Transferred Cold Atmospheric Plasma Treatment on Melanoma Skin Cancer Cells with/without Catalase Enzyme In Vitro
Abstract
1. Introduction
2. Materials and Methods
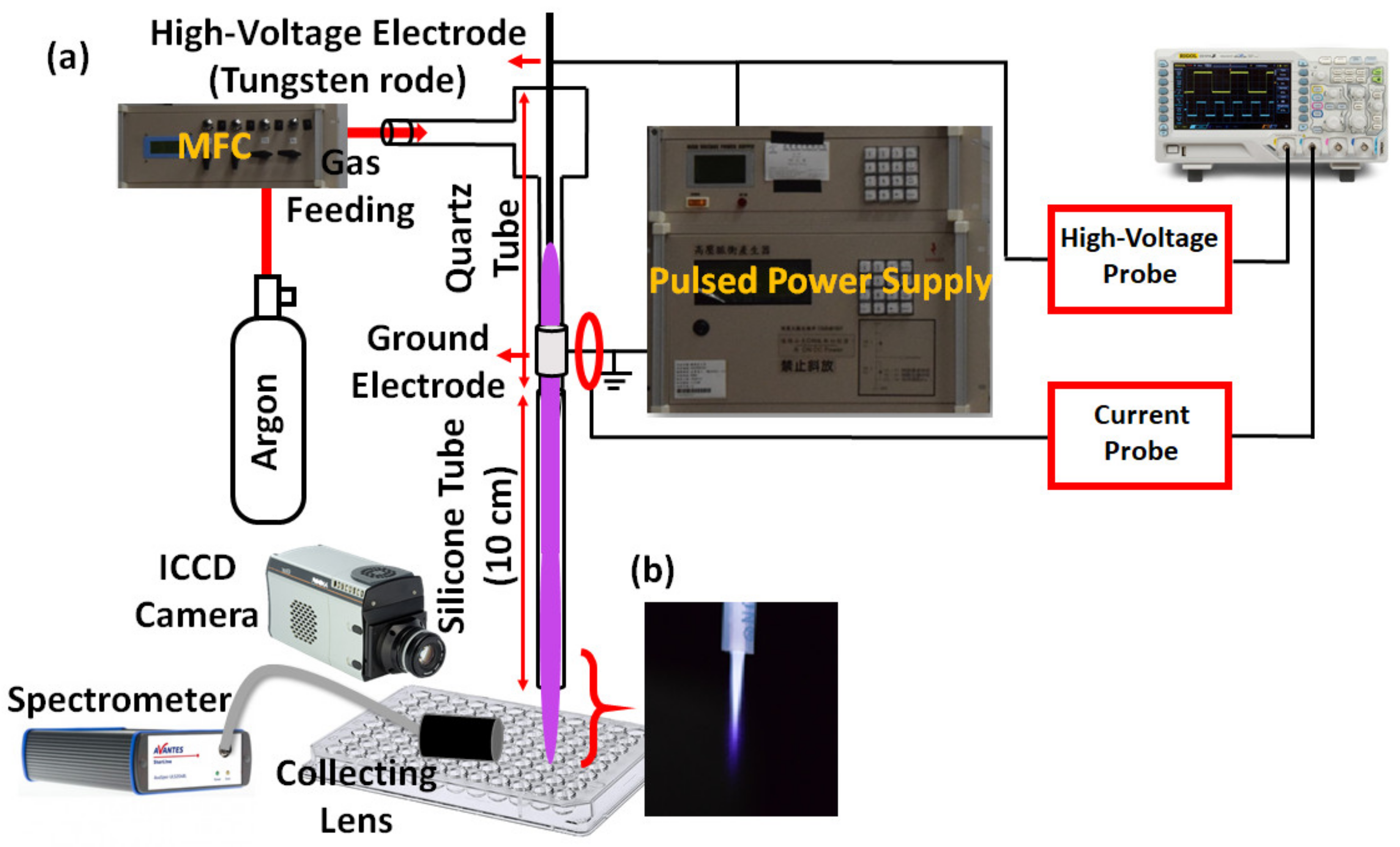
2.1. Transfer-CAP System
2.2. Transfer-CAP Generation
2.2.1. Electrical Measurement
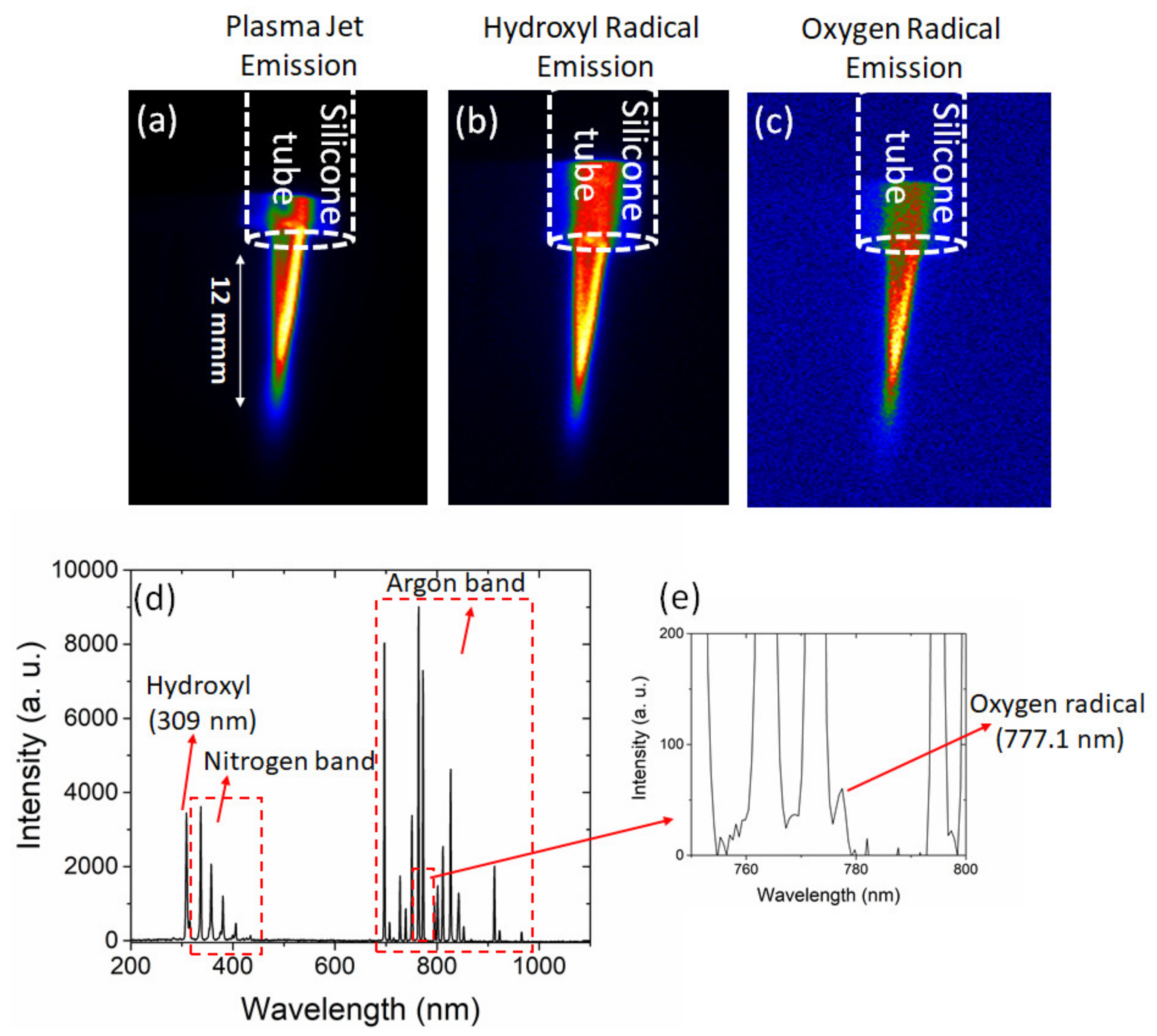
2.2.2. Emission and Spectroscopic Measurements
2.3. Cell Experiments
2.3.1. Cell Viability Analysis
2.3.2. Live/Dead Experiment
2.3.3. Fluorescence RONS and Catalase Level Analysis in Cells
2.3.4. Statistical Analysis
3. Results and Discussion
3.1. Transfer-CAP Characterization
Gas Temperature
3.2. Cell Viability
3.3. Live/Dead Experiment (Control, 30 s, and 30 s with CAT)
3.4. Cell RONS Analysis (Control, 30 s, and 30 s with CAT)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erdei, E.; Torres, S.M. A new understanding in the epidemiology of melanoma. Expert Rev. Anticancer. Ther. 2010, 10, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 1, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Ikegawa, S.; Saida, T.; Obayashi, H.; Sasaki, A.; Esumi, H.; Ikeda, S.; Kiyohara, Y.; Hayasaka, K.; Ishihara, K. Cisplatin combination chemotherapy in squamous cell carcinoma and adenoid cystic carcinoma of the skin. J. Dermatol. 1989, 16, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Kalal, B.S.; Upadhya, D.; Pai, V.R. Chemotherapy resistance mechanisms in advanced skin cancer. Oncol. Rev. 2017, 11, 326. [Google Scholar] [CrossRef]
- Abdullah, Z.; Mohtar, J.A.; Zaaba, S.K. Review on Melanoma Skin Cancer Treatment by Cold Atmospheric Plasma. J. Telecommun. Electron. Comput. Eng. JTEC 2018, 10, 97–100. [Google Scholar]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193. [Google Scholar] [CrossRef]
- Burm, K. Plasma: The fourth state of matter. Plasma Chem. Plasma Process. 2012, 32, 401–407. [Google Scholar] [CrossRef]
- Laroussi, M. Cold plasma in medicine and healthcare: The new frontier in low temperature plasma applications. Front. Phys. 2020, 8, 74. [Google Scholar] [CrossRef]
- Duarte, S.; Panariello, B.H. Comprehensive biomedical applications of low temperature plasmas. Arch. Biochem. Biophys. 2020, 693, 108560. [Google Scholar] [CrossRef] [PubMed]
- Martines, E. Special issue “Plasma Technology for Biomedical Applications”; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2020. [Google Scholar]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.-D.; Wende, K.; Bogaerts, A.; Bekeschus, S. Ros from physical plasmas: Redox chemistry for biomedical therapy. Oxidative Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Borges, A.C.; Kostov, K.G.; Pessoa, R.S.; de Abreu, G.M.A.; Lima, G.d.G.; Figueira, L.W.; Koga-Ito, C.Y. Applications of Cold Atmospheric Pressure Plasma in Dentistry. Appl. Sci. 2021, 11, 1975. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Bogaerts, A. Plasma in Cancer Treatment; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2020. [Google Scholar]
- Stope, M.B. Plasma oncology-Physical plasma as innovative tumor therapy. J. Cancer 2020, 53, 56. [Google Scholar]
- Keidar, M.; Shashurin, A.; Volotskova, O.; Stepp, M.A.; Srinivasan, P.; Sandler, A.; Trinket, B. Cold atmospheric plasma in cancer therapy. Phys. Plasmas 2013, 20, 057101. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 2017, 26, 053001. [Google Scholar] [CrossRef]
- Robert, E.; Vandamme, M.; Brullé, L.; Lerondel, S.; le Papebe, A.; Sarrona, V.; Rièsa, D.; Darnya, T.; Doziasa, S.; Colletaf, G.; et al. Perspectives of endoscopic plasma applications. Clin. Plasma Med. 2013, 1, 8–16. [Google Scholar] [CrossRef]
- Onyshchenko, I.; de Geyter, N.; Nikiforov, A.Y.; Morent, R. Atmospheric pressure plasma penetration inside flexible polymeric tubes. Plasma Process. Polym. 2015, 12, 271–284. [Google Scholar] [CrossRef]
- Prysiazhnyi, V.; Saturnino, V.F.; Kostov, K.G. Transferred plasma jet as a tool to improve the wettability of inner surfaces of polymer tubes. Int. J. Polym. Anal. Charact. 2017, 22, 215–221. [Google Scholar] [CrossRef]
- Schweigert, I.; Zakrevsky, D.; Gugin, P.; Yelak, E.; Golubitskaya, E.; Troitskaya, O.; Koval, O. Interaction of cold atmospheric argon and helium plasma jets with bio-target with grounded substrate beneath. Appl. Sci. 2019, 9, 4528. [Google Scholar] [CrossRef]
- Wang, S.; der Gathen, V.S.; Döbele, H. Discharge comparison of nonequilibrium atmospheric pressure Ar/O2 and He/O2 plasma jets. Appl. Phys. Lett. 2003, 83, 3272–3274. [Google Scholar] [CrossRef]
- Fridman, G.; Shereshevsky, A.; Jost, M.M.; Brooks, A.D.; Fridman, A.; Gutsol, A.; Vasilets, V.; Friedman, G. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem. Plasma Process. 2007, 27, 163–176. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Golpour, M.; Sohbatzadeh, F.; Hadavi, S.; Bekeschus, S.; Niaki, H.A.; Valadan, R.; Rafiei, A. Cold atmospheric plasma is a potent tool to improve chemotherapy in melanoma in vitro and in vivo. Biomolecules 2020, 10, 1011. [Google Scholar] [CrossRef]
- Zucker, S.N.; Zirnheld, J.; Bagati, A.; DiSanto, T.M.; des Soye, B.; Wawrzyniak, J.A.; Etemadi, K.; Nikiforov, M.; Berezney, R. Preferential induction of apoptotic cell death in melanoma cells as compared with normal keratinocytes using a non-thermal plasma torch. Cancer Biol. Ther. 2012, 13, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Cloux, A.-J.; Aubry, D.; Heulot, M.; Widmann, C.; ElMokh, O.; Piacente, F.; Cea, M.; Nencioni, A.; Bellotti, A.; Bouzourène, K.; et al. Reactive oxygen/nitrogen species contribute substantially to the antileukemia effect of APO866, a NAD lowering agent. Oncotarget 2019, 10, 6723. [Google Scholar] [CrossRef][Green Version]
- Chwa, M.; Atilano, S.R.; Reddy, V.; Jordan, N.; Kim, D.W.; Kenney, M.C. Increased stress-induced generation of reactive oxygen species and apoptosis in human keratoconus fibroblasts. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1902–1910. [Google Scholar] [CrossRef]
- Rafiei, A.; Sohbatzadeh, F.; Hadavi, S.; Bekeschus, S.; Alimohammadi, M.; Valadan, R. Inhibition of murine melanoma tumor growth in vitro and in vivo using an argon-based plasma jet. Clin. Plasma Med. 2020, 19, 100102. [Google Scholar] [CrossRef]
- Cullen, P.; Milosavljević, V. Spectroscopic characterization of a radio-frequency argon plasma jet discharge in ambient air. Prog. Theor. Exp. Phys. 2015, 2015. [Google Scholar] [CrossRef]
- Lim, J.-P.; Uhm, H.S.; Li, S.-Z. Influence of oxygen in atmospheric-pressure argon plasma jet on sterilization of Bacillus atrophaeous spores. Phys. Plasmas 2007, 14, 093504. [Google Scholar] [CrossRef]
- Lietz, M.A.; Kushner, M.J. Molecular admixtures and impurities in atmospheric pressure plasma jets. J. Appl. Phys. 2018, 124, 153303. [Google Scholar] [CrossRef]
- Yarmolenko, P.S.; Moon, E.J.; Landon, C.; Manzoor, A.; Hochman, D.W.; Viglianti, B.L.; Dewhirst, M.W. Thresholds for thermal damage to normal tissues: An update. Int. J. Hyperth. 2011, 27, 320–343. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Gao, Z.; Zhang, Y.; Wu, H.; You, C.; Wang, S.; An, P.; Suna, C.; Sun, B. Enhanced highly toxic reactive oxygen species levels from iron oxide core–shell mesoporous silica nanocarrier-mediated Fenton reactions for cancer therapy. J. Mater. Chem. B 2018, 6, 5876–5887. [Google Scholar] [CrossRef]
- Nantapong, N.; Murata, R.; Trakulnaleamsai, S.; Kataoka, N.; Yakushi, T.; Matsushita, K. The effect of reactive oxygen species (ROS) and ROS-scavenging enzymes, superoxide dismutase and catalase, on the thermotolerant ability of Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2019, 103, 5355–5366. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.A.; Averill-Bates, D. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta BBA Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-R.; Xu, G.-M.; Shi, X.-M.; Zhang, G.-J. Low temperature plasma promoting fibroblast proliferation by activating the NF-κB pathway and increasing cyclinD1 expression. Sci. Rep. 2017, 7, 11698. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxidative Med. Cell. Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Hsieh, J.-H.; Wang, I.-T.; Jheng, P.-R.; Yeh, Y.-Y.; Lee, J.-W.; Bolouki, N.; Chuang, E.-Y. Transferred Cold Atmospheric Plasma Treatment on Melanoma Skin Cancer Cells with/without Catalase Enzyme In Vitro. Appl. Sci. 2021, 11, 6181. https://doi.org/10.3390/app11136181
Chen Y-H, Hsieh J-H, Wang I-T, Jheng P-R, Yeh Y-Y, Lee J-W, Bolouki N, Chuang E-Y. Transferred Cold Atmospheric Plasma Treatment on Melanoma Skin Cancer Cells with/without Catalase Enzyme In Vitro. Applied Sciences. 2021; 11(13):6181. https://doi.org/10.3390/app11136181
Chicago/Turabian StyleChen, Yun-Hsuan, Jang-Hsing Hsieh, I-Te Wang, Pei-Ru Jheng, Yi-Yen Yeh, Jyh-Wei Lee, Nima Bolouki, and Er-Yuan Chuang. 2021. "Transferred Cold Atmospheric Plasma Treatment on Melanoma Skin Cancer Cells with/without Catalase Enzyme In Vitro" Applied Sciences 11, no. 13: 6181. https://doi.org/10.3390/app11136181
APA StyleChen, Y.-H., Hsieh, J.-H., Wang, I.-T., Jheng, P.-R., Yeh, Y.-Y., Lee, J.-W., Bolouki, N., & Chuang, E.-Y. (2021). Transferred Cold Atmospheric Plasma Treatment on Melanoma Skin Cancer Cells with/without Catalase Enzyme In Vitro. Applied Sciences, 11(13), 6181. https://doi.org/10.3390/app11136181







