Abstract
Cemented implant fixation in total joint arthroplasty has been proven to be safe and reliable with good long-term results. However, aseptic loosening is one of the main reasons for revision, potentially caused by poor cementation with low penetration depth in the cancellous bone. Aim of this prospective laboratory study was, to compare impact pressure and cleaning effects of pulsatile saline lavage to novel carbon dioxide lavage in a standardized carbon foam setup, to determine whether or not additional use of carbon dioxide lavage has any impact on cleaning volume or cleaning depth in cancellous bone. Carbon specimens simulating human cancellous bone were filled with industrial grease and then underwent a standardized cleaning procedure. Specimens underwent computed tomography pre- and post-cleaning. Regarding the impact pressure, isolated carbon dioxide lavage showed significant lower pressure compared to pulsatile saline lavage. Even though the combination of carbon dioxide lavage and pulsatile saline lavage had a positive cleaning effect compared to the isolated use of pulsatile saline lavage or carbon dioxide lavage, this was not significant in terms of cleaning volume or cleaning depth.
1. Introduction
Cemented implant fixation in total joint arthroplasty has demonstrated to be a reliable and safe procedure with excellent long-term results [1,2,3,4]. However, there are many regional differences if cemented or non-cemented fixation is performed. Regarding cemented fixation in total hip arthroplasty, those differences vary from 76% (Sweden) to 29% (Denmark) [5]. National registries demonstrate slight differences in the technique for total knee arthroplasty as well. In Germany, the Netherlands and New Zealand, 95% and more of total knee arthroplasties are performed using the cemented technique [6,7,8], whereas in Sweden it was only 92% in 2019 [9].
Despite regional differences in the choice of technique, the procedure for cemented arthroplasty is standardized. In total knee arthroplasty this includes to use high-pressure pulsatile saline lavage irrigation, drilling holes into the tibia, drying the bone and applying vacuum-mixed-cement to both, implant and bone [10]. In total hip arthroplasty third generation cementing technique is performed. This includes preparing bone bed properly by aggressive rasping, using high-pressure pulsatile saline lavage irrigation, using a distal cement restrictor, applying vacuum-mixed-cement in retrograde technique into the femur via cement gun, pressurizing the cement and inserting the stem with a distal centralizer [11]. Despite improved technique of cementing, aseptic loosening is still the main reason for revision after cemented total knee and hip arthroplasty [6,12,13].
Implant stability can be increased by adequate cleaning of the bone bed prior to cement application. In addition, pulsatile lavage can have a protective effect on implant stability in case of reduced bone density [14]. This preparation requires removal of bone marrow containing residual bone material, blood and fat. Even the presence of blood between bone and cement can reduce integrity of bone-cement interface [15]. Bone cement interface and penetration depth of cement are directly related to prior cleaning. For stable anchorage, desired cement thickness is described as 2–5 mm [16,17]. With this cement penetration depth, implant failure rates are significantly lower compared to cement thickness below 2 mm [18]. In addition, harmful thermal effects of polymerizing cement are not observed, which are described for a cement thickness of more than 5 mm [19,20].
A reliable method for this crucial cleaning procedure of cancellous bone is using saline high-pressure pulsatile lavage and subsequent drying of bone bed with abdominal cloth and suction. However, after this procedure liquid and fatty material often remains and may prevent cement from penetrating in the desired depth into the cancellous bone. Many studies have shown, that cleaning cancellous bone using pulsatile lavage is more efficient than manual rinse cleaning by bladder syringe alone [21,22,23,24,25]. For the application of pulsatile lavage, different rinsing pressures are recommended in the literature depending on the area of application or tissue type, since pulsatile lavage is used to clean contaminated wounds as well. When comparing pressures of these procedures, it is important to distinguish whether the pressure specifications are “output pressure” or “impact pressure”. The US Department of Health and Human Services recommends an impact pressure between 0.03 N/mm² (4 PSI) and 0.10 N/mm² (15 PSI) for cleaning wounds [26]. This recommendation is based on a number of studies that have shown a pressure of less than 0.03 N/mm² being inefficient in removing surface pathogens. Also, pressure of more than 0.10 N/mm² can potentially facilitate bacterial contamination in deeper tissue layers or cause traumatic soft tissue damage. Regarding the topic of this study, much higher pressures for cleaning cancellous bone have been reported in the literature. They reach from 0.48 N/mm² (70 PSI) to 0.59 N/mm² (85 PSI) [27,28].
A novel type of high-pressure carbon dioxide lavage, which is recommended for usage after standard saline lavage, is designed to advance cleaning and simultaneous drying of the bone [29]. A cadaveric study demonstrated that a combination of pressurized carbon dioxide and common pulsatile saline lavage produced a significantly deeper bone cement penetration than saline lavage alone [30]. Another study from 2019 showed that by using sterile compressed CO2 in addition to the established pulsatile lavage, a significantly deeper cement penetration in three of seven defined zones (Knee Society Radiographic Evaluation System) could be achieved in 303 total knee endoprosthesis [31]. In addition bone cement interface was stronger in an experimental study using human radii [32]. However, cases of embolic events using carbon dioxide lavage in intramedullary cleaning during cemented hip arthroplasty have been reported [33,34].
Aim of this prospective laboratory study was to compare impact pressure and cleaning effects of pulsatile saline lavage to novel carbon dioxide lavage in a standardized carbon foam setup and to determine whether or not additional use of carbon dioxide lavage has any impact on cleaning volume or cleaning depth in cancellous bone.
2. Materials and Methods
First, we investigated impact pressure to the surface of two different devices. This was followed by measurements of cleaning volume and cleaning depth of the two devices and their combination in a standardized laboratory setting.
2.1. Devices and Investigated Carbon Specimens
2.1.1. Lavage and Cleaning Systems
In an in vitro study, we investigated the bone cleaning effect of carbon dioxide lavage compared to pulsatile lavage and in addition to pulsatile bone cleaning. We used the InterPulse (Stryker, Kalamazoo, MI, USA) for conventional bone cleaning with saline solution and the CarboJet (Kinamed, Camarillo, CA, USA) for carbon dioxide cleaning (Figure 1). The bone cleaning tip (REF 0210-010-00) for the InterPulse and the supplied Wide-Angle-Knee-Nozzle of the CarboJet were used for the experiments (Figure 1). To investigate the impaction pressure, the cleaning systems were divided into the InterPulse and CarboJet group. For the analysis of the cleaning effect, the two systems were investigated separately and in combination. This resulted in the groups InterPulse (A), CarboJet (B) and InterPulse + CarboJet (C).

Figure 1.
(left) Handpiece of InterPulse by Stryker. (right) Handpiece of CO2 lavage CarboJet by KINAMED.
2.1.2. Determination Impact Pressure
The impact pressure of both investigated lavage systems was determined under standardized condition. Therefore, we used a custom-made setup with mounting platform for the lavage systems and a target with integrated force measuring plate (Figure 2). Each lavage system was firmly fixed on the movable mounting platform. This was necessary to place the tip with splash shield for the InterPulse and the nozzle for the CarboJet at a defined distance of 2 mm in front of the force measuring plate (Figure 2). A distance of 2 mm was maintained to avoid interference between the force plate and the tip due to the flushing liquid or carbon dioxide. The flushing medium was applied centrally onto the force plate. The distance of the tip of the saline lavage device to the force measuring plate was specified by the splash shield (Figure 1, left). For the InterPulse this was 17 mm (15 + 2 mm) and in case of the CarboJet, the distance was 2 mm because there was no splash shield.

Figure 2.
Force measuring plate with 2 mm distance to the CO2 nozzle.
The impact pressure was calculated by dividing the force and the area of the nozzle orifice (Figure 3). The nozzle openings of the two lavage systems investigated were measured using a calibrated digital microscope (Digital Microscope VHX-500 by Keyence, Osaka, Japan). Both lavage systems were tested four times within the first minute of powering up. The force values were recorded at a sampling rate of 1000 Hz and the mean values were calculated.
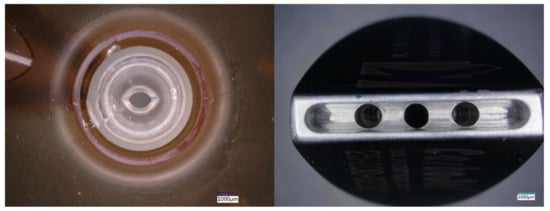
Figure 3.
Top view of: (left) Stryker- InterPuls bone cleaning tip, (right) KINAMED—CarboJet Wide-Angle Knee—Nozzle.
2.1.3. Determination of the Cleaning Effect
The two lavage systems were divided into group InterPulse (A), CarboJet (B) and InterPulse + CarboJet (C) as described. A sample size of 10 specimens per group (30 in total) were used. In case of the InterPulse a saline irrigation volume of one liter per specimen was used. The cleaning distance was determined by the splash shield. Following the manufacturer’s specifications, a cleaning duration of 30 s was used for the CarboJet.
The determination of the cleaning effect of the investigated lavage systems were performed using validated and standardized carbon foam specimens (RVC foam; ERG Materials and Aerospace, Oakland, CA, USA) as substitutes for human cancellous bone [35]. The carbon specimens showed a porosity of 30 PPI (pores per inch), which corresponds to 1.2 PPM (pores per millimeter). During the manufacturing process, the specimens were compressed twice, resulting in similar trabecular bone structure (Figure 4, right). The carbon foam specimens were filled with standardized technical fat (Bechem Rhus FA 37; Carl Bechem GmbH, Hagen, Germany), to simulate human bone marrow [36]. One ingredient of the fat was an aluminum-complex soap which we used as contrast medium for the radiological analysis. The fat-filled carbon specimens were coated with a shrink-on tube simulating the cortex (Figure 4, left).

Figure 4.
(Left) Fat filled carbon specimen with shrink tube, (right) close up of Carbon foam.
The evaluation of cleaning effects was determined by using computer tomography scans (SOMATOM Emotion, Siemens Healthcare GmbH, Erlangen, Germany). For this purpose, the fat-filled specimens were scanned before and after cleaning with a slice thickness of 0.75 mm. For volume determination, a reference volume was recorded for each scan. The segmentation and calculation of the cleaning volume was performed with the software itk-Snap [37]. The mean flushing depth was calculated using the volume and the geometric parameters. Exclusion criteria were air inclusions or if specimens would not have been fully filled with fat. One specimen in Group A had to be excluded due to air inclusions that would have biased the cleaning effect. Therefore, only 9 out of 10 samples could be analyzed for Group A. Beyond that, no other specimens had to be excluded.
2.2. Statistics
Using SPSS V.25 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp., Armonk, NY, USA) a descriptively data analysis was performed. A Shapiro- Wilk test was used to test for normal distribution. A t-test was done to detect significances in impact pressure. Analysis of variance (ANOVA) was used to determine if there are significant differences regarding cleaning effects. p < 0.05 was set to be the significance level.
3. Results
3.1. Impact Pressure
The t-test revealed a statistically significant difference for the impact pressure between the InterPulse 0.76 ± 0.02 N/mm² and CarboJet 0.12 ± 0.01 N/mm² group, t(3.4) = 59.0, p < 0.001, (95% CI [0.61–0.68]). Whereas this was a mean difference increase of 0.64 N/mm² for the InterPulse system (Figure 5).
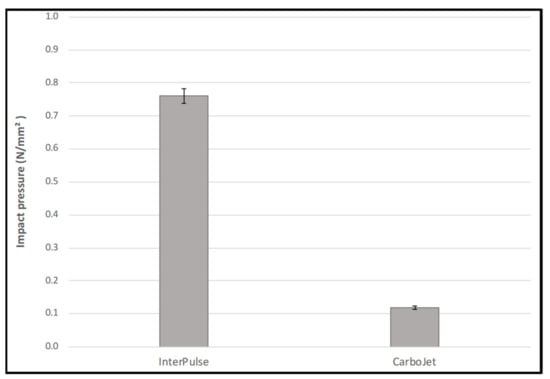
Figure 5.
Impact pressure in N/mm².
3.2. Cleaning Effect
The ANOVA-test comparing cleaning volumes showed no statistically significant difference between the InterPulse, CarboJet and the combination of both, F(2,26) = 1.14, p = 0.334 (Table 1, Figure 6). Cleaning depth showed also no significant differences between the investigated groups, F(2,26) = 1.07, p = 0.259 (Table 1, Figure 7).

Table 1.
Mean cleaning volume and cleaning depth in carbon foam specimens.
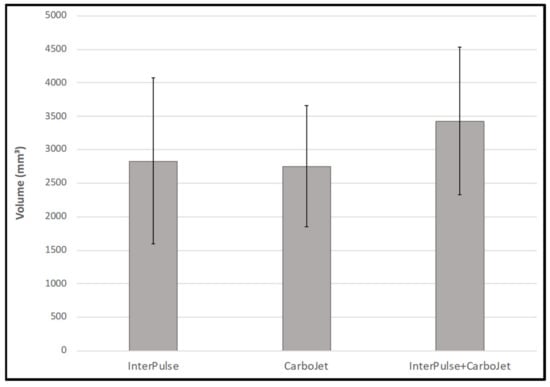
Figure 6.
Volume of removed grease in standardized carbon foam specimens.
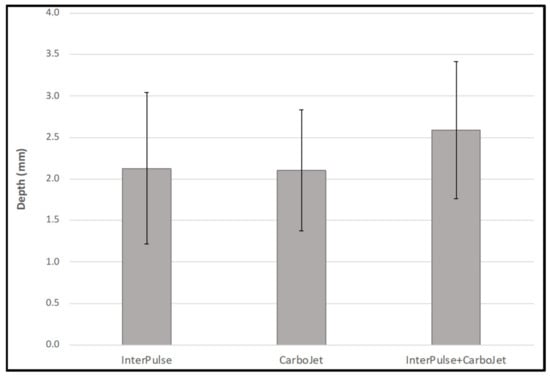
Figure 7.
Cleaning depth in mm in standardized carbon foam specimens.
4. Discussion
The use of pulsatile lavage is known to enhance the fixation of cemented total knee arthroplasty [38]. Highly significant improvements of cement penetration into cancellous bone can be demonstrated in vitro comparing manual rinsing and pulsed saline lavage [25]. In addition, cleaning of cancellous bone leads to improved cement penetration and therefore to a stronger bone cement interface, which reduces the probability of aseptic loosening [18,39]. However, aseptic loosening is the main reason for revision in cemented total knee and hip arthroplasty [12,13]. Modern cementing technique requires pulsed saline lavage, drying bone by abdominal cloth and suction, drilling holes in the tibia and applying vacuum-mixed-cement either on both, metal and bone in total knee arthroplasty, or filling the femur in retrograde technique using a cement gun in total hip arthroplasty. Considering this, many factors besides the cancellous bone cleaning result can affect cementation. Bone density or sclerotic bone also can cause reasonable differences [40]. Cement storage temperature, humidity, cement viscosity or timing of cement application are other aspects that have to be considered [41,42].
In our experimental in vitro study, we compared a standard high-pressure pulsatile saline lavage to a novel lavage system using compressed carbon dioxide to enhance cleaning efficiency of cancellous bone under standardized conditions. Cancellous bone was simulated by standardized fat-filled carbon foam. In this experimental setup, impact pressure was applied to a force-measuring-plate. We detected significantly higher pressures in pulsatile saline lavage (InterPulse) than in pressurized carbon dioxide lavage (CarboJet). Although we showed higher cleaning volume and deeper penetration in carbon foam, when using carbon dioxide lavage additionally to high-pressure pulsatile saline lavage (Group C) these differences were not significant. Similar results were obtained for the single use of carbon dioxide lavage (Group B) in comparison to the single use of pulsatile saline lavage (Group A) (Figure 6 and Figure 7).
So far, there is only limited data available about carbon dioxide lavage. A cadaveric study in 10 knees in 2016 demonstrated that a combination of pressurized carbon dioxide and common pulsatile saline lavage produced a significantly deeper bone cement penetration than saline lavage alone [30]. In addition, another study demonstrated that bone cement interface was stronger [32]. In 2019, Gapinski et al. revealed that using sterile compressed carbon dioxide in addition to the established pulsatile jet lavage, resulted in a deeper cement penetration in six of seven defined zones (Knee Society Radiographic Evaluation System) in 303 total knee endoprostheses. This difference was significant in three out of seven zones [31].
In this standardized laboratory setup, which was established in earlier studies [35,36], no significant difference between the three experimental groups was detected although higher cleaning volumes and cleaning depth using additional carbon dioxide lavage (Group C) was discovered. In this study the aspect of preparing bone bed prior to cementation was investigated. Other aspects such as pressure during cementing, viscosity of cement, remaining blood or fat in the interface or the timing of cement-application were excluded from the investigations. With this test setup, the ability of carbon dioxide lavage to clean cancellous bone was examined closely. Whether or not the slightly enhanced cleaning ability will result in deeper cement penetration remains uncertain. The use of additional carbon dioxide lavage needs extra devices, sterilization and carbon dioxide gas cylinders, which need adequate storage and the exact following of safety instructions. Moreover, the additional time needed for the procedure extends duration of operation and time of anesthesia. Since we saw similar results for single use carbon dioxide lavage (Group B) compared to single use of pulsatile lavage (Group A) it has to be considered, that using carbon dioxide lavage only, might be faster, less wet and more controlled to saline lavage. Studies showed that using high-pressure lavage to clean contaminated wounds can cause irreversible insult to tissue, resulting in myonecrosis and dystrophic calcification [43,44]. Even bacteria can be propagated into soft tissue or significant damage to the architecture of the bone can occur [45,46]. Therefore, lower impact pressure as shown in our investigation can hold advantages. Despite these potential advantages case reports are published showing gas embolisms occurred during femoral intramedullary usage of carbon dioxide lavage [33,34]. Further investigation focusing on in vivo impact can help revealing, if a specific range of pressure orchestrates all benefits while avoiding complications.
In this experimental in vitro study where we compared high-pressure saline lavage to carbon dioxide lavage and its combination, we detected significant lower impact pressure and a deeper cleaning effect with carbon dioxide lavage used additionally to high-pressure pulsatile saline lavage in standardized carbon foam specimens representing cancellous bone. However, these effects were statistically not significant. We found no evidence that using carbon dioxide lavage reduces the effect of saline lavage. Using carbon dioxide lavage only had similar outcomes compared to high-pressure pulsatile lavage. Since aseptic loosening still is the main reason for implant failure due to poor cementing, better cleaning of cancellous bone prior to cementing will have a positive effect on implant durability. In conclusion, using a combination of high-pressure saline lavage followed by carbon dioxide lavage showed a clear tendency of resulting in improved cleaning effects prior to cementing and therefore seems to have a positive effect on stability of endoprosthesis. Further investigation in human bone and larger clinical trials will determine, if using additional carbon dioxide lavage will confirm its positive effect on bone cement interface and therefore on success of endoprosthesis implantation itself.
Author Contributions
Conceptualization, K.K. and S.J.; Data curation, K.K., M.S., C.S. and S.J.; Formal analysis, K.K., S.J. and C.S.; Methodology, K.K. and S.J.; Project administration, K.K., S.J. and M.I.; Writing—original draft, K.K. and S.J.; Writing—review & editing, K.K., C.S., M.I., M.S., T.G., T.R. and S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no pertinent conflict of interest. S.J. reports grants from B Braun Aesculap, Johnson & Johnson Depuy Synthes, Heraeus Medical, Waldemar Link, Peter Brehm and Zimmer Biomet that are not related to the current study. T.G. reports personal fees paid to T.G. during the conduct of the study from Zimmer Biomet, Europe and from Depuy Synthes Orthopädie GmbH, Peter Brehm GmbH, ImplanTec GmbH outside the submitted work. Research grants paid to the institution during the conduct of the study from Zimmer Biomet, Europe, Mathys AG Switzerland, Anika Therapeutics outside the submitted work that are not related to the current study. TR reports research funding/travel expenses and/or paid speaking engagements by the German Federal Ministry of Education and Research (BMBF), The German Federal Ministry for Economic Cooperation and Development (BMZ), Otto Bock Foundation, Stiftung Oskar-Helene-Heim Berlin, DePuy Int, Zimmer, Aesculap/B. Braun, the Vielberth Foundation, German Society of Orthopaedics and Traumatology (DGOU) and the German Association of Orthopaedics and Orthopaedic Surgery (DGOOC). TR is associate editor of “Der Orthopäde” and “Der Unfallchirurg” (Springer Heidelberg, Berlin, New York) and member of the International Advisory Board of the Journal of the American Academy of Orthopaedic Surgeons (AAOS).
References
- Dalury, D.F. Cementless total knee arthroplasty: Current concepts review. Bone Jt. J. 2016, 98, 867–873. [Google Scholar] [CrossRef] [Green Version]
- Ritter, M.A.; Keating, E.M.; Sueyoshi, T.; Davis, K.E.; Barrington, J.W.; Emerson, R.H. Twenty-Five-Years and Greater, Results After Nonmodular Cemented Total Knee Arthroplasty. J. Arthroplast. 2016, 31, 2199–2202. [Google Scholar] [CrossRef]
- Herberts, P.; Malchau, H. Long-term registration has improved the quality of hip replacement: A review of the Swedish THR Register comparing 160,000 cases. Acta Orthop. Scand. 2000, 71, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Malchau, H.; Herberts, P.; Eisler, T.; Garellick, G.; Soderman, P. The Swedish Total Hip Replacement Register. J. Bone Jt. Surg. Am. 2002, 84 (Suppl. 2), 2–20. [Google Scholar] [CrossRef] [PubMed]
- Bunyoz, K.I.; Malchau, E.; Malchau, H.; Troelsen, A. Has the Use of Fixation Techniques in THA Changed in This Decade? The Uncemented Paradox Revisited. Clin. Orthop. Relat. Res. 2020, 478, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A.; Jansson, V.; Melsheimer, O.; Steinbrück, A. German Arthroplasty Registry (EPRD)—2019 Annual Report. 2019. Available online: https://www.eprd.de/fileadmin/user_upload/Dateien/Publikationen/Berichte/EPRD_Jahresbericht_2019_EN_doppelseitig_F_Web-Stand-251120.pdf (accessed on 30 June 2021).
- The New Zealand Joint Registry—Fifteen Year Report January 1999 to December 2013; NZOA: Christchurch, New Zealand, 2014; Volume 15, Available online: https://nzoa.org.nz/sites/default/files/NZJR2014Report.pdf (accessed on 30 June 2021).
- Gademan, M.G.J.; van Steenbergen, L.N.; Cannegieter, S.C.; Nelissen, R.; Marang-Van De Mheen, P.J. Population-based 10-year cumulative revision risks after hip and knee arthroplasty for osteoarthritis to inform patients in clinical practice: A competing risk analysis from the Dutch Arthroplasty Register. Acta Orthop. 2021, 92, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Robertsson, O. (Ed.) The Swedish Knee Arthroplasty Register—Anual Report 2020; Skånes University Hospital: Lund, Sweden, 2020. [Google Scholar]
- Refsum, A.M.; Nguyen, U.V.; Gjertsen, J.E.; Espehaug, B.; Fenstad, A.M.; Lein, R.K.; Ellison, P.; Høl, P.J.; Furnes, O. Cementing technique for primary knee arthroplasty: A scoping review. Acta Orthop. 2019, 90, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Niculescu, M.; Solomon, B.L.; Viscopoleanu, G.; Antoniac, I.V.; Viscopoleanu, G.; Antoniac, I.V. Evolution of Cementation Techniques and Bone Cements in Hip Arthroplasty. In Handbook of Bioceramics and Biocomposites; Springer International Publishing: Cham, Switzerland, 2016; pp. 859–899. [Google Scholar]
- Khan, M.; Osman, K.; Green, G.; Haddad, F.S. The epidemiology of failure in total knee arthroplasty: Avoiding your next revision. Bone Jt. J. 2016, 98 (Suppl. 1), 105–112. [Google Scholar] [CrossRef] [Green Version]
- Kochbati, R.; Rbai, H.; Jlailia, M.; Makhlouf, H.; Bouguira, A.; Daghfous, M.S. Predictive factors of aseptic loosening of cemented total hip prostheses. Pan. Afr. Med. J. 2016, 24, 260. [Google Scholar]
- Jaeger, S.; Rieger, J.S.; Bruckner, T.; Kretzer, J.P.; Clarius, M.; Bitsch, R.G. The protective effect of pulsed lavage against implant subsidence and micromotion for cemented tibial unicompartmental knee components: An experimental cadaver study. J. Arthroplast. 2014, 29, 727–732. [Google Scholar] [CrossRef]
- Benjamin, J.B.; Gie, G.A.; Lee, A.J.; Ling, R.S.; Volz, R.G. Cementing technique and the effects of bleeding. J. Bone Jt. Surg. Br. 1987, 69, 620–624. [Google Scholar] [CrossRef]
- Wetzels, T.; van Erp, J.; Brouwer, R.W.; Bulstra, S.K.; van Raay, J. Comparing Cementing Techniques in Total Knee Arthroplasty: An In Vitro Study. J. Knee Surg. 2019, 32, 886–890. [Google Scholar] [CrossRef]
- Huiskes, R. Some fundamental aspects of human joint replacement. Analyses of stresses and heat conduction in bone-prosthesis structures. Acta Orthop. Scand. Suppl. 1980, 185, 1–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampton, C.B.; Berliner, Z.P.; Nguyen, J.T.; Mendez, L.; Smith, S.S.; Joseph, A.D.; Padgett, D.E.; Rodriguez, J.A. Aseptic Loosening at the Tibia in Total Knee Arthroplasty: A Function of Cement Mantle Quality? J. Arthroplast. 2020, 35, S190–S196. [Google Scholar] [CrossRef] [PubMed]
- Cawley, D.T.; Kelly, N.; McGarry, J.P.; Shannon, F.J. Cementing techniques for the tibial component in primary total knee replacement. Bone Jt. J. 2013, 95, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Sih, G.C.; Connelly, G.M.; Berman, A.T. The effect of thickness and pressure on the curing of PMMA bone cement for the total hip joint replacement. J. Biomech. 1980, 13, 347–352. [Google Scholar] [CrossRef]
- Clarius, M.; Hauck, C.; Seeger, J.B.; James, A.; Murray, D.W.; Aldinger, P.R. Pulsed lavage reduces the incidence of radiolucent lines under the tibial tray of Oxford unicompartmental knee arthroplasty: Pulsed lavage versus syringe lavage. Int. Orthop. 2009, 33, 1585–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breusch, S.J.; Norman, T.L.; Schneider, U.; Reitzel, T.; Blaha, J.D.; Lukoschek, M. Lavage technique in total hip arthroplasty: Jet lavage produces better cement penetration than syringe lavage in the proximal femur. J. Arthroplast. 2000, 15, 921–927. [Google Scholar] [CrossRef]
- Seeger, J.B.; Jaeger, S.; Bitsch, R.G.; Mohr, G.; Rohner, E.; Clarius, M. The effect of bone lavage on femoral cement penetration and interface temperature during Oxford unicompartmental knee arthroplasty with cement. J. Bone Jt. Surg. Am. 2013, 95, 48–53. [Google Scholar] [CrossRef]
- Helwig, P.; Konstantinidis, L.; Hirschmuller, A.; Miltenberger, V.; Kuminack, K.; Sudkamp, N.P.; Hauschild, O. Tibial cleaning method for cemented total knee arthroplasty: An experimental study. Indian J. Orthop. 2013, 47, 18–22. [Google Scholar] [CrossRef]
- Kalteis, T.; Pforringer, D.; Herold, T.; Handel, M.; Renkawitz, T.; Plitz, W. An experimental comparison of different devices for pulsatile high-pressure lavage and their relevance to cement intrusion into cancellous bone. Arch. Orthop. Trauma Surg. 2007, 127, 873–877. [Google Scholar] [CrossRef]
- Granick, M.S.; Tenenhaus, M.; Knox, K.R.; Ulm, J.P. Comparison of wound irrigation and tangential hydrodissection in bacterial clearance of contaminated wounds: Results of a randomized, controlled clinical study. Ostomy Wound Manag. 2007, 53, 64–66, 68–70, 72. [Google Scholar]
- Sobel, J.W.; Goldberg, V.M. Pulsatile irrigation in orthopedics. Orthopedics 1985, 8, 1019–1022. [Google Scholar] [CrossRef]
- Morgan, J.; Holder, G.; Desoutter, G. The measurement and comparison of jet characteristics of surgical pulse lavage devices. J. Arthroplast. 2003, 18, 45–50. [Google Scholar] [CrossRef]
- Goldstein, W.M.; Gordon, A.; Goldstein, J.M.; Berland, K.; Branson, J.; Sarin, V.K. Improfement of Cement mantle thickness with pressurized carbon dioxide lavage. In Proceedings of the 20th Annual Meeting of the International Society for Technology in Arthroplasty 2007, Paris, France, 28 July 2007. [Google Scholar]
- Boontanapibul, K.; Ruangsomboon, P.; Charoencholvanich, K.; Pornrattanamaneewong, C. Effectiveness Testing of Combined Innovative Pressurized Carbon Dioxide Lavage and Pulsatile Normal Saline Irrigation to Enhance Bone Cement Penetration in Total Knee Replacement: A Cadaveric Study. J. Med. Assoc. Thai. 2016, 99, 1198–1202. [Google Scholar]
- Gapinski, Z.A.; Yee, E.J.; Kraus, K.R.; Deckard, E.R.; Meneghini, R.M. The Effect of Tourniquet Use and Sterile Carbon Dioxide Gas Bone Preparation on Cement Penetration in Primary Total Knee Arthroplasty. J. Arthroplast. 2019, 34, 1634–1639. [Google Scholar] [CrossRef]
- Ravenscroft, M.J.; Charalambous, C.P.; Mills, S.P.; Woodruff, M.J.; Stanley, J.K. Bone-cement interface strength in distal radii using two medullary canal preparation techniques: Carbon dioxide jet cleaning versus syringed saline. Hand Surg. 2010, 15, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Lax-Prez, R.; Ferrero-Manzanal, F.; Marin-Penia, O.; Murcia-Asensio, A. Gas embolism after the use of the CarboJet lavage system during hip hemiarthroplasty. Clinical case and literature review. Acta Ortop. Mex. 2014, 28, 45–48. [Google Scholar] [PubMed]
- Garcia Martinez, M.R.; Ruiz, G.M.V.; Perez, J.M.R.; Perez, R.L. Air embolism after cleaning the intramedullary canal with a high pressure CO2 wash in hip fracture. Rev. Esp. Anestesiol. Reanim 2013, 60, 353–354. [Google Scholar] [PubMed]
- Jaeger, S.; Rieger, J.S.; Obermeyer, B.; Klotz, M.C.; Kretzer, J.P.; Bitsch, R.G. Cement applicator use for hip resurfacing arthroplasty. Med. Eng. Phys. 2015, 37, 447–452. [Google Scholar] [CrossRef]
- Bitsch, R.G.; Loidolt, T.; Heisel, C.; Schmalzried, T.P. Cementing techniques for hip resurfacing arthroplasty: Development of a laboratory model. J. Bone Jt. Surg. Am. 2008, 90 (Suppl. 3), 102–110. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, U.J.; Siewe, J.; Delank, K.S.; Eysel, P.; Puschel, K.; Morlock, M.M.; de Uhlenbrock, A.B. Pulsed lavage improves fixation strength of cemented tibial components. Int. Orthop. 2011, 35, 1165–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.A.; Terbush, M.J.; Goodheart, J.R.; Izant, T.H.; Mann, K.A. Increased initial cement-bone interlock correlates with reduced total knee arthroplasty micro-motion following in vivo service. J. Biomech. 2014, 47, 2460–2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitsch, R.G.; Jager, S.; Lurssen, M.; Loidolt, T.; Schmalzried, T.P.; Clarius, M. Influence of bone density on the cement fixation of femoral hip resurfacing components. J. Orthop. Res. 2010, 28, 986–991. [Google Scholar] [CrossRef]
- Buller, L.T.; Rao, V.; Chiu, Y.F.; Nam, D.; McLawhorn, A.S. Primary Total Knee Arthroplasty Performed Using High-Viscosity Cement is Associated With Higher Odds of Revision for Aseptic Loosening. J. Arthroplast. 2020, 35 (Suppl. 6), S182–S189. [Google Scholar] [CrossRef] [PubMed]
- Koh, B.T.; Tan, J.H.; Ramruttun, A.K.; Wang, W. Effect of storage temperature and equilibration time on polymethyl methacrylate (PMMA) bone cement polymerization in joint replacement surgery. J. Orthop. Surg. Res. 2015, 10, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiaramonti, A.M.; Robertson, A.D.; Nguyen, T.P.; Jaffe, D.E.; Hanna, E.L.; Holmes, R.; Barfield, W.R.; Fourney, W.L.; Stains, J.P.; Pellegrini, V.D., Jr. Pulsatile Lavage of Musculoskeletal Wounds Causes Muscle Necrosis and Dystrophic Calcification in a Rat Model. J. Bone Jt. Surg. Am. 2017, 99, 1851–1858. [Google Scholar] [CrossRef]
- Boyd, J.I., 3rd; Wongworawat, M.D. High-pressure pulsatile lavage causes soft tissue damage. Clin. Orthop. Relat. Res. 2004, 427, 13–17. [Google Scholar] [CrossRef]
- Bhandari, M.; Adili, A.; Lachowski, R.J. High pressure pulsatile lavage of contaminated human tibiae: An in vitro study. J. Orthop. Trauma 1998, 12, 479–484. [Google Scholar] [CrossRef]
- Hassinger, S.M.; Harding, G.; Wongworawat, M.D. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop. Relat. Res. 2005, 439, 27–31. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).