Sugarcane Bagasse Torrefaction for Fluidized Bed Gasification
Abstract
:1. Introduction
2. The Torrefaction Process
3. Materials and Methods
3.1. Characterization of the Sugarcane Bagasse
3.2. Thermogravimetric Analysis (TGA)
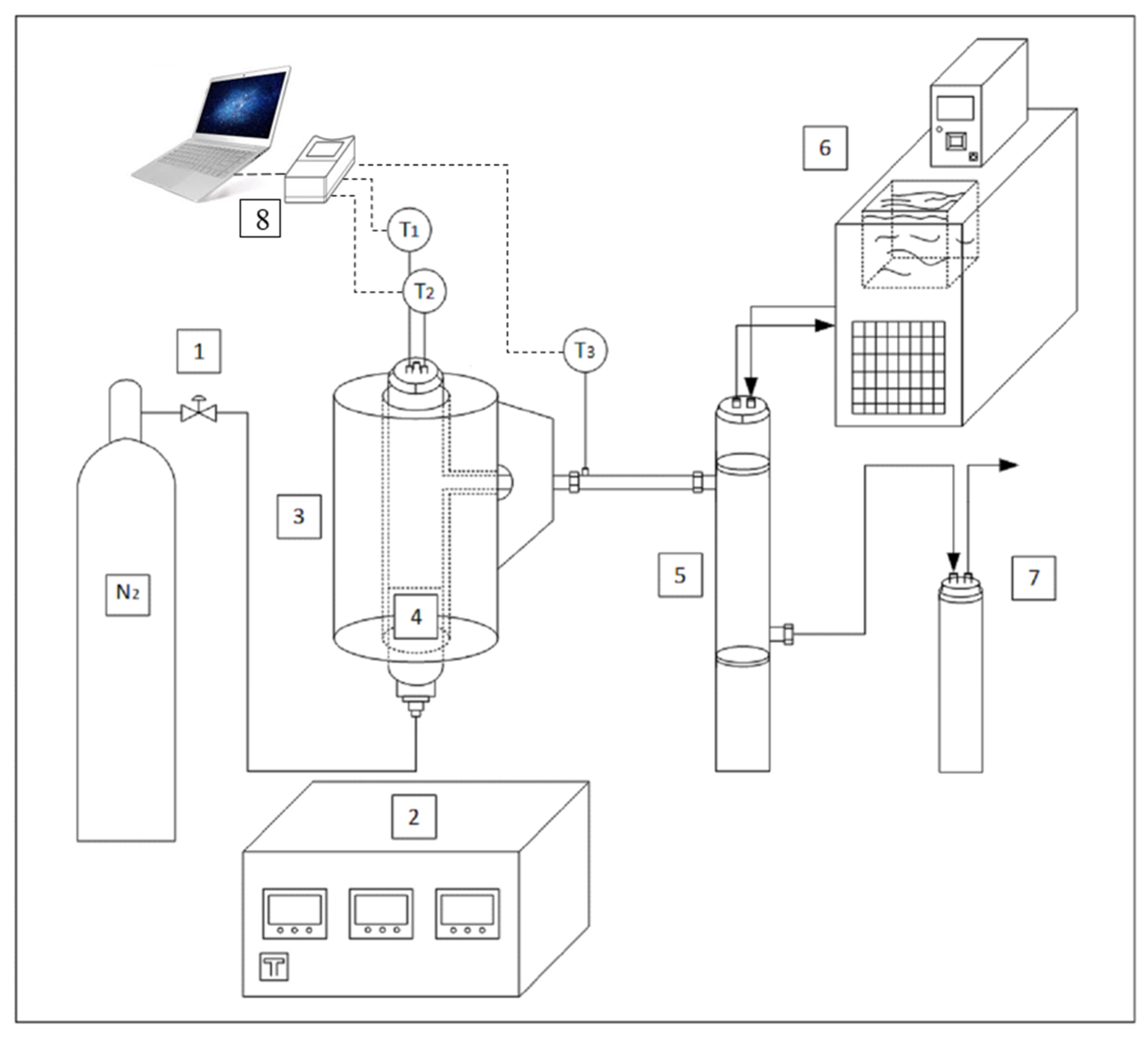
3.3. Experimental Setup
4. Results and Discussion
4.1. Thermogravimetric Analysis (TGA)
4.2. Proximate Analysis of Unprocessed Sugarcane Bagasse
4.3. Ultimate Analysis of Unprocessed Sugarcane Bagasse
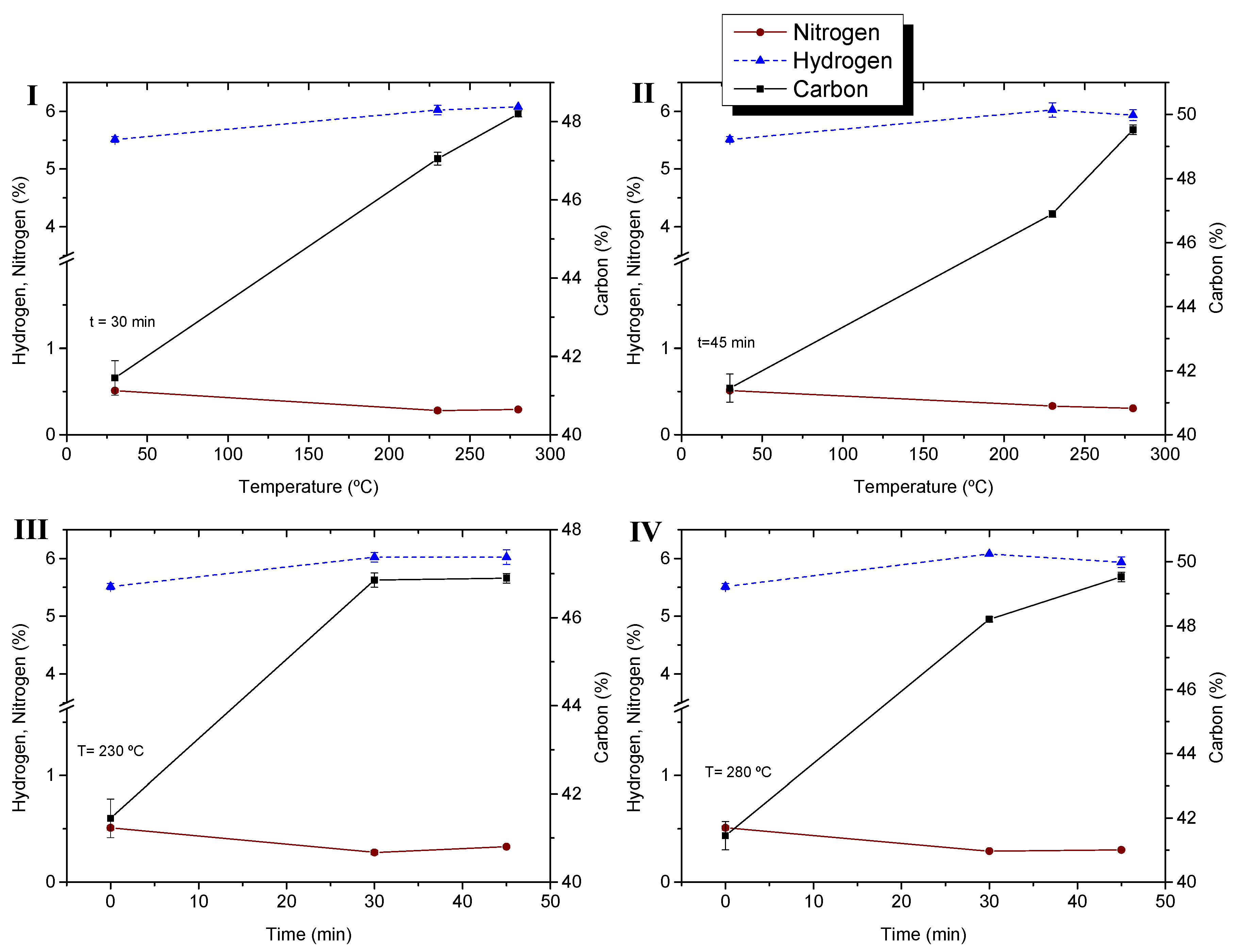
4.4. Improving Energy Properties of Sugarcane Bagasse by Torrefaction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, R.D. 2016 Outlook of the U.S. and World Sugar Markets, 2016–2025. Agribus. Appl. Econ. Rep. 2016, 751. [Google Scholar] [CrossRef]
- de Sousa, E.L.L.; de Carvalho Macedo, I. Ethanol and Bioelectricity: Sugarcane in the Future of the Energy Matrix; Unica: São Paulo, Brazil, 2011. [Google Scholar]
- EPE Balanço Energético Nacional. 2019. Available online: https://www.epe.gov.br/sites-pt/publicacoes-dados-abertos/publicacoes/PublicacoesArquivos/publicacao-377/topico-494/BEN%202019%20Completo%20WEB.pdf (accessed on 2 January 2020).
- De Filippis, P.; Borgianni, C.; Paolucci, M.; Pochetti, F. Gasification process of Cuban bagasse in a two-stage reactor. Biomass Bioenergy 2004, 27, 247–252. [Google Scholar] [CrossRef]
- FAO; OECD. OECD-FAO Agricultural Outlook 2018–2027; FAO-OECD: Rome, Italy, 2018; ISBN 978-92-5-130501-0. [Google Scholar]
- USDA. Sugar: World Markets and Trade; USDA: Washington, DC, USA, 2021.
- Pernalete, Z.; Piña, F.; Suarez, M.; Ferrer, A.; Aiello, C. Fraccionamiento del Bagazo de Caña de Azúcar Mediante Tratamiento Amoniacal: Efecto de la Humedad del Bagazo y la Carga de Amoníaco. Bioagro 2008, 20, 3–10. [Google Scholar]
- Barroso, J.; Barreras, F.; Amaveda, H.; Lozano, A. On the optimization of boiler efficiency using bagasse as fuel. Fuel 2003, 82, 1451–1463. [Google Scholar] [CrossRef]
- Brito, A.L.; Beaton, P.A.; Ballester, J.; Dopazo, C. Novel Approach for the Analysis of Heat Transfer in Bagasse-Fired Furnaces; Overend, R.P., Chornet, E., Eds.; Pergamon: Oxford, UK, 1999; ISBN 0080430198. [Google Scholar]
- Larson, E.D.; Williams, R.H.; Leal, M.R.L. A review of biomass integrated-gasifier/gas turbine combined cycle technology and its application in sugarcane industries, with an analysis for Cuba. Energy Sustain. Dev. 2001, 5, 54–76. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Jana, K.; De, S. Biomass integrated gasification combined cogeneration with or without CO2 capture—A comparative thermodynamic study. Renew. Energy 2014, 72, 243–252. [Google Scholar] [CrossRef]
- Babu, S. Thermal gasification of biomass technology developments: End of task report for 1992 to 1994. Biomass Bioenergy 1995, 9, 271–285. [Google Scholar] [CrossRef]
- Gómez, E.O.; Cortez, L.A.B.; Alarcon, G.R.; Rocha, G.J.D.M.; Da Silva, V.F.N.; De Almeida, E. Some simplified geometrical properties of elephant grass and sugarcane trash particles. Fuel Process. Technol. 2012, 104, 234–244. [Google Scholar] [CrossRef]
- Driemeier, C.; Oliveira, M.M.; Mendes, F.M.; Gómez, E.O. Characterization of sugarcane bagasse powders. Powder Technol. 2011, 214, 111–116. [Google Scholar] [CrossRef]
- Alarcon, G.; Olivares Gómez, E.; Barbosa Cortez, L.; Glauco Sánchez, C.; Alarcon, G.; Olivares Gómez, E.; Barbosa Cortez, L.G.S. Caracterización del bagazo de la caña de azúcar. Parte I: Características físicas. In Proceedings of the VI Encontro Energia no Meio Rural AGRENER 2006, Campinas, Brazil, 6–8 June 2006; NIPE/Unicamp: Campinas, Brazil, 2006; p. 10. [Google Scholar]
- Rasul, M.; Rudolph, V.; Carsky, M. Physical properties of bagasse. Fuel 1999, 78, 905–910. [Google Scholar] [CrossRef]
- van den Enden, P.J.; Lora, E.S. Design approach for a biomass fed fluidized bed gasifier using the simulation software CSFB. Biomass Bioenergy 2004, 26, 281–287. [Google Scholar] [CrossRef]
- Pellegrini, L.; Deoliveirajr, S. Exergy analysis of sugarcane bagasse gasification. Energy 2007, 32, 314–327. [Google Scholar] [CrossRef]
- Gómez, E.O.; Cortez, L.A.B.; Lora, E.E.S.; Sanchez, C.G.; Bauen, A. Preliminary tests with a sugarcane bagasse fueled fluidized-bed air gasifier. Energy Convers. Manag. 1999, 40, 205–214. [Google Scholar] [CrossRef]
- Gabra, M.; Salman, H.; Kjellström, B. Development of a sugar cane residue feeding system for a cyclone gasifier. Biomass Bioenergy 1998, 15, 143–153. [Google Scholar] [CrossRef]
- Gabra, M.; Pettersson, E.; Backman, R.; Kjellström, B. Evaluation of cyclone gasifier performance for gasification of sugar cane residue—Part 1: Gasification of bagasse. Biomass Bioenergy 2001, 21, 351–369. [Google Scholar] [CrossRef]
- Basu, P. (Ed.) Torrefaction. In Biomass Gasification, Pyrolysis and Torrefaction; Academic Press: New York, NY, USA, 2013; pp. 93–154. ISBN 9780128129920. [Google Scholar]
- Leal-Arcas, R.; Minas, S. Renewable energy. Res. Handb. Int. Law Nat. Resour. 2016, 261–280. [Google Scholar] [CrossRef] [Green Version]
- Pach, M.; Zanzi, R.; Björnbom, E. Torrefied biomass a substitue for wood and charcoal. In Proceedings of the 6th Asia-Pacific Internatioonal Symposium on Combustion and Energy Utilization, Kuala Lumpur, Malaysia, 20–22 May 2002; Faculty of Mechanical Engineering, Universiti Teknologi Malaysia, Ed.; Universiti Teknologi Malaysia: Skudai, Malaysia, 2002; p. 6. [Google Scholar]
- Anukam, A.; Mamphweli, S.; Reddy, P.; Meyer, E.; Okoh, O. Pre-processing of sugarcane bagasse for gasification in a downdraft biomass gasifier system: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 66, 775–801. [Google Scholar] [CrossRef] [Green Version]
- Blanco Machin, E. Analise Técnica, Econômica e Ecológica da Incorporação de Sistemas de Gaseificação de Bagaço de Cana-de-Açúcar No Setor Sucroalcooleiro: Uso de Ciclos Combinados Para o Aumento da Oferta de Eletricidade; Universidade Estadual Paulista (UNESP): Guaratinguetá, Brazil, 2015. [Google Scholar]
- Pedroso, D.T.; Machin, E.B.; Perez, N.P.; Braga, L.B.; Silveira, J.L. Technical assessment of the Biomass Integrated Gasification/Gas Turbine Combined Cycle (BIG/GTCC) incorporation in the sugarcane industry. Renew. Energy 2017, 114, 464–479. [Google Scholar] [CrossRef] [Green Version]
- Manatura, K. Inert torrefaction of sugarcane bagasse to improve its fuel properties. Case Stud. Therm. Eng. 2020, 19, 100623. [Google Scholar] [CrossRef]
- Granados, D.A.; Ruiz, R.; Vega, L.; Chejne, F. Study of reactivity reduction in sugarcane bagasse as consequence of a torrefaction process. Energy 2017, 139, 818–827. [Google Scholar] [CrossRef]
- Shehzad, M.; Asghar, A.; Ramzan, N.; Aslam, U.; Bello, M.M. Impacts of non-oxidative torrefaction conditions on the fuel properties of indigenous biomass (bagasse). Waste Manag. Res. 2020, 38, 1284–1294. [Google Scholar] [CrossRef]
- Bourgois, J.; Guyonnet, R. Characterization and analysis of torrefied wood. Wood Sci. Technol. 1988, 22, 143–155. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. More efficient biomass gasification via torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Zanatta, E.R.; Reinehr, T.O.; Awadallak, J.A.; Kleinübing, S.J.; Santos, J.; Bariccatti, R.A.; Arroyo, P.; Da Silva, E.A. Kinetic studies of thermal decomposition of sugarcane bagasse and cassava bagasse. J. Therm. Anal. Calorim. 2016, 125, 437–445. [Google Scholar] [CrossRef]
- Mohomane, S.M.; Motaung, T.E.; Revaprasadu, N. Thermal Degradation Kinetics of Sugarcane Bagasse and Soft Wood Cellulose. Materials 2017, 10, 1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory, 2nd ed.; Elsevier: New York, NY, USA, 2013; ISBN 9780123964885. [Google Scholar]
- Wang, L.; Barta-Rajnai, E.; Skreiberg, Ø.; Khalil, R.; Czégény, Z.; Jakab, E.; Barta, Z.; Grønli, M. Effect of torrefaction on physiochemical characteristics and grindability of stem wood, stump and bark. Appl. Energy 2018, 227, 137–148. [Google Scholar] [CrossRef]
- Bergman, P.C.A.; Kiel, J.H.A. Torrefaction for biomass upgrading. In Proceedings of the 14th European Biomass Conference, Paris, France, 17–21 October 2005; pp. 17–21. [Google Scholar]
- Basu, P. Introduction. In Biomass Gasification, Pyrolysis and Torrefaction; Academic Press: New York, NY, USA, 2013; pp. 1–27. [Google Scholar]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood. Part 1. Weight loss kinetics. J. Anal. Appl. Pyrolysis 2006, 77, 28–34. [Google Scholar] [CrossRef]
- van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Channiwala, S.; Parikh, P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Chen, W.-H.; Du, S.-W.; Tsai, C.-H.; Wang, Z.-Y. Torrefied biomasses in a drop tube furnace to evaluate their utility in blast furnaces. Bioresour. Technol. 2012, 111, 433–438. [Google Scholar] [CrossRef]
- Itoh, T.; Fujiwara, N.; Iwabuchi, K.; Narita, T.; Mendbayar, D.; Kamide, M.; Niwa, S.; Matsumi, Y. Effects of pyrolysis temperature and feedstock type on particulate matter emission characteristics during biochar combustion. Fuel Process. Technol. 2020, 204, 106408. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Wang, K.; Luo, Z. Influence of the interaction of components on the pyrolysis behavior of biomass. J. Anal. Appl. Pyrolysis 2011, 91, 183–189. [Google Scholar] [CrossRef]
- Munir, S.; Daood, S.S.; Nimmo, W.; Cunliffe, A.; Gibbs, B. Thermal analysis and devolatilization kinetics of cotton stalk, sugar cane bagasse and shea meal under nitrogen and air atmospheres. Bioresour. Technol. 2009, 100, 1413–1418. [Google Scholar] [CrossRef]
- Santos, K.G.; Lira, T.; Gianesella, M.; Lobato, F.S.; Murata, V.V.; Barrozo, M. Bagasse pyrolysis: A comparative study of kinetic models. Chem. Eng. Commun. 2012, 199, 109–121. [Google Scholar] [CrossRef]
- Cheng, K.; Winter, W.T.; Stipanovic, A.J. A modulated-TGA approach to the kinetics of lignocellulosic biomass pyrolysis/combustion. Polym. Degrad. Stab. 2012, 97, 1606–1615. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, M.; Zhao, L.; Zhu, L. Influence of Interactions among Three Biomass Components on the Pyrolysis Behavior. Ind. Eng. Chem. Res. 2018, 57, 5241–5249. [Google Scholar] [CrossRef]
- Rein, P. Cane Sugar Engineering; Verlag Dr. Albert Bartens KG: Berlin, Germany, 2017; ISBN 9783870401672. [Google Scholar]
- Camargo, C.; Hakuo Ushima, A. Conservação de Energia na Indústria do Açúcar e do álcool: Manual de Recomendações; Instituto de Pesquisas Tecnológicas, Ed.; Instituto de Pesquisas Tecnológicas: São Paulo, Brazil, 1990. [Google Scholar]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Garcìa-Pèrez, M.; Chaala, A.; Yang, J.; Roy, C. Co-pyrolysis of sugarcane bagasse with petroleum residue. Part I: Thermogravimetric analysis. Fuel 2001, 80, 1245–1258. [Google Scholar] [CrossRef]
- Miles, T.R.; Baxter, L.L.; Bryers, R.W.; Jenkins, B.M.; Oden, L.L. Boilers. Fuels and deposit formation. In Alkali Deposits Found in Biomass Power Plants; National Techrucd Momation Service (NTIS): Golden, CO, USA, 1995; Volume I, pp. 1–122. ISBN 9788578110796. [Google Scholar]
- Agblevor, F.A.; Besler-Guran, S.; Montane, D.; Wiselogel, A.E. Biomass Feedstock Variability and Its Effect on Biocrude Oil Properties. In Developments in Thermochemical Biomass Conversion; Springer: Dordrecht, The Netherlands, 1997; pp. 741–755. [Google Scholar]
- Natarajan, E.; Öhman, M.; Gabra, M.; Nordin, A.; Liliedahl, T.; Rao, A. Experimental determination of bed agglomeration tendencies of some common agricultural residues in fluidized bed combustion and gasification. Biomass Bioenergy 1998, 15, 163–169. [Google Scholar] [CrossRef]
- Felfli, F.F.; Luengo, C.A.; Bezzon, G.; Soler, P.B. Bench unit for biomass residues torrefaction. In Proceedings of the 10th European Conference Biomass for Energy & Industry, Würzburg, Germany, 8–11 June 1998; EU: Würzburg, Germany, 1998; p. 12. [Google Scholar]
- Basu, P. Chapter 2. Biomass Characteristics. In Biomass Gasification and Pyrolysis: Practical Design and Theory; Academic Press: New York, NY, USA, 2010; ISBN 9780123749888. [Google Scholar]
- Basu, P. Gasification Theory. In Biomass Gasification, Pyrolysis and Torrefaction; Academic Press: New York, NY, USA, 2013; pp. 199–248. [Google Scholar]
- Anukam, A.; Mamphweli, S.; Reddy, P.; Okoh, O.; Meyer, E. An Investigation into the Impact of Reaction Temperature on Various Parameters during Torrefaction of Sugarcane Bagasse Relevant to Gasification. J. Chem. 2015, 2015, 235163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniyanto, S.; Deendarlianto, B.A. Torrefaction of Indonesian Sugar-cane Bagasse to Improve Bio-syngas Quality for Gasification Process. Energy Procedia 2015, 68, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Pimchuai, A.; Dutta, A.; Basu, P. Torrefaction of Agriculture Residue to Enhance Combustible Properties. Energy Fuels 2010, 24, 4638–4645. [Google Scholar] [CrossRef]
- Zaharioiu, A.; Bucura, F.; Ionete, E.I.; Ionete, R.E.; Ebrasu, D.; Sandru, C.; Marin, F.; Oancea, S.; Niculescu, V.; Miricioiu, M.G.; et al. Thermochemical Decomposition of Sewage Sludge—An Eco-Friendly Solution for a Sustainable Energy Future by Using Wastes. Rev. Chim. 2020, 71, 171–181. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
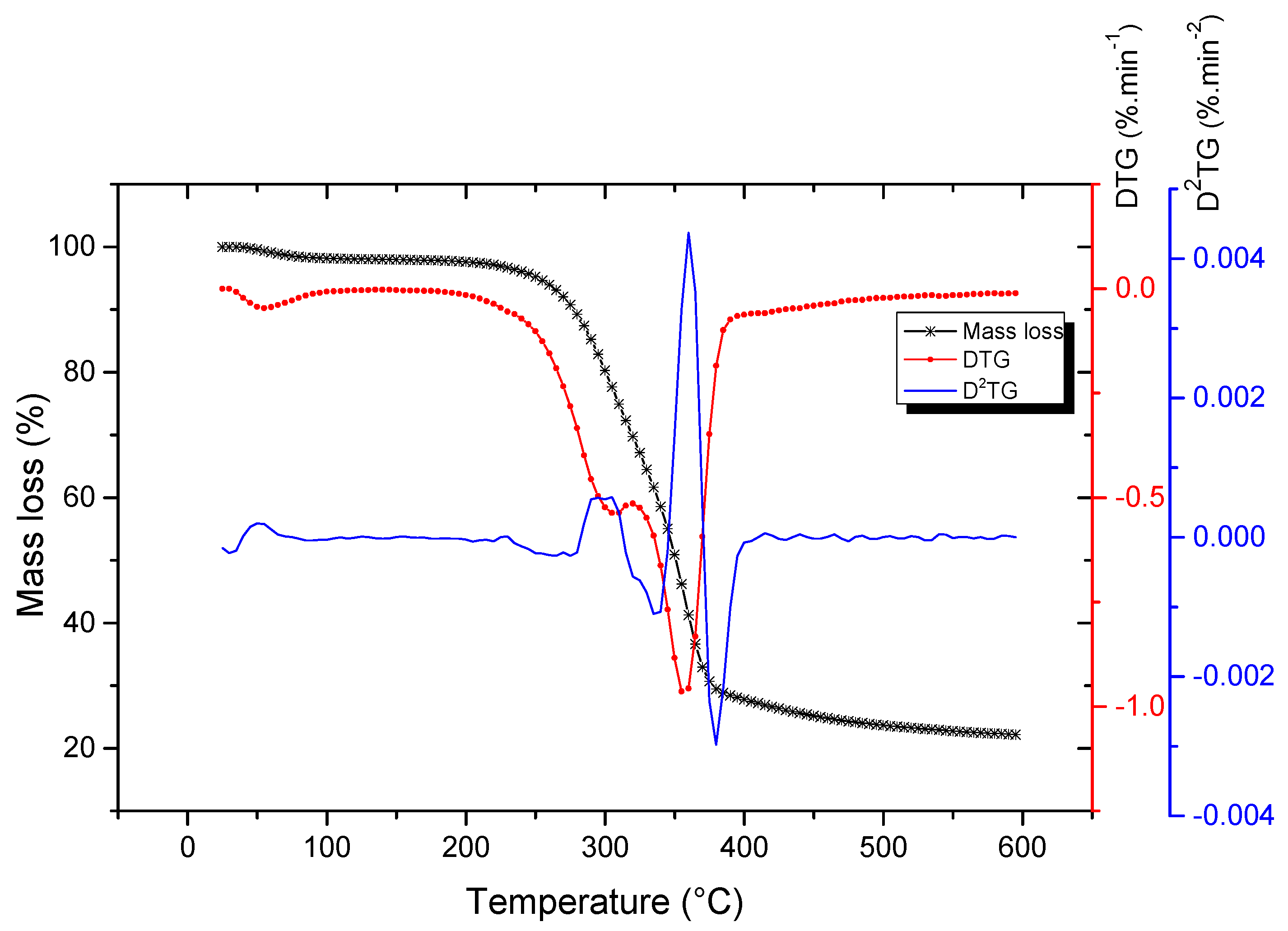

| Sugarcane Bagasse | Temperature (°C) | Weight Loss (%) | Char (%) | ||
|---|---|---|---|---|---|
| TONSET | TPEAK | TOFFSET | |||
| First stage | 30 | 58 | 113 | 2.3 | |
| Second stage | 178 | 307 | 321 | 28.8 | 22.1 |
| Third stage | 322 | 356 | 478 | 45.1 | |
| Present Study | [51] | [52] | [53] | [4] | [54] | [55] | [56] | [57] | |
|---|---|---|---|---|---|---|---|---|---|
| Volatile matter (wt.%, dry basis) | 88.48 | 87.06 | 83.0 | 85.61 | 88.7 | 82.1 | 85.43 | 79.35 | n.a. |
| Ash (wt.%, dry basis) | 2.1 | n.a. | 4.0 | 2.44 | 2.0 | 1.6 | 1.68 | 3.66 | 6.8 |
| Fixed Carbon (wt.%, dry basis) | 9.41 | 12.94 | 13.0 | 11.95 | 9.3 | 16.3 | 12.89 | 17.88 | n.a. |
| Higher heating value (MJ. kg−1, dry basis) | 15.96 | 18.6 | 18.9 | 18.99 | 18.7 | 19.19 | 19.14 | 19.41 | 18.85 |
| Element | Present Study | [51] | [52] | [53] | [4] | [54] | [55] | [56] | [57] |
|---|---|---|---|---|---|---|---|---|---|
| C | 41.45 | 47.0 | 46.3 | 48.65 | 42.9 | 48.81 | 49.0 | 48.4 | 46.7 |
| H | 5.51 | 5.9 | 6.4 | 5.87 | 5.9 | 6 | 5.87 | 6.01 | 6.2 |
| O * | 50.37 | 45.81 | 43.0 | 42.82 | 49.0 | 43.1 | 43.27 | 41.61 | 39.8 |
| N | 0.51 | 0.33 | n.a. | 0.16 | 0.20 | 0.46 | 0.1 | 0.17 | 0.2 |
| S | 0.054 | 0.05 | 0.1 | 0.04 | n.a. | 0.1 | 0.06 | 0.02 | 0.02 |
| Cl | n.a. | n.a. | n.a. | 0.03 | n.a. | <0.01 | 0.02 | n.a. | 0.06 |
| Torrefaction Time (min) | |||
|---|---|---|---|
| Property | Raw Bagasse | 30 | 45 |
| Volatiles (%) | 88.48 | 85.67 | 85.71 |
| Ash (%) | 2.11 | 5.97 | 5.48 |
| Fixed Carbon (%) | 9.41 | 8.36 | 8.81 |
| C (%) | 41.45 | 47.07 | 46.90 |
| H (%) | 5.514 | 6.02 | 6.02 |
| O (%) * | 50.37 | 40.62 | 41.21 |
| N (%) | 0.509 | 0.28 | 0.33 |
| C/O | 0.823 | 1.159 | 1.138 |
| H/C | 0.133 | 0.128 | 0.128 |
| Increase C/O ratio (%) | - | 40.82 | 38.29 |
| Decrease H/C ratio (%) | - | 3.75 | 3.75 |
| Higher heating value (HHV) (MJ/kg product) | 15.96 | 19.19 | 19.08 |
| Mass yield DAF (%) | - | 82.21 | 81.03 |
| Energy yield DAF (%) | - | 98.85 | 96.87 |
| Energy density (% Energy yield/% Mass yield) | - | 1.20 | 1.19 |
| Rise in HHV (%) | - | 20.24 | 19.55 |
| Torrefaction Time (min) | |||
|---|---|---|---|
| Property | Raw Bagasse | 30 | 45 |
| Volatiles (%) | 88.48 | 81.32 | 79.47 |
| Ash (%) | 2.11 | 6.34 | 5.32 |
| Fixed Carbon (%) | 9.41 | 12.34 | 15.21 |
| C (%) | 41.45 | 48.20 | 49.53 |
| H (%) | 5.51 | 6.08 | 5.94 |
| O (%) * | 50.37 | 39.04 | 39.16 |
| N (%) | 0.51 | 0.29 | 0.01 |
| C/O | 0.82 | 1.24 | 1.27 |
| H/C | 0.133 | 0.126 | 0.120 |
| Increase C/O ratio (%) | - | 50.05 | 53.71 |
| Decrease H/C ratio (%) | - | 5.26 | 9.77 |
| Higher heating value (HHV) (MJ/kg product) | 15.96 | 19.81 | 20.12 |
| Mass yield DAF (%) | - | 71.01 | 72.60 |
| Energy yield DAF (%) | - | 88.10 | 91.41 |
| Energy density (% Energy yield/% Mass yield) | - | 1.24 | 1.26 |
| Rise in HHV (%) | - | 24.11 | 26.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedroso, D.T.; Machin, E.B.; Cabrera-Barjas, G.; Flores, M.; Urra, H.G.; de Carvalho, F.S.; Santos, M.I.S.d.; Machín, A.B.; Canettieri, E.V.; Pérez, N.P.; et al. Sugarcane Bagasse Torrefaction for Fluidized Bed Gasification. Appl. Sci. 2021, 11, 6105. https://doi.org/10.3390/app11136105
Pedroso DT, Machin EB, Cabrera-Barjas G, Flores M, Urra HG, de Carvalho FS, Santos MISd, Machín AB, Canettieri EV, Pérez NP, et al. Sugarcane Bagasse Torrefaction for Fluidized Bed Gasification. Applied Sciences. 2021; 11(13):6105. https://doi.org/10.3390/app11136105
Chicago/Turabian StylePedroso, Daniel Travieso, Einara Blanco Machin, Gustavo Cabrera-Barjas, Mauricio Flores, Héctor Grandón Urra, Felipe Solferini de Carvalho, Maria Isabel Silva dos Santos, Adrian Blanco Machín, Eliana Vieira Canettieri, Néstor Proenza Pérez, and et al. 2021. "Sugarcane Bagasse Torrefaction for Fluidized Bed Gasification" Applied Sciences 11, no. 13: 6105. https://doi.org/10.3390/app11136105
APA StylePedroso, D. T., Machin, E. B., Cabrera-Barjas, G., Flores, M., Urra, H. G., de Carvalho, F. S., Santos, M. I. S. d., Machín, A. B., Canettieri, E. V., Pérez, N. P., Teixeira Lacava, P., Ribeiro dos Santos, L., & Andrade de Carvalho Júnior, J. (2021). Sugarcane Bagasse Torrefaction for Fluidized Bed Gasification. Applied Sciences, 11(13), 6105. https://doi.org/10.3390/app11136105








