A Study of Predictive Models for Early Outcomes of Post-Prostatectomy Incontinence: Machine Learning Approach vs. Logistic Regression Analysis Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Magnetic Resonance Images and Measurements
2.3. Assessment of Incontinence
2.4. Data Split for Model Development and Testing
2.5. Building Process of Prediction Models Using Machine Learning Algorithms
2.6. Statistical Analysis
3. Results
3.1. Subsection Baseline Characteristics of the Development and Test Cohorts
3.2. Comparison between PPI Early-Recovery and Consistent Group and Logistic Regression Analysis
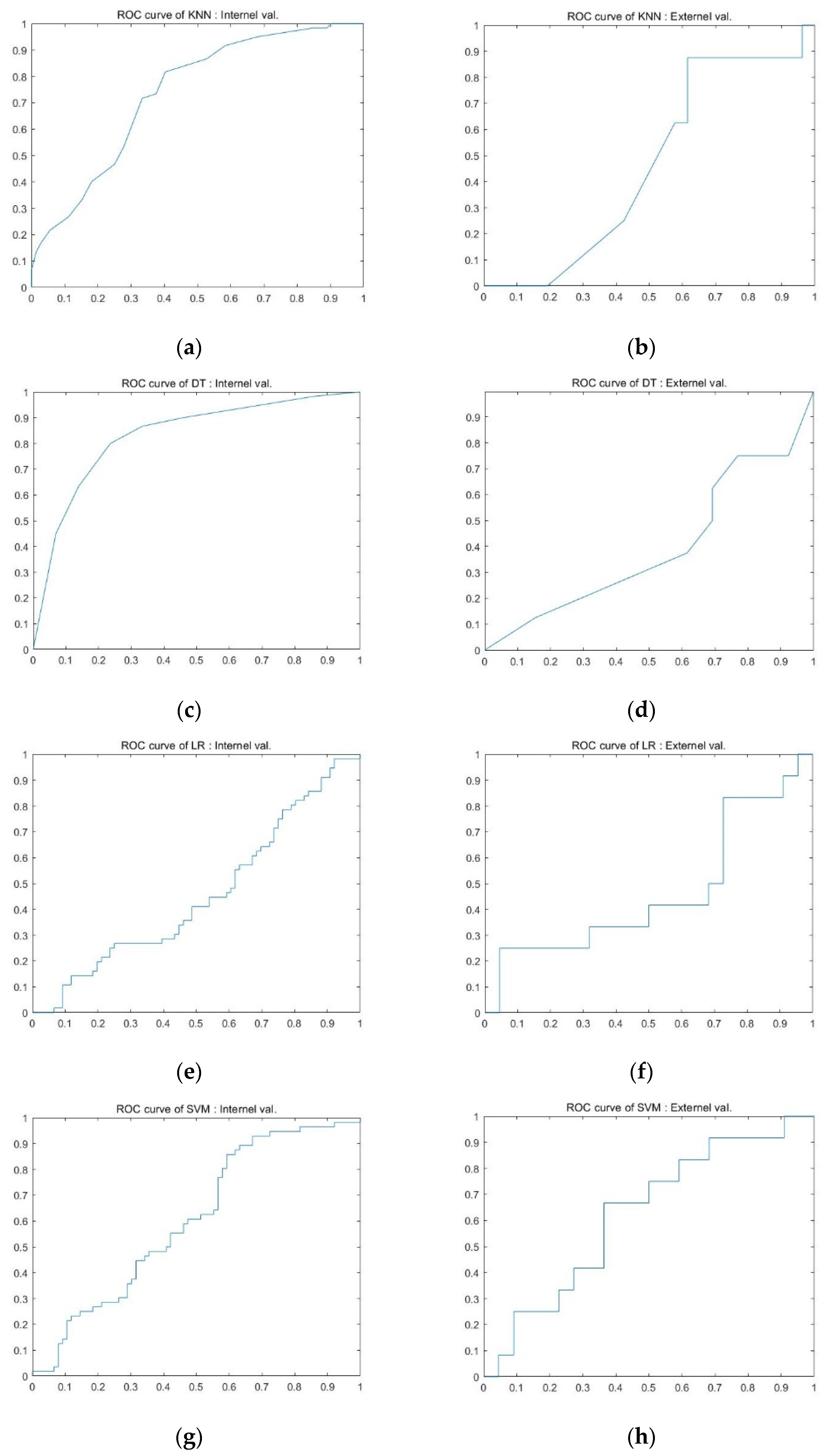
3.3. Diagnostic Performance of the Predictive Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
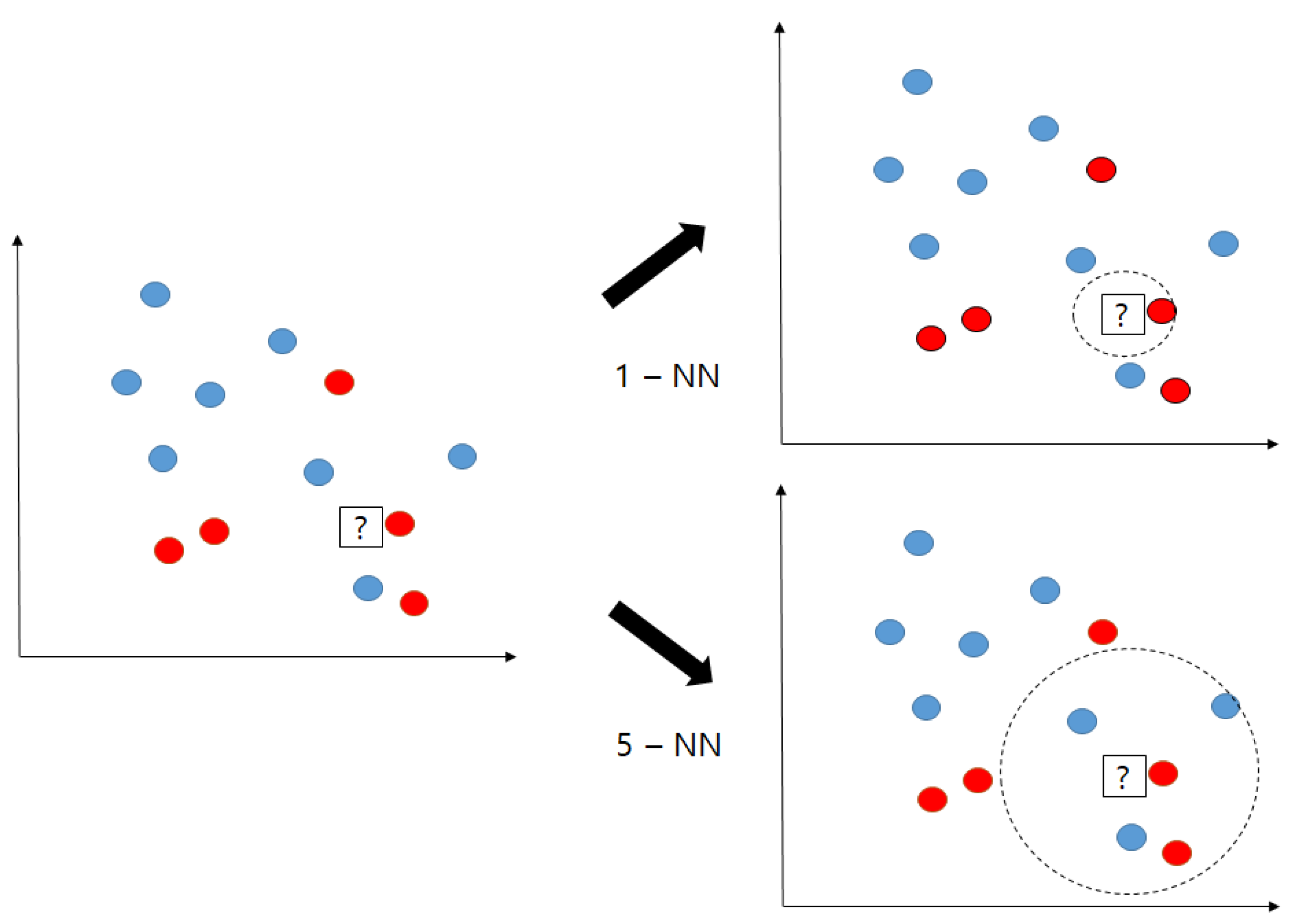
Appendix A.1. K-Nearest Neighborhood
Appendix A.2. Decision Tree
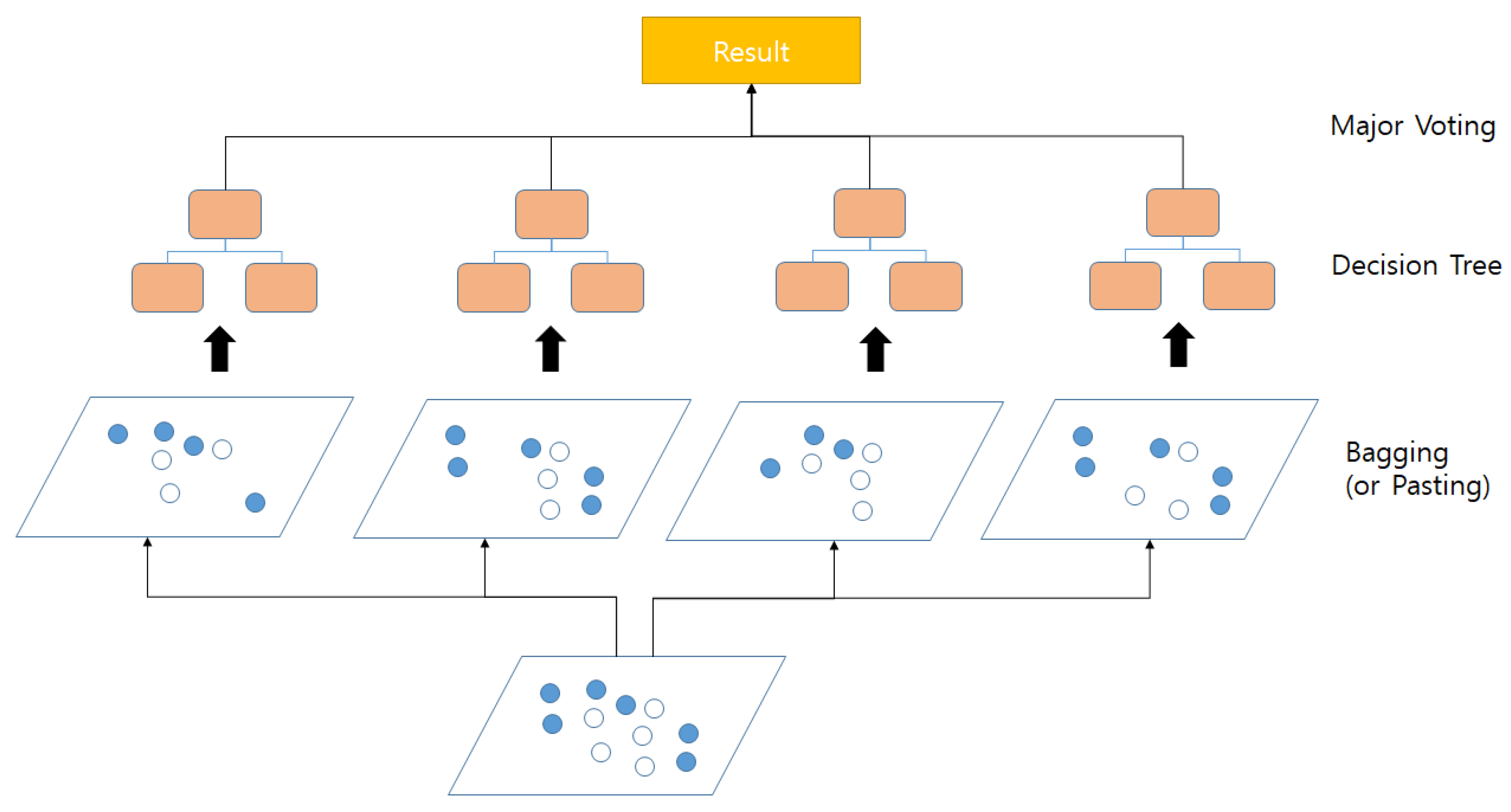
Appendix A.3. Random Forest
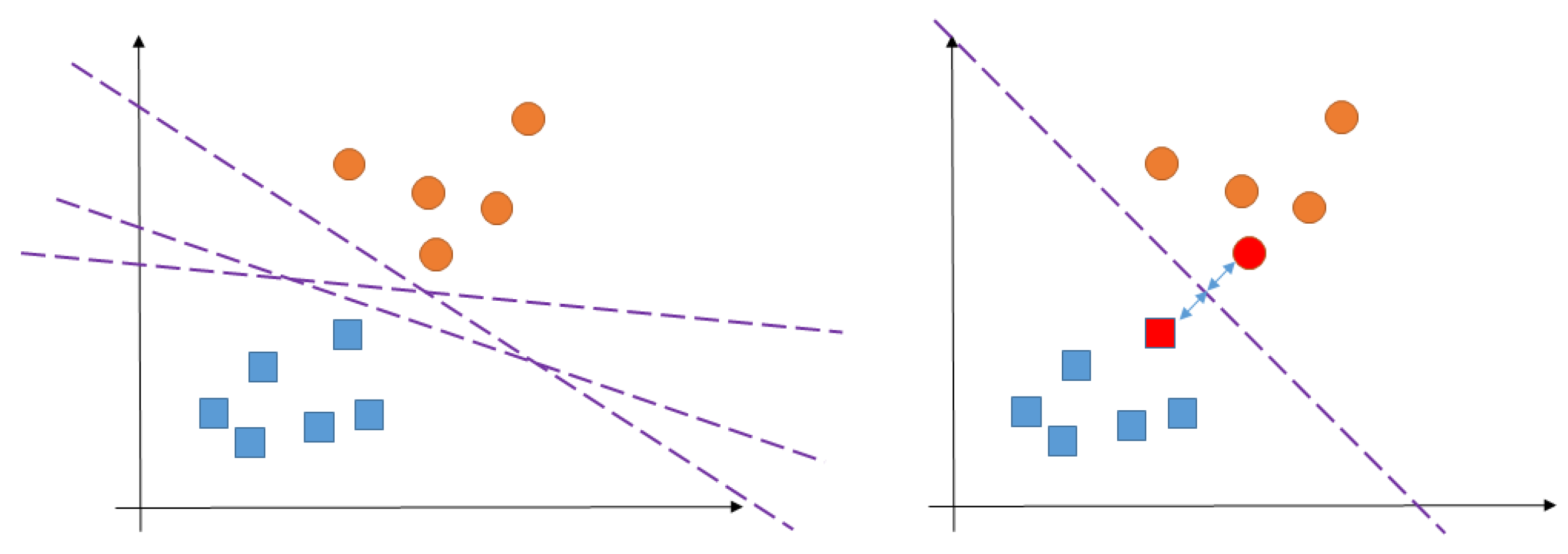
Appendix A.4. Support Vector Machine
Appendix B
| KNN | DT | SVM | RF | LR | ||
|---|---|---|---|---|---|---|
| Internal validation cohort | KNN | 1.000 | - | - | - | - |
| DT | 0.984 | 1.000 | - | - | - | |
| SVM | 0.225 | 0.140 | 1.000 | - | - | |
| RF | 0.001 | <0.001 | <0.001 | 1.000 | - | |
| LR | <0.001 | <0.001 | <0.001 | <0.001 | 1.000 | |
| External validation cohort | KNN | 1.000 | - | - | - | - |
| DT | 0.854 | 1.000 | - | - | - | |
| SVM | 0.002 | 0.002 | 1.000 | - | - | |
| RF | 0.533 | 0.631 | 0.014 | 1.000 | - | |
| LR | 0.375 | 0.251 | <0.001 | 0.121 | 1.000 | |
References
- Punnen, S.; Cowan, J.E.; Chan, J.M.; Carroll, P.R.; Cooperberg, M.R. Long-Term Health-Related Quality of Life after Primary Treatment for Localized Prostate Cancer: Results from the CaPSURE Registry. Eur. Urol. 2015, 68, 600–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liss, M.A.; Osann, K.; Canvasser, N.; Chu, W.; Chang, A.; Gan, J.; Li, R.; Santos, R.; Skarecky, D.; Finley, D.S.; et al. Continence Definition after Radical Prostatectomy Using Urinary Quality of life: Evaluation of Patient Reported Validated Questionnaires. J. Urol. 2010, 183, 1464–1468. [Google Scholar] [CrossRef] [PubMed]
- Sanda, M.G.; Dunn, R.L.; Michalski, J.; Sandler, H.M.; Northouse, L.; Hembroff, L.; Lin, X.; Greenfield, T.K.; Litwin, M.S.; Saigal, C.S.; et al. Quality of Life and Satisfaction with Outcome among Prostate-Cancer Survivors. N. Engl. J. Med. 2008, 358, 1250–1261. [Google Scholar] [CrossRef]
- Ficarra, V.; Novara, G.; Rosen, R.C.; Artibani, W.; Carroll, P.R.; Costello, A.; Menon, M.; Montorsi, F.; Patel, V.R.; Stolzenburg, J.U.; et al. Systematic Review and Meta-Analysis of Studies Reporting Urinary Continence Recovery after Robot-Assisted Radical Prostatectomy. Eur. Urol. 2012, 62, 405–417. [Google Scholar] [CrossRef]
- Sadahira, T.; Mitsui, Y.; Araki, M.; Maruyama, Y.; Wada, K.; Edamura, K.; Kobayashi, Y.; Watanabe, M.; Watanabe, T.; Nasu, Y. Pelvic Magnetic Resonance Imaging Parameters Predict Urinary Incontinence after Robot-Assisted Radical Prostatectomy. LUTS Low. Urin. Tract Symptoms 2019, 11, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Kent, M.T.; Vickers, A.J.; von Bodman, C.; Bernstein, M.; Touijer, K.A.; Coleman, J.A.; Laudone, V.T.; Scardino, P.T.; Eastham, J.A.; et al. Preoperative Predictive Model of Recovery of Urinary Continence after Radical Prostatectomy. BJU Int. 2015, 116, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Shikanov, S.; Desai, V.; Razmaria, A.; Zagaja, G.P.; Shalhav, A.L. Robotic Radical Prostatectomy for Elderly Patients: Probability of Achieving Continence and Potency 1 Year after Surgery. J. Urol. 2010, 183, 1803–1807. [Google Scholar] [CrossRef]
- Song, W.; Kim, C.K.; Park, B.K.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Choi, H.Y.; Lee, H.M. Impact of Preoperative and Postoperative Membranous Urethral Length Measured by 3 Tesla Magnetic Resonance Imaging on Urinary Continence Recovery after Robotic-Assisted Radical Prostatectomy. Can. Urol. Assoc. J. 2017, 11, e93–e99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, K.V.; Nitti, V.W. Prevention and Management of Incontinence Following Radical Prostatectomy. Urol. Clin. North Am. 2001, 28, 595–612. [Google Scholar] [CrossRef]
- Lee, S.E.; Byun, S.S.; Lee, H.J.; Song, S.H.; Chang, I.H.; Kim, Y.J.; Gill, M.C.; Hong, S.K. Impact of Variations in Prostatic Apex Shape on Early Recovery of Urinary Continence after Radical Retropubic Prostatectomy. Urology 2006, 68, 137–141. [Google Scholar] [CrossRef]
- Deliveliotis, C.; Protogerou, V.; Alargof, E.; Varkarakis, J. Radical Prostatectomy: Bladder Neck Preservation and Puboprostatic Ligament Sparing--Effects on Continence and Positive Margins. Urology 2002, 60, 855–858. [Google Scholar] [CrossRef]
- Van Randenborgh, H.; Paul, R.; Kübler, H.; Breul, J.; Hartung, R. Improved Urinary Continence after Radical Retropubic Prostatectomy with Preparation of a Long, Partially Intraprostatic Portion of the Membraneous Urethra: An analysis of 1013 Consecutive Cases. Prostate Cancer Prostatic Dis. 2004, 7, 253–257. [Google Scholar] [CrossRef]
- Takenaka, A.; Soga, H.; Sakai, I.; Nakano, Y.; Miyake, H.; Tanaka, K.; Fujisawa, M. Influence of Nerve-Sparing Procedure on Early Recovery of Urinary Continence after Laparoscopic Radical Prostatectomy. J. Endourol. 2009, 23, 1115–1119. [Google Scholar] [CrossRef]
- Sandhu, J.S.; Eastham, J.A. Factors Predicting Early Return of Continence after Radical Prostatectomy. Curr. Urol. Rep. 2010, 11, 191–197. [Google Scholar] [CrossRef]
- Koppie, T.M.; Guillonneau, B. Predictors of Incontinence after Radical Prostatectomy: Where do We Stand? Eur. Urol. 2007, 52, 22–23; discussion 24–25. [Google Scholar] [CrossRef]
- Jeong, S.J.; Yeon, J.S.; Lee, J.K.; Cha, W.H.; Jeong, J.W.; Lee, B.K.; Lee, S.C.; Jeong, C.W.; Kim, J.H.; Hong, S.K.; et al. Development and Validation of Nomograms to Predict the Recovery of Urinary Continence after Radical Prostatectomy: Comparisons between Immediate, Early, and Late Continence. World J. Urol. 2014, 32, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Paparel, P.; Akin, O.; Sandhu, J.S.; Otero, J.R.; Serio, A.M.; Scardino, P.T.; Hricak, H.; Guillonneau, B. Recovery of Urinary Continence after Radical Prostatectomy: Association with Urethral Length and Urethral Fibrosis Measured by Preoperative and Postoperative Endorectal Magnetic Resonance Imaging. Eur. Urol. 2009, 55, 629–637. [Google Scholar] [CrossRef]
- Shao, I.H.; Chang, Y.H.; Hou, C.M.; Lin, Z.F.; Wu, C.T. Predictors of Short-Term and Long-Term Incontinence after Robot-Assisted Radical Prostatectomy. J. Int. Med. Res. 2018, 46, 421–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, S.; Kagebayashi, Y.; Iemura, Y.; Matsumura, Y.; Samma, S. Postoperative Cystogram Findings Predict Recovery of Urinary Continence after Robot-Assisted Laparoscopic Radical Prostatectomy. LUTS Low. Urin. Tract Symptoms 2019, 11, 143–150. [Google Scholar] [CrossRef]
- Abdollah, F.; Sun, M.; Suardi, N.; Gallina, A.; Bianchi, M.; Tutolo, M.; Passoni, N.; Tian, Z.; Salonia, A.; Colombo, R.; et al. Prediction of Functional Outcomes after Nerve-Sparing Radical Prostatectomy: Results of Conditional Survival Analyses. Eur. Urol. 2012, 62, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Nevin, L.; On behalf of The PLOS Medicine Editors. Advancing the beneficial use of machine learning in health care and medicine: Toward a community understanding. PLoS Med. 2018, 15, e1002708. [Google Scholar] [CrossRef] [Green Version]
- Obermeyer, Z.; Emanuel, E.J. Predicting the Future-Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 2016, 375, 1216–1219. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging-Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and Elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.T.; Dunn, R.L.; Litwin, M.S.; Sandler, H.M.; Sanda, M.G. Development and Validation of the Expanded Prostate Cancer index Composite (EPIC) for Comprehensive Assessment of Health-Related Quality of Life in Men with Prostate Cancer. Urology 2000, 56, 899–905. [Google Scholar] [CrossRef] [Green Version]
- Vabalas, A.; Gowen, E.; Poliakoff, E.; Casson, A.J. Machine Learning Algorithm Validation with a Limited Sample Size. PLoS ONE 2019, 14, e0224365. [Google Scholar] [CrossRef] [PubMed]
- Moons, K.G.M.; Kengne, A.P.; Grobbee, D.E.; Royston, P.; Vergouwe, Y.; Altman, D.G.; Woodward, M. Risk Prediction Models: II. External Validation, Model Updating, and Impact Assessment. Heart 2012, 98, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.P. Machine Learning: A Probabilistic Perspective; MIT Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Géron, A. Hands-on Machine Learning with Scikit-Learn, Keras, and TensorFlow: Concepts, Tools, and Techniques to Build Intelligent Systems; O’Reilly Media: Sebastopol, CA, USA, 2019. [Google Scholar]
- MathWorks. Available online: https://kr.mathworks.com/help/stats/hyperparameter-optimization-in-classification-learner-app.html (accessed on 24 March 2021).
- Von Bodman, C.; Matsushita, K.; Savage, C.; Matikainen, M.P.; Eastham, J.A.; Scardino, P.T.; Rabbani, F.; Akin, O.; Sandhu, J.S. Recovery of urinary function after radical prostatectomy: Predictors of Urinary Function on Preoperative Prostate Magnetic Resonance Imaging. J. Urol. 2012, 187, 945–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlomm, T.; Heinzer, H.; Steuber, T.; Salomon, G.; Engel, O.; Michl, U.; Haese, A.; Graefen, M.; Huland, H. Full Functional-Length Urethral Sphincter Preservation during Radical Prostatectomy. Eur. Urol. 2011, 60, 320–329. [Google Scholar] [CrossRef]
- Eastham, J.A.; Kattan, M.W.; Rogers, E.; Goad, J.R.; Ohori, M.; Boone, T.B.; Scardino, P.T. Risk Factors for Urinary Incontinence after Radical Prostatectomy. J. Urol. 1996, 156, 1707–1713. [Google Scholar] [CrossRef]
- Dubbelman, Y.D.; Groen, J.; Wildhagen, M.F.; Rikken, B.; Bosch, J.L. Urodynamic Quantification of Decrease in Sphincter Function after Radical Prostatectomy: Relation to Postoperative Continence Status and the Effect of Intensive Pelvic Floor Muscle Exercises. Neurourol. Urodyn. 2012, 31, 646–651. [Google Scholar] [CrossRef]
- Mungovan, S.F.; Sandhu, J.S.; Akin, O.; Smart, N.A.; Graham, P.L.; Patel, M.I. Preoperative Membranous Urethral Length Measurement and Continence Recovery Following Radical Prostatectomy: A Systematic Review and Meta-analysis. Eur. Urol. 2017, 71, 368–378. [Google Scholar] [CrossRef] [Green Version]
- Greco, K.A.; Meeks, J.J.; Wu, S.; Nadler, R.B. Robot-Assisted Radical Prostatectomy in Men Aged > or = 70 Years. BJU Int. 2009, 104, 1492–1495. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.E.; Schumacher, M.C.; Johansson, E.; Carlsson, S.; Stranne, J.; Nyberg, T.; Wiklund, N.P.; Steineck, G. Age at Surgery, Educational Level and Long-Term Urinary Incontinence after Radical Prostatectomy. BJU Int. 2011, 108, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Eastham, J.A. Does Neurovascular Bundle Preservation at the Time of Radical Prostatectomy Improve Urinary Continence? Nat. Clin. Pr. Urol. 2007, 4, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Burkhard, F.C.; Kessler, T.M.; Fleischmann, A.; Thalmann, G.N.; Schumacher, M.; Studer, U.E. Nerve Sparing Open Radical Retropubic Prostatectomy--Does it Have an Impact on Urinary Continence? J. Urol. 2006, 176, 189–195. [Google Scholar] [CrossRef]
- Braslis, K.G.; Petsch, M.; Lim, A.; Civantos, F.; Soloway, M.S. Bladder Neck Preservation Following Radical Prostatectomy: Continence and Margins. Eur. Urol. 1995, 28, 202–208. [Google Scholar] [CrossRef]
- Menon, M.; Muhletaler, F.; Campos, M.; Peabody, J.O. Assessment of Early Continence after Reconstruction of the Periprostatic Tissues in Patients Undergoing Computer Assisted (Robotic) Prostatectomy: Results of a 2 Group Parallel Randomized Controlled Trial. J. Urol. 2008, 180, 1018–1023. [Google Scholar] [CrossRef]
- Vickers, A.; Savage, C.; Bianco, F.; Mulhall, J.; Sandhu, J.; Guillonneau, B.; Cronin, A.; Scardino, P. Cancer Control and Functional Outcomes after Radical Prostatectomy as Markers of Surgical Quality: Analysis of Heterogeneity between Surgeons at a Single Cancer Center. Eur. Urol. 2011, 59, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Sidey-Gibbons, J.A.M.; Sidey-Gibbons, C.J. Machine Learning in Medicine: A Practical Introduction. BMC Med Res. Methodol. 2019, 19, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Nitti, V.W.; Mourtzinos, A.; Brucker, B.M.; SUFU Pad Test Study Group. Correlation of Patient Perception of Pad Use with Objective Degree of Incontinence Measured by Pad Test in Men with Post-Prostatectomy Incontinence: The SUFU Pad Test Study. J. Urol. 2014, 192, 836–842. [Google Scholar] [CrossRef] [PubMed]


| Optimizable Hyper-Parameters | |
|---|---|
| Decision Tree | Maximum number of splits Split criterion |
| KNN | Number of neighbors Distance metric Distance weight Standardization |
| SVM | Kernel function Box constraint level Kernel scale Standardize data |
| Random Forest | Maximum number of splits Ensemble method Number of learners |
| All Population (n = 166) | Development Group (n = 109) | Test Group (n = 57) | p-Value | |
|---|---|---|---|---|
| Age at surgery, year | 71.6 (50, 87) | 71.9 (52, 87) | 71.0 (50, 87) | 0.440 ‡ |
| BMI (kg/m2) | 24.2 (15.5, 32.7) | 24.3 (18.5, 32.7) | 24.0 (15.5, 30.5) | 0.924 ‡ |
| PSA (ng/mL) | 17.9 (1.2, 426.6) | 21.5 (2.7, 426.6) | 11.0 (1.2, 57.4) | 0.351 ‡ |
| History of TURP, n | 7 (4.2) | 4 (3.7) | 3 (5.3) | 0.628 § |
| ISUP category and biopsy Gleason score | 0.732 § | |||
| 1, 6 (3 + 3) | 23 (13.9) | 13 (11.9) | 10 (17.5) | |
| 2, 7 (3 + 4) | 40 (24.1) | 25 (22.9) | 15 (26.3) | |
| 3, 7 (4 + 3) | 50 (30.1) | 36 (33.0) | 14 (24.6) | |
| 4, 8 | 31 (18.7) | 21 (19.3) | 10 (17.5) | |
| 5, 9 | 22 (13.3) | 14 (12.8) | 8 (14.0) | |
| Surgical approach, n | <0.001 § | |||
| Open | 33 (19.9) | 74(67.9) | 41(71.9) | |
| Laparoscopic | 18 (10.8) | 29 (26.6) | 4 (7.0) | |
| Robotic | 115 (69.3) | 6 (5.5) | 12 (21.1) | |
| Continence at 3 months | 90 (54.2) | 56 (51.4) | 20 (35.1) | 0.045 § |
| Anatomic finding on MRI | ||||
| PV (mm3) | 44.1 (18.7, 149.7) | 44.6 (18.7, 149.7) | 43.2 (19.9, 108.5) | 0.656 ‡ |
| MUL (mm) | 14.7 (5.1, 24.8) | 15.5 (7.9, 24.8) | 13.2 (5.1, 24.2) | 0.000 † |
| LAM (mm) | 7.7 (4.3, 11.6) | 7.6 (4.5, 11.3) | 8.0 (4.3, 11.6) | 0.154 † |
| UWT (mm) | 10.1 (5.2, 13.6) | 10.0 (5.2, 13.6) | 10.4 (7.4, 13.0) | 0.094 † |
| ASM (mm) | 3.1 (1.0, 6.4) | 3.0 (1.6, 6.4) | 3.3 (1.0, 5.2) | 0.347 ‡ |
| OIM (mm) | 17.8 (8.4, 49.5) | 17.8 (9.3, 23.7) | 17.8 (8.4, 49.5) | 0.019 ‡ |
| PPI Early-Recovery Group (n = 76) | PPI Consistent Group (n = 90) | p-Value | |
|---|---|---|---|
| Age at surgery, year | 70.1 (50, 87) | 72.8 (56, 87) | 0.009 ‡ |
| BMI, kg/m2 | 24.5 (18.6, 32.2) | 23.9 (15.5, 32.7) | 0.208 ‡ |
| PSA, ng/mL | 17.1 (1.2, 194.8) | 18.5 (2.7, 426.6) | 0.203 ‡ |
| History of TURP, n | 1 (1.3) | 6 (6.7) | 0.087 § |
| ISUP category and biopsy Gleason score | 0.596 § | ||
| 1, 6 (3 + 3) | 11 (14.5) | 12 (13.3) | |
| 2, 7 (3 + 4) | 19 (25.0) | 21 (23.3) | |
| 3, 7 (4 + 3) | 26 (34.2) | 24 (26.7) | |
| 4, 8 | 13 (17.1) | 18 (20.0) | |
| 5, 9 | 7 (9.2) | 15 (16.7) | |
| Surgical approach, n | 0.191 § | ||
| Open | 58 (76.3) | 57 (63.3) | |
| Laparoscopic | 12 (15.8) | 21 (23.3) | |
| Robotic | 6 (7.9) | 12 (13.3) | |
| Anatomic finding on MRI | |||
| PV (mm3) | 42.4 (19.3, 149.7) | 45.6 (18.7, 120.7) | 0.133 ‡ |
| MUL (mm) | 15.7 (8.4, 24.8) | 13.9 (5.1, 24.2) | 0.002 † |
| LAM (mm) | 7.9 (4.5, 11.3) | 7.6 (4.3, 11.6) | 0.169 † |
| UWT (mm) | 10.2 (7.3, 13.6) | 10.0 (5.2, 13.0) | 0.297 † |
| ASM (mm) | 3.2 (1.0, 6.4) | 3.0 (1.2, 5.3) | 0.274 ‡ |
| OIM (mm) | 18.2 (9.3, 23.7) | 17.5 (8.4, 49.5) | 0.021 ‡ |
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age at surgery, year | 1.06 | 1.01, 1.12 | 0.011 | 1.07 | 1.02, 1.13 | 0.007 |
| BMI, kg/m2 | 0.93 | 0.84, 1.04 | 0.215 | |||
| PSA, ng/mL | 1.00 | 0.99, 1.01 | 0.814 | |||
| History of TURP | 5.36 | 0.63, 45.52 | 0.124 | |||
| ISUP category and biopsy Gleason score | ||||||
| 1, 6 (3 + 3) | 0.605 | |||||
| 2, 7 (3 + 4) | 1.01 | 0.36, 2.83 | 0.980 | |||
| 3, 7 (4 + 3) | 0.85 | 0.31, 2.27 | 0.740 | |||
| 4, 8 | 1.27 | 0.43, 3.76 | 0.667 | |||
| 5, 9 | 1.96 | 0.58, 6.61 | 0.276 | |||
| Surgical approach | ||||||
| Open | ref | 0.196 | ||||
| Laparoscopic | 1.78 | 0.80, 3.95 | 0.156 | |||
| Robotic | 2.04 | 0.72, 5.79 | 0.183 | |||
| Anatomic finding on MRI | ||||||
| PV, mm3 | 1.01 | 0.99, 1.02 | 0.323 | |||
| MUL, mm | 0.88 | 0.81, 0.96 | 0.003 | 0.87 | 0.80, 0.95 | 0.002 |
| LAM, mm | 0.86 | 0.70, 1.07 | 0.169 | |||
| UWT, mm | 0.89 | 0.71, 1.11 | 0.296 | |||
| ASM, mm | 0.82 | 0.59, 1.15 | 0.259 | |||
| OIM, mm | 0.95 | 0.87, 1.04 | 0.248 | |||
| Title 1 | Sensitivity | Specificity | Accuracy | AUC | p-Value * | |
|---|---|---|---|---|---|---|
| Internal validation cohort | KNN | 72.5% ± 0.13 | 63.0% ± 0.17 | 68.3% ± 0.10 | 0.73 ± 0.09 | 0.3 |
| DT | 76.9% ± 0.09 | 64.0% ± 0.12 | 71.0% ± 0.05 | 0.73 ± 0.07 | 0.497 | |
| SVM | 72.6% ± 0.06 | 57.7% ± 0.11 | 65.9% ± 0.03 | 0.72 ± 0.03 | 0.457 | |
| RF | 79.0% ± 0.10 | 68.4% ± 0.11 | 74.2% ± 0.08 | 0.80 ± 0.10 | 0.552 | |
| LR | 79.3% ± 0.11 | 49.1% ± 0.23 | 65.7% ± 0.05 | 0.63 ± 0.04 | 0.25 | |
| External validation cohort | KNN | 62.4% ± 0.15 | 50.2% ± 0.16 | 56.1% ± 0.08 | 0.60 ± 0.08 | 0.358 |
| DT | 66.7% ± 0.13 | 49.4% ± 0.16 | 58.4% ± 0.07 | 0.61 ± 0.07 | 0.323 | |
| SVM | 68.8% ± 0.12 | 51.3% ± 0.15 | 60.2% ± 0.07 | 0.65 ± 0.07 | 0.394 | |
| RF | 65.1% ± 0.11 | 53.6% ± 0.14 | 59.5% ± 0.07 | 0.61 ± 0.08 | 0.464 | |
| LR | 71.7% ± 0.15 | 40.0% ± 0.22 | 56.5% ± 0.07 | 0.59 ± 0.07 | 0.339 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Byun, J. A Study of Predictive Models for Early Outcomes of Post-Prostatectomy Incontinence: Machine Learning Approach vs. Logistic Regression Analysis Approach. Appl. Sci. 2021, 11, 6225. https://doi.org/10.3390/app11136225
Park S, Byun J. A Study of Predictive Models for Early Outcomes of Post-Prostatectomy Incontinence: Machine Learning Approach vs. Logistic Regression Analysis Approach. Applied Sciences. 2021; 11(13):6225. https://doi.org/10.3390/app11136225
Chicago/Turabian StylePark, Seongkeun, and Jieun Byun. 2021. "A Study of Predictive Models for Early Outcomes of Post-Prostatectomy Incontinence: Machine Learning Approach vs. Logistic Regression Analysis Approach" Applied Sciences 11, no. 13: 6225. https://doi.org/10.3390/app11136225
APA StylePark, S., & Byun, J. (2021). A Study of Predictive Models for Early Outcomes of Post-Prostatectomy Incontinence: Machine Learning Approach vs. Logistic Regression Analysis Approach. Applied Sciences, 11(13), 6225. https://doi.org/10.3390/app11136225





