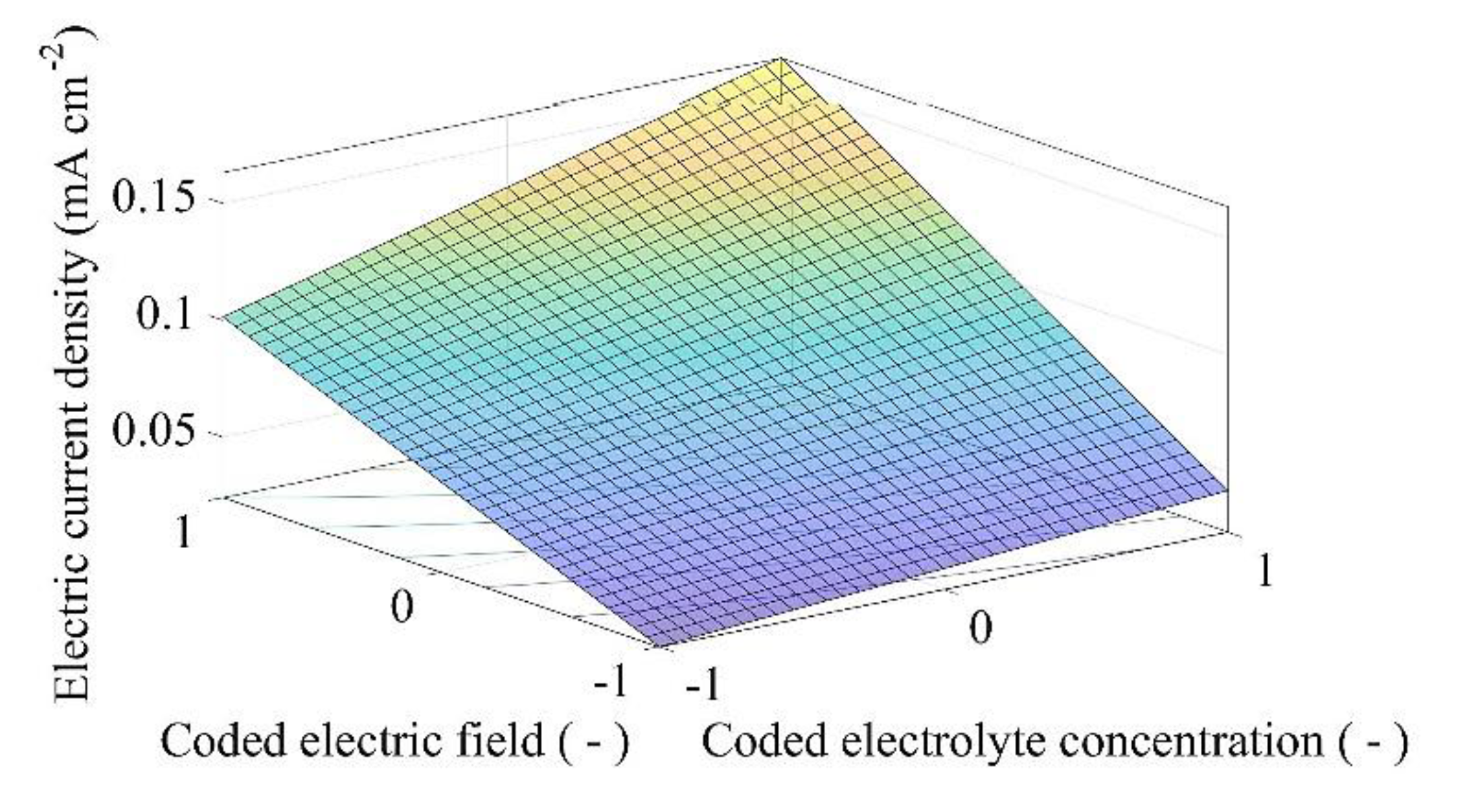
3.3. Electromining Process Optimization
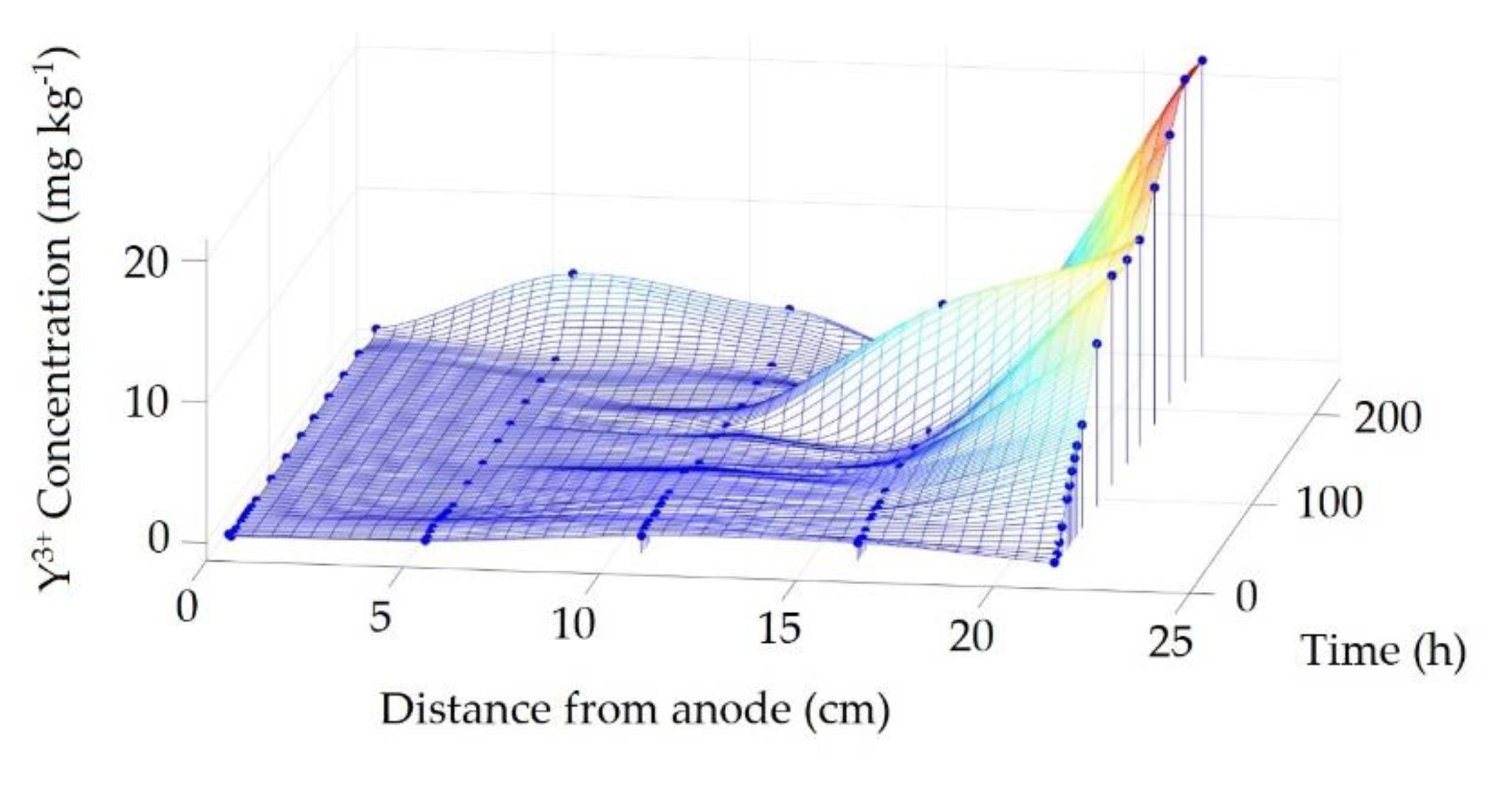
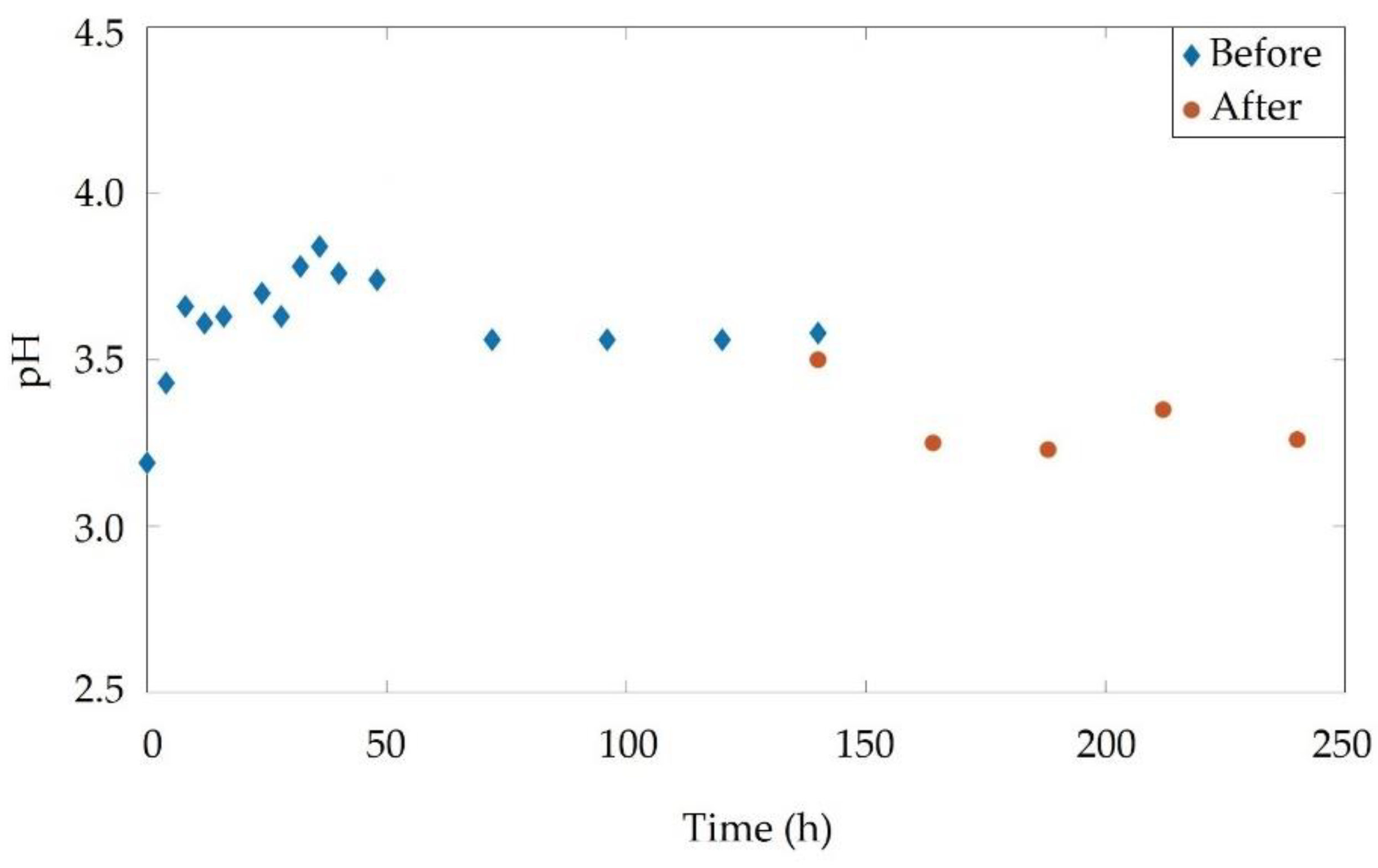
3.4. Real Soil Electromining
3.4.1. Real Soil pH
3.4.2. Y3+ Migrational Profile in the Reactor Cathodic Chamber
3.4.3. Cost of Y3+ Produced
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, V.; Chakravarty, S.; Finkelman, R.B.; Varma, A.K. Geochemistry of Rare Earth Elements in Lower Gondwana Coals of the Talchir Coal Basin, India. J. Geochem. Explor. 2019, 204, 43–56. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Yu, Y.; Zhao, L.; Wang, C.; Feng, Z.; Cui, D.; Long, Z. Towards cleaner production of rare earth elements from bastnaesite in China. J. Clean. Prod. 2017, 165, 231–242. [Google Scholar] [CrossRef]
- Lefticariu, L.; Klitzing, K.L.; Kolker, A. Rare Earth Elements and Yttrium (REY) in coal mine drainage from the Illinois Basin, USA. Int. J. Coal Geol. 2020, 217, 103327. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Lima, A.T.; Ottosen, L. Recovering rare earth elements from contaminated soils: Critical overview of current remediation technologies. Chemosphere 2020, 265, 129163. [Google Scholar] [CrossRef]
- Guedes, P.; Lopes, V.; Couto, N.; Mateus, E.P.; Pereira, C.S.; Ribeiro, A.B. Electrokinetic remediation of contaminants of emergent concern in clay soil: Effect of operating parameters. Environ. Pollut. 2019, 253, 625–635. [Google Scholar] [CrossRef]
- Preinfalk, C.; Morteani, G. The Industrial Applications of Rare Earth Elements. In Lanthanides, Tantalum and Niobium; Möller, P., Černý, P., Saupé, F., Eds.; Special Publication No. 7 of the Society for Geology Applied to Mineral Deposits; Springer: Berlin/Heidelberg, Germany, 1989; Volume 7, pp. 359–370. [Google Scholar] [CrossRef]
- Khanchi, A.R.; Sedighi, H.; Ansar, S.; Fasihi, J. Preconcentration of rare earth elements from Iranian monazite ore by spiral separator using multi-response optimization method. Int. J. Min. Sci. Technol. 2014, 24, 117–121. [Google Scholar] [CrossRef]
- Amato, A.; Becci, A.; Birloaga, I.; De Michelis, I.; Ferella, F.; Innocenzi, V.; Ippolito, N.M.; Pillar-Jimenez-Gomez, C.; Vegliò, F.; Beolchini, F. Sustainability analysis of innovative technologies for the rare earth elements recovery. Renew. Sustain. Energy Rev. 2019, 106, 41–53. [Google Scholar] [CrossRef]
- Couto, N.; Ferreira, A.R.; Lopes, V.; Peters, S.; Mateus, E.P.; Ribeiro, A.B.; Pamukcu, S. Electrodialytic Recovery of Rare Earth Elements from Coal Ashes. Electrochim. Acta 2020, 136934. [Google Scholar] [CrossRef]
- Takehara, L.; Silveira, F.V.; Santos, R.V. Potentiality of Rare Earth Elements in Brazil. Rare Earths Ind. 2016, 57–72. [Google Scholar] [CrossRef]
- Swain, N.; Mishra, S.; Acharya, M.R. Hydrometallurgical route for recovery and separation of samarium (III) and cobalt (II) from simulated waste solution using tri-n-octyl phosphine oxide—A novel pathway for synthesis of samarium and cobalt oxides nanoparticles. J. Alloys Compd. 2020, 815. [Google Scholar] [CrossRef]
- Vahidi, E.; Navarro, J.; Zhao, F. An initial life cycle assessment of rare earth oxides production from ion-adsorption clays. Resour. Conserv. Recycl. 2016, 113, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vahidi, E.; Zhao, F. Environmental life cycle assessment on the separation of rare earth oxides through solvent extraction. J. Environ. Manag. 2017, 203, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Tunsu, C.; Menard, Y.; Eriksen, D.Ø.; Ekberg, C.; Petranikova, M. Recovery of critical materials from mine tailings: A comparative study of the solvent extraction of rare earths using acidic, solvating and mixed extractant systems. J. Clean. Prod. 2019, 218, 425–437. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Zhang, D.; Gao, K.; Xu, W.; Wang, H.; Geng, J.; Ma, X.; Huang, L. Clean production technology of selective decomposition of Bayan Obo rare earth concentrate by NaOH. J. Clean. Prod. 2019, 236. [Google Scholar] [CrossRef]
- Omodara, L.; Pitkäaho, S.; Turpeinen, E.M.; Saavalainen, P.; Oravisjärvi, K.; Keiski, R.L. Recycling and substitution of light rare earth elements, cerium, lanthanum, neodymium, and praseodymium from end-of-life applications—A review. J. Clean. Prod. 2019, 236, 117573. [Google Scholar] [CrossRef]
- Innocenzi, V.; De Michelis, I.; Kopacek, B.; Vegliò, F. Yttrium recovery from primary and secondary sources: A review of main hydrometallurgical processes. Waste Manag. 2014, 34, 1237–1250. [Google Scholar] [CrossRef]
- Pires, C.M.G.; de Ponte, H.A.; Pereira, J.T.; de Ponte, M.J.J.S. Yttrium extraction from soils by electric field assisted mining applying the evolutionary operation technique. J. Clean. Prod. 2019, 227, 272–279. [Google Scholar] [CrossRef]
- Auerbach, R.; Bokelmann, K.; Stauber, R.; Gutfleisch, O.; Schnell, S.; Ratering, S. Critical raw materials—Advanced recycling technologies and processes: Recycling of rare earth metals out of end of life magnets by bioleaching with various bacteria as an example of an intelligent recycling strategy. Miner. Eng. 2019, 134, 104–117. [Google Scholar] [CrossRef]
- Mancheri, N.A. World trade in rare earths, Chinese export restrictions, and implications. Resour. Policy 2015, 46, 262–271. [Google Scholar] [CrossRef]
- Mancheri, N.A.; Sprecher, B.; Bailey, G.; Ge, J.; Tukker, A. Effect of Chinese policies on rare earth supply chain resilience. Resour. Conserv. Recycl. 2019, 142, 101–112. [Google Scholar] [CrossRef]
- Zhang, K.; Kleit, A.N.; Nieto, A. An economics strategy for criticality—Application to rare earth element Yttrium in new lighting technology and its sustainable availability. Renew. Sustain. Energy Rev. 2017, 77, 899–915. [Google Scholar] [CrossRef]
- Acar, Y.B.; Gale, R.J.; Alshawabkeh, A.N.; Marks, R.E.; Puppala, S.; Bricka, M.; Parker, R. Electrokinetic remediation: Basics and technology status. J. Hazard. Mater. 1995, 40, 117–137. [Google Scholar] [CrossRef]
- Yang, J.S.; Kwon, M.J.; Choi, J.; Baek, K.; O’Loughlin, E.J. The transport behavior of As, Cu, Pb, and Zn during electrokinetic remediation of a contaminated soil using electrolyte conditioning. Chemosphere 2014, 117, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.; Kim, D.H.; Park, S.W.; Ryu, B.G.; Bajargal, T.; Yang, J.S. Electrolyte conditioning-enhanced electrokinetic remediation of arsenic-contaminated mine tailing. J. Hazard. Mater. 2009, 161, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tan, X.Y.; Da Wu, X.; Pan, C.; Xu, P. Effects of electrolyte characteristics on soil conductivity and current in electrokinetic remediation of lead-contaminated soil. Sep. Purif. Technol. 2014, 135, 14–21. [Google Scholar] [CrossRef]
- Song, Y.; Cang, L.; Xu, H.; Wu, S.; Zhou, D. Migration and decomplexation of metal-chelate complexes causing metal accumulation phenomenon after chelate-enhanced electrokinetic remediation. J. Hazard. Mater. 2019, 377, 106–112. [Google Scholar] [CrossRef]
- Mohamadi, S.; Saeedi, M.; Mollahosseini, A. Enhanced electrokinetic remediation of mixed contaminants from a high buffering soil by focusing on mobility risk. J. Environ. Chem. Eng. 2019, 7, 103470. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, B.; Guo, P.; Wang, S.; Guo, S. Enhanced electrokinetic remediation and simulation of cadmium-contaminated soil by superimposed electric field. Chemosphere 2019, 233, 17–24. [Google Scholar] [CrossRef]
- Kumar, A.; Pinto, M.C.; Candeias, C.; Dinis, P.A. Baseline maps of potentially toxic elements in the soils of Garhwal Himalayas, India: Assessment of their eco-environmental and human health risks. Land Degrad. Dev. 2021, 1–14. [Google Scholar] [CrossRef]
- Arora, J.S. Introduction to Optimum Design, 4th ed.; Elsevier: New York, NY, USA, 2017. [Google Scholar]
- De Sousa, T.L.; da Silva, J.; Pereira, J.T. Indirect identification of the complex poisson’s ratio in fractional viscoelasticity. Lat. Am. J. Solids Struct. 2018, 15. [Google Scholar] [CrossRef]
- Li, P.; Peng, D.; Tan, Z.; Deng, K. Study of probability integration method parameter inversion by the genetic algorithm. Int. J. Min. Sci. Technol. 2017, 27, 1073–1079. [Google Scholar] [CrossRef]
- Paz-García, J.M.; Johannesson, B.; Ottosen, L.M.; Ribeiro, A.B.; Rodríguez-Maroto, J.M. Modeling of electrokinetic processes by finite element integration of the Nernst-Planck-Poisson system of equations. Sep. Purif. Technol. 2011, 79, 183–192. [Google Scholar] [CrossRef]
- Acar, Y.B.; Alshawabkeh, A.N. Principles of electrokinetic remediation. Environ. Sci. Technol. 1993, 27, 2638–2647. [Google Scholar] [CrossRef]
- Bentouhami, E.; Bouet, G.M.; Meullemeestre, J.; Vierling, F.; Khan, M.A. Physicochemical study of the hydrolysis of Rare-Earth elements (III) and thorium (IV). Comptes Rendus Chim. 2004, 7, 537–545. [Google Scholar] [CrossRef]
- Scheberl, L.; Scharenbroch, B.C.; Werner, L.P.; Prater, J.R.; Fite, K.L. Evaluation of soil pH and soil moisture with different field sensors: Case study urban soil. Urban For. Urban Green. 2019, 38, 267–279. [Google Scholar] [CrossRef]
- Bargrizan, S.; Smernik, R.J.; Fitzpatrick, R.W.; Mosley, L.M. The application of a spectrophotometric method to determine pH in acidic (pH < 5) soils. Talanta 2018, 186, 421–426. [Google Scholar] [CrossRef]
- Feher, J. Electrochemical potential and free energy. Quant. Hum. Physiol. 2012, 63–68. [Google Scholar] [CrossRef]
- Sprocati, R.; Masi, M.; Muniruzzaman, M.; Rolle, M. Modeling electrokinetic transport and biogeochemical reactions in porous media: A multidimensional Nernst–Planck–Poisson approach with PHREEQC coupling. Adv. Water Resour. 2019, 127, 134–147. [Google Scholar] [CrossRef]
- Reddy, K.R. Technical challenges to in situ remediation of polluted sites. Geotech. Geol. Eng. 2010, 28, 211–221. [Google Scholar] [CrossRef]
- Yeung, A.T.; Datla, S. Fundamental formulation of electrokinetic extraction of contaminants from soil. Can. Geotech. J. 1995, 32, 569–583. [Google Scholar] [CrossRef]
- Paz-Garcia, J.M.; Baek, K.; Alshawabkeh, I.D.; Alshawabkeh, A.N. A generalized model for transport of contaminants in soil by electric fields. J. Environ. Sci. Health Part A Toxic 2012, 47, 308–318. [Google Scholar] [CrossRef] [PubMed]

| Real Soil Composition (%) | ||||||
|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | TiO2 | Nb2O5 | SnO2 | ZrO2 |
| 60.7 | 25.6 | 2.0 | 1.9 | 1.3 | 1.3 | 0.3 |
| Ta2O5 | K2O | MnO | SO3 | P2O5 | Y2O3 | |
| 0.1 | 0.1 | 0.1 | 0.1 | <0.1 | <0.1 | |
| Soil | Resistivity (Ω cm) | pH | ||||
| Real | 50,253.8 ± 2,124.0 | 5.25 | ||||
| Synthetic | 2622.2 ± 112.4 | 4.26 | ||||
| Physicochemical Analyses of Real Soil | ||||||
| Porosity | 0.43 | |||||
| Cation exchange capacity | 4.12 cmol dm−3 | |||||
| Total organic carbon | 2.42 g dm−3 | |||||
| Sandy | 60.00% | |||||
| Silt | 3.36% | |||||
| Clay | 36.64% | |||||
| Soil texture | Sandy clay | |||||
| Experiment | C (mol L−1) | ε (V cm−1) | (-) | (-) |
|---|---|---|---|---|
| EM-01 | 0.10 | 0.1818 | −1.00 | −1.00 |
| EM-02 | 1.00 | 0.1818 | 1.00 | −1.00 |
| EM-03 | 0.10 | 0.5454 | −1.00 | 1.00 |
| EM-04 | 1.00 | 0.5454 | 1.00 | 1.00 |
| EM-05 | 0.55 | 0.3636 | 0.00 | 0.00 |
| Experiment | CY (mol L−1) | mY (mg) | ξ (%) | Im (mA cm−2) |
|---|---|---|---|---|
| EM-01 | 55.175 | 8.276 | 21.221 | 0.022 |
| EM-02 | 41.281 | 6.192 | 15.877 | 0.040 |
| EM-03 | 110.828 | 16.624 | 42.626 | 0.099 |
| EM-04 | 37.402 | 5.610 | 14.385 | 0.161 |
| EM-05 | 65.845 | 9.877 | 25.325 | 0.091 |
| Optimization Problem | Electric Current Density Limit (mA cm−2) | Coded Electric Field | Electric Field (V cm−1) | Coded Electrolyte Concentration | Electrolyte Concentration (mol L−1) | Total Mass of Y3 + Extracted (mg) |
|---|---|---|---|---|---|---|
| PY1 | 0.0500 | −0.3299 | 0.3036 | −1.0000 | 0.1000 | 11.2135 |
| PY2 | 0.0750 | 0.3164 | 0.4211 | −1.0000 | 0.1000 | 13.9113 |
| PY3 | 0.1000 | 0.9628 | 0.5386 | −1.0000 | 0.1000 | 16.6090 |
| PY4 | 0.1300 | 1.0000 | 0.5454 | −1.0000 | 0.1000 | 16.7644 |
| PY5 | 0.1600 | 1.0000 | 0.5454 | −1.0000 | 0.1000 | 16.7644 |
| PY6 | Unlimited | 1.0000 | 0.5454 | −1.0000 | 0.1000 | 16.7644 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, C.M.G.; Pereira, J.T.; Ribeiro, A.B.; Ponte, H.A.; Ponte, M.J.J.S. Optimization of Electric Field Assisted Mining Process Applied to Rare Earths in Soils. Appl. Sci. 2021, 11, 6316. https://doi.org/10.3390/app11146316
Pires CMG, Pereira JT, Ribeiro AB, Ponte HA, Ponte MJJS. Optimization of Electric Field Assisted Mining Process Applied to Rare Earths in Soils. Applied Sciences. 2021; 11(14):6316. https://doi.org/10.3390/app11146316
Chicago/Turabian StylePires, Carolina M. G., Jucélio T. Pereira, Alexandra B. Ribeiro, Haroldo A. Ponte, and Maria José J. S. Ponte. 2021. "Optimization of Electric Field Assisted Mining Process Applied to Rare Earths in Soils" Applied Sciences 11, no. 14: 6316. https://doi.org/10.3390/app11146316
APA StylePires, C. M. G., Pereira, J. T., Ribeiro, A. B., Ponte, H. A., & Ponte, M. J. J. S. (2021). Optimization of Electric Field Assisted Mining Process Applied to Rare Earths in Soils. Applied Sciences, 11(14), 6316. https://doi.org/10.3390/app11146316








