Vascular and Cardiac Prognostic Determinants in Patients with Gynecological Cancers: A Six-Year Follow-up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Flow-Mediated Dilation Measurement
2.2. Carotid Intima-Media Thickness Measurement
2.3. Infrarenal Abdominal Aorta Diameter Measurement
2.4. Evaluation of Cardiac Function
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- AIOM AIRTUM. I Numeri del Cancro in Italia. 2014. Available online: www.registri-tumori.it/PDF/AIOM2014/I_numeri_del_cancro_2014.pdf (accessed on 16 January 2020).
- Sant, M.; Allemani, C.; Santaquilani, M.; Knijn, A.; Marchesi, F.; Capocaccia, R.; EUROCARE Working Group. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur. J. Cancer 2009, 45, 931–991. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Huang, H.; Feng, Y.L.; Wan, T.; Zhang, Y.N.; Cao, X.P.; Huang, Y.W.; Xiong, Y.; Huang, X.; Zheng, M.; Li, Y.F.; et al. Effectiveness of Sequential Chemoradiation vs Concurrent Chemoradiation or Radiation Alone in Adjuvant Treatment After Hysterectomy for Cervical Cancer: The STARS Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 361–369. [Google Scholar] [CrossRef]
- Wang, B.; Tan, Y.; Yang, X.; Man, X. Survival outcomes of neoadjuvant chemotherapy-related strategies compared with concurrent chemoradiotherapy for locally advanced cervical cancer: A meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 2021. [Google Scholar] [CrossRef]
- Bisch, S.P.; Jago, C.A.; Kalogera, E.; Ganshorn, H.; Meyer, L.A.; Ramirez, P.T.; Dowdy, S.C.; Nelson, G. Outcomes of enhanced recovery after surgery (ERAS) in gynecologic oncology—A systematic review and meta-analysis. Gynecol. Oncol. 2021, 161, 46–55. [Google Scholar] [CrossRef]
- Botteri, E.; Iodice, S.; Maisonneuve, P.; Alfieri, M.; Burzoni, N.; Manghi, L.; Martinetti, M.; Montanari, B.; Albertazzi, E.; Bazolli, B.; et al. Case mix at the European Institute of Oncology: First report of the Tumour Registry, 2000–2002. Ecancermedicalscience 2009, 3, 149. [Google Scholar] [CrossRef][Green Version]
- Brancaccio, M.; Pirozzi, F.; Hirsch, E.; Ghigo, A. Mechanisms underlying the cross-talk between heart and cancer. J. Physiol. 2020, 598, 3015–3027. [Google Scholar] [CrossRef]
- Moslehi, J.; Zhang, Q.; Moore, K.J. Crosstalk Between the Heart and Cancer: Beyond Drug Toxicity. Circulation 2020, 142, 684–687. [Google Scholar] [CrossRef]
- Meijers, W.C.; Maglione, M.; Bakker, S.J.L.; Oberhuber, R.; Kieneker, L.M.; de Jong, S.; Haubner, B.J.; Nagengast, W.B.; Lyon, A.R.; van der Vegt, B.; et al. Heart Failure Stimulates Tumor Growth by Circulating Factors. Circulation 2018, 138, 678–691. [Google Scholar] [CrossRef]
- Rhea, I.B.; Uppuluri, S.; Sawada, S.; Schneider, B.P.; Feigenbaum, H. Incremental prognostic value of echocardiographic strain and its association with mortality in cancer patients. J. Am. Soc. Echocardiogr. 2015, 28, 667–673. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Bilianou, E.; Balbarini, A.; Gesualdo, M.; Ghiadoni, L.; Metra, M.; Palmiero, P.; Pedrinelli, R.; Salvetti, M.; Scicchitano, P.; et al. Task force on: ‘Early markers of atherosclerosis: Influence of age and sex’. J. Cardiovasc. Med. 2013, 14, 757–766. [Google Scholar] [CrossRef]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1063–1093. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Favale, S.; Bhuva, A.; Scicchitano, P.; Caragnano, V.; Lavopa, C.; De Pergola, G.; Loverro, G. Antero-posterior diameter of the infrarenal abdominal aorta is higher in women with polycystic ovary syndrome. Vasc. Health Risk Manag. 2009, 5, 561–566. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Cohen, J. The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educ. Psychol. Meas. 1973, 33, 613–619. [Google Scholar] [CrossRef]
- Di Fiore, R.; Suleiman, S.; Ellul, B.; O’Toole, S.A.; Savona-Ventura, C.; Felix, A.; Napolioni, V.; Conlon, N.T.; Kahramanoglu, I.; Azzopardi, M.J.; et al. GYNOCARE Update: Modern Strategies to Improve Diagnosis and Treatment of Rare Gynecologic Tumors-Current Challenges and Future Directions. Cancers 2021, 13, 493. [Google Scholar] [CrossRef]
- Kim, E.K.; Cho, J.; Kim, J.Y.; Chang, S.A.; Park, S.J.; Choi, J.O.; Lee, S.C.; Ahn, J.S.; Park, S.W.; Im, Y.H.; et al. Early Decline in Left Ventricular Ejection Fraction Can Predict Trastuzumab-Related Cardiotoxicity in Patients with Breast Cancer: A Study Using 13 Years of Registry Data. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2019, 51, 727–736. [Google Scholar] [CrossRef]
- López-Sendón, J.; Álvarez-Ortega, C.; Zamora Auñon, P.; Buño Soto, A.; Lyon, A.R.; Farmakis, D.; Cardinale, D.; Canales Albendea, M.; Feliu Batlle, J.; Rodríguez Rodríguez, I.; et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: The CARDIOTOX registry. Eur. Heart J. 2020, 41, 1720–1729. [Google Scholar] [CrossRef]
- Arbuck, S.G.; Strauss, H.; Rowinsky, E.; Christian, M.; Suffness, M.; Adams, J.; Oakes, M.; McGuire, W.; Reed, E.; Gibbs, H.; et al. A reassessment of cardiac toxicity associated with Taxol. J. Natl. Cancer Inst. Monogr. 1993, 15, 117–130. [Google Scholar]
- Rowinsky, E.K.; McGuire, W.P.; Guarnieri, T.; Fisherman, J.S.; Christian, M.C.; Donehower, R.C. Cardiac disturbances during the administration of taxol. J. Clin. Oncol. 1991, 9, 1704–1712. [Google Scholar] [CrossRef]
- Liu, B.J.; Li, X.P.; Wang, J.L.; Wu, Y.; Wei, L.H. Analysis of cardiotoxicity of chemotherapy in 30 cases with gynecological cancer. Zhonghua Fu Chan Ke Za Zhi 2011, 46, 884–887. [Google Scholar]
- Cheng, C.F.; Juan, S.H.; Chen, J.J.; Chao, Y.C.; Chen, H.H.; Lian, W.S.; Lu, C.Y.; Chang, C.I.; Chiu, T.H.; Lin, H. Pravastatin attenuates carboplatin-induced cardiotoxicity via inhibition of oxidative stress associated apoptosis. Apoptosis 2008, 13, 883–894. [Google Scholar] [CrossRef]
- Altin, C.; Sade, L.E.; Demirtas, S.; Karacaglar, E.; Kanyilmaz, S.; Simsek, V.; Ayhan, A.; Muderrisoglu, H. Effects of Paclitaxel and Carboplatin combination on mechanical myocardial and microvascular functions: A transthoracic Doppler echocardiography and two-dimensional strain imaging study. Echocardiography 2015, 32, 238–247. [Google Scholar] [CrossRef]
- Abu-Khalaf, M.M.; Safonov, A.; Stratton, J.; Wang, S.; Hatzis, C.; Park, E.; Pusztai, L.; Gross, C.P.; Russell, R. Examining the cost-effectiveness of baseline left ventricular function assessment among breast cancer patients undergoing anthracycline-based therapy. Breast Cancer Res. Treat. 2019, 176, 261–270. [Google Scholar] [CrossRef]
- Peddi, P.; Master, S.R.; Dwary, A.D.; Ravipati, H.P.; Patel, A.H.; Pasam, A.; Katikaneni, P.K.; Shi, R.; Burton, G.V.; Chu, Q.D. Utility of routine pretreatment evaluation of left ventricular ejection fraction in breast cancer patients receiving anthracyclines. Breast J. 2019, 25, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Truong, S.R.; Barry, W.T.; Moslehi, J.J.; Baker, E.L.; Mayer, E.L.; Partridge, A.H. Evaluating the Utility of Baseline Cardiac Function Screening in Early-Stage Breast Cancer Treatment. Oncologist 2016, 21, 666–670. [Google Scholar] [CrossRef]
- O’Brien, P.; Matheson, K.; Jeyakumar, A.; Anderson, K.; Younis, T. The clinical utility of baseline cardiac assessments prior to adjuvant anthracycline chemotherapy in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2019, 174, 357–363. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Sugiyama, S.; Sumida, H.; Sugamura, K.; Nozaki, T.; Ohba, K.; Matsubara, J.; Kurokawa, H.; Fujisue, K.; Konishi, M.; et al. Peripheral endothelial function and cardiovascular events in high-risk patients. J. Am. Heart Assoc. 2013, 2, e000426. [Google Scholar] [CrossRef]
- Shechter, M.; Matetzky, S.; Arad, M.; Feinberg, M.S.; Freimark, D. Vascular endothelial function predicts mortality risk in patients with advanced ischaemic chronic heart failure. Eur. J. Heart Fail. 2009, 11, 588–593. [Google Scholar] [CrossRef]
- Kirchmair, R.; Walter, D.H.; Ii, M.; Rittig, K.; Tietz, A.B.; Murayama, T.; Emanueli, C.; Silver, M.; Wecker, A.; Amant, C.; et al. Antiangiogenesis mediates cisplatin-induced peripheral neuropathy: Attenuation or reversal by local vascular endothelial growth factor gene therapy without augmenting tumor growth. Circulation 2005, 111, 2662–2670. [Google Scholar] [CrossRef]
- Yoshikawa, A.; Saura, R.; Matsubara, T.; Mizuno, K. A mechanism of cisplatin action: Antineoplastic effect through inhibition of neovascularization. Kobe J. Med. Sci. 1997, 43, 109–120. [Google Scholar] [PubMed]
- Belotti, D.; Vergani, V.; Drudis, T.; Borsotti, P.; Pitelli, M.R.; Viale, G.; Giavazzi, R.; Taraboletti, G. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin. Cancer Res. 1996, 2, 1843–1849. [Google Scholar] [PubMed]
- Sandoo, A.; Kitas, G.D.; Carmichael, A.R. Endothelial dysfunction as a determinant of trastuzumab-mediated cardiotoxicity in patients with breast cancer. Anticancer Res. 2014, 34, 1147–1151. [Google Scholar] [PubMed]
- Wolf, M.B.; Baynes, J.W. The anti-cancer drug, doxorubicin, causes oxidant stress-induced endothelial dysfunction. Biochim. Biophys. Acta 2006, 1760, 267–271. [Google Scholar] [CrossRef]
- Giordano, P.; Muggeo, P.; Delvecchio, M.; Carbonara, S.; Romano, A.; Altomare, M.; Ricci, G.; Valente, F.; Zito, A.; Scicchitano, P.; et al. Endothelial dysfunction and cardiovascular risk factors in childhood acute lymphoblastic leukemia survivors. Int. J. Cardiol. 2017, 228, 621–627. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Iacoviello, M.; Puzzovivo, A.; Scicchitano, P.; Monitillo, F.; De Crescenzo, F.; Caragnano, V.; Sassara, M.; Quistelli, G.; Guida, P.; et al. Clinical correlates of endothelial function in chronic heart failure. Clin. Res. Cardiol. 2011, 100, 515–521. [Google Scholar] [CrossRef]
- Scicchitano, P.; Cortese, F.; Gesualdo, M.; De Palo, M.; Massari, F.; Giordano, P.; Ciccone, M.M. The role of endothelial dysfunction and oxidative stress in cerebrovascular diseases. Free Radic. Res. 2019, 53, 579–595. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Zhang, R. Associations between endothelial nitric oxide synthase gene polymorphisms and the risk of coronary artery disease: A systematic review and meta-analysis of 132 case-control studies. Eur. J. Prev. Cardiol. 2019, 26, 160–170. [Google Scholar] [CrossRef]
- Mensah, S.A.; Nersesyan, A.A.; Ebong, E.E. Endothelial Glycocalyx-Mediated Intercellular Interactions: Mechanisms and Implications for Atherosclerosis and Cancer Metastasis. Cardiovasc. Eng. Technol. 2021, 12, 72–90. [Google Scholar] [CrossRef]
| Characteristics | All Patients | Survivors | Non-Survivors | p-Values |
|---|---|---|---|---|
| Total number of patients (n, %) | 47 (100) | 27 (100) | 20 (100) | |
| Mean age (years) | 58.6 ± 13.1 | 55.3 ± 11.3 | 62.9 ± 14.4 | 0.048 |
| Hypertension (n, %) | 21 (44.7) | 13 (48.1) | 8 (40) | 0.59 |
| Diabetes (n, %) | 2 (4.3) | 1 (3.7) | 1 (5) | 0.83 |
| Dyslipidemia (n, %) | 6 (12.8) | 5 (18.5) | 1 (5) | 0.18 |
| Ischemic heart disease (n, %) | 2 (4.3) | 1 (3.7) | 1 (5) | 0.83 |
| Peripheral artery disease (n, %) | 2 (4.3) | 0 (0) | 2 (10) | 0.10 |
| Previous stroke or TIA (n, %) | 2 (4.3) | 0 (0) | 2 (10) | 0.10 |
| Family history CVD (n, %) | 19 (40.4) | 13 (48.1) | 6 (30) | 0.22 |
| Smoking (n, %) | 3 (6.4) | 1 (3.7) | 2 (10) | 0.39 |
| Chemotherapy (n, %) | 32 (68.1) | 15 (55.5) | 17 (85) | 0.03 |
| Type of chemotherapy | ||||
| Doxorubicin alone (n, %) | 1 (2.1) | 0 (0) | 1 (5) | 0.25 |
| Paclitaxel alone (n, %) | 1 (2.1) | 1 (3.7) | 0 (0) | 0.39 |
| Carboplatin + paclitaxel (n, %) | 21 (44.7) | 13 (48.1) | 8 (40) | 0.59 |
| Carboplatin + paclitaxel + bevacizumab (n, %) | 9 (19.1) | 7 (25.9) | 2 (10) | 0.17 |
| Surgical intervention (n, %) | 18 (38.3) | 14 (51.8) | 4 (20) | 0.03 |
| Type of cancer | ||||
| Ovarian cancer diagnosis (n, %) | 25 (53.2) | 14 (51.8) | 11 (55) | |
| Endometrial cancer diagnosis (n, %) | 13 (27.7) | 8 (29.6) | 5 (25) | |
| Uterine cervix cancer diagnosis (n, %) | 7 (14.9) | 5(18.5) | 2 (10) | |
| Vulvar cancer diagnosis (n, %) | 2 (4.3) | 0 (0) | 2 (10) | |
| Follow-up duration (days) | 1364.3 ± 676.1 | 1870.5 ± 120.1 | 680.9 ± 480.1 | <0.0001 |
| Laboratory evaluation | ||||
| RBC (×106/mm3) | 4.21 ± 0.37 | 4.25 ± 0.32 | 4.15 ± 0.43 | 0.39 |
| WBC (×103/mm3) | 6.39 ± 2.24 | 6.58 ± 2.31 | 6.14 ± 2.18 | 0.51 |
| Hb (g/dL) | 12.0 ± 1.2 | 11.9 ± 1.2 | 12.1 ± 1.2 | 0.61 |
| HCT (%) | 36.2 ± 3.4 | 35.7 ± 2.9 | 37.0 ± 3.8 | 0.20 |
| MCH (µg/mL) | 28.6 ± 2.7 | 28.0 ± 2.6 | 29.5 ± 2.6 | 0.06 |
| MCHC (µg/mL) | 33.2 ± 1.5 | 33.4 ± 1.4 | 32.9 ± 1.6 | 0.32 |
| MCV (fL) | 86.4 ± 6.8 | 84.0 ± 5.9 | 89.5 ± 6.9 | 0.006 |
| PLT (×103/mm3) | 268.1 ± 92.2 | 253.6 ± 79.1 | 287.5 ± 106.4 | 0.22 |
| Total cholesterol (mg/dL) | 179.2 ± 28.4 | 179.1 ± 31.4 | 179.3 ± 24.6 | 0.98 |
| TG (mg/dL) | 109.4 ± 28.4 | 109.6 ± 31.1 | 109.0 ± 26.4 | 0.94 |
| HDL-C (mg/dL) | 48.9 ± 8.8 | 49.0 ± 7.7 | 48.7 ± 10.4 | 0.93 |
| LDL-C (mg/dL) | 108.5 ± 29.1 | 108.2 ± 30.7 | 108.8 ± 27.6 | 0.95 |
| Fasting glycemia (mg/dL) | 96.9 ± 27.1 | 97.5 ± 32.3 | 96.2 ± 18.7 | 0.88 |
| Creatinine (mg/dL) | 0.71 ± 0.21 | 0.71 ± 0.18 | 0.71 ± 0.24 | 0.97 |
| Characteristics | All Patients (n = 47) | Survivors (n = 27) | Non-Survivors (n = 20) | p-Values |
|---|---|---|---|---|
| FMD (%) | 8.2 ± 3.6 | 9.71 ± 3.53 | 6.13 ± 2.62 | <0.001 |
| Mean C-IMT (mm) | 0.69 ± 0.15 | 0.68 ± 0.13 | 0.71 ± 0.17 | 0.48 |
| APAO (cm) | 1.58 ± 0.27 | 1.61 ± 0.29 | 1.54 ± 0.23 | 0.45 |
| LV EDD (mm) | 43.0 ± 8.9 | 40.76 ± 3.71 | 46.07 ± 12.56 | 0.043 |
| LVESD (mm) | 28.2 ± 5.0 | 26.30 ± 4.03 | 30.79 ± 5.02 | 0.0014 |
| IVS (mm) | 11.1 ± 1.8 | 10.9 ± 1.7 | 11.3 ± 1.8 | 0.42 |
| LA APD (mm) | 36.7 ± 5.2 | 36.0 ± 5.8 | 37.7 ± 4.3 | 0.30 |
| AoR (mm) | 29.5 ± 3.2 | 28.6 ± 3.0 | 30.7 ± 3.2 | 0.03 |
| LVEF (%) | 59.5 ± 3.9 | 60.8 ± 3.0 | 57.8 ± 4.4 | 0.009 |
| Left E (cm/s) | 67.96 ± 22.29 | 61.41 ± 13.68 | 76.8 ± 28.36 | 0.02 |
| Left A (cm/s) | 76.04 ± 20.99 | 79.98 ± 18.98 | 70.71 ± 22.84 | 0.14 |
| Left E/A ratio | 1.00 ± 0.62 | 0.79 ± 0.19 | 1.29 ± 0.85 | 0.0048 |
| Left e’ (cm/s) | 7.39 ± 2.32 | 7.24 ± 2.75 | 7.60 ± 1.64 | 0.60 |
| Left a’ (cm/s) | 9.37 ± 3.14 | 9.37 ± 2.41 | 9.38 ± 3.98 | 0.99 |
| Left s’ (cm/s) | 7.11 ± 1.75 | 7.25 ± 1.68 | 6.92 ± 1.87 | 0.53 |
| Left IVRT (ms) | 71.49 ± 19.75 | 71.48 ± 20.13 | 71.50 ± 10.74 | 0.99 |
| Left ET(ms) | 282.77 ± 39.31 | 284.07 ± 34.67 | 281.00 ± 45.73 | 0.79 |
| Left IVCT (ms) | 60.32 ± 17.52 | 57.78 ± 16.25 | 63.75 ± 18.98 | 0.25 |
| Left Tei index | 0.47 ± 0.12 | 0.46 ± 0.12 | 0.49 ± 0.13 | 0.45 |
| Left E/e’ ratio | 10.05 ± 4.73 | 9.55 ± 4.11 | 10.72 ± 5.50 | 0.41 |
| TAPSE (mm) | 22.7 ± 4.1 | 23.1 ± 4.1 | 22.3 ± 4.1 | 0.52 |
| Right E (cm/s) | 50.26 ± 10.73 | 50.10 ± 10.21 | 50.47 ± 11.66 | 0.91 |
| Right A (cm/s) | 51.40 ± 14.33 | 52.60 ± 12.37 | 49.78 ± 16.83 | 0.51 |
| Right E/A ratio | 1.04 ± 0.36 | 0.99 ± 0.27 | 1.12 ± 0.45 | 0.23 |
| Right e’ (cm/s) | 11.56 ± 3.71 | 10.98 ± 2.90 | 12.36 ± 4.55 | 0.21 |
| Right a’ (cm/s) | 15.96 ± 5.00 | 16.25 ± 4.50 | 15.57 ± 5.69 | 0.65 |
| Right s’ (cm/s) | 12.91 ± 3.23 | 12.94 ± 3.13 | 12.88 ± 3.45 | 0.95 |
| Right IVRT (ms) | 63.08 ± 18.75 | 62.59 ± 21.05 | 63.75 ± 15.63 | 0.84 |
| Right ET (ms) | 284.04 ± 46.28 | 281.11 ± 42.55 | 288.00 ± 51.77 | 0.62 |
| Right IVCT (ms) | 57.77 ± 15.94 | 56.30 ± 16.67 | 59.75 ± 15.08 | 0.47 |
| Right Tei index | 0.44 ± 0.12 | 0.43 ± 0.14 | 0.44 ± 0.10 | 0.85 |
| Right E/e’ ratio | 4.65 ± 1.50 | 4.84 ± 1.58 | 4.40 ± 1.38 | 0.32 |
| Parameter | Survivors (n = 27) | Non-Survivors (n = 20) | Cut-off | Sensibility (%) | Specificity (%) | AUC | p |
|---|---|---|---|---|---|---|---|
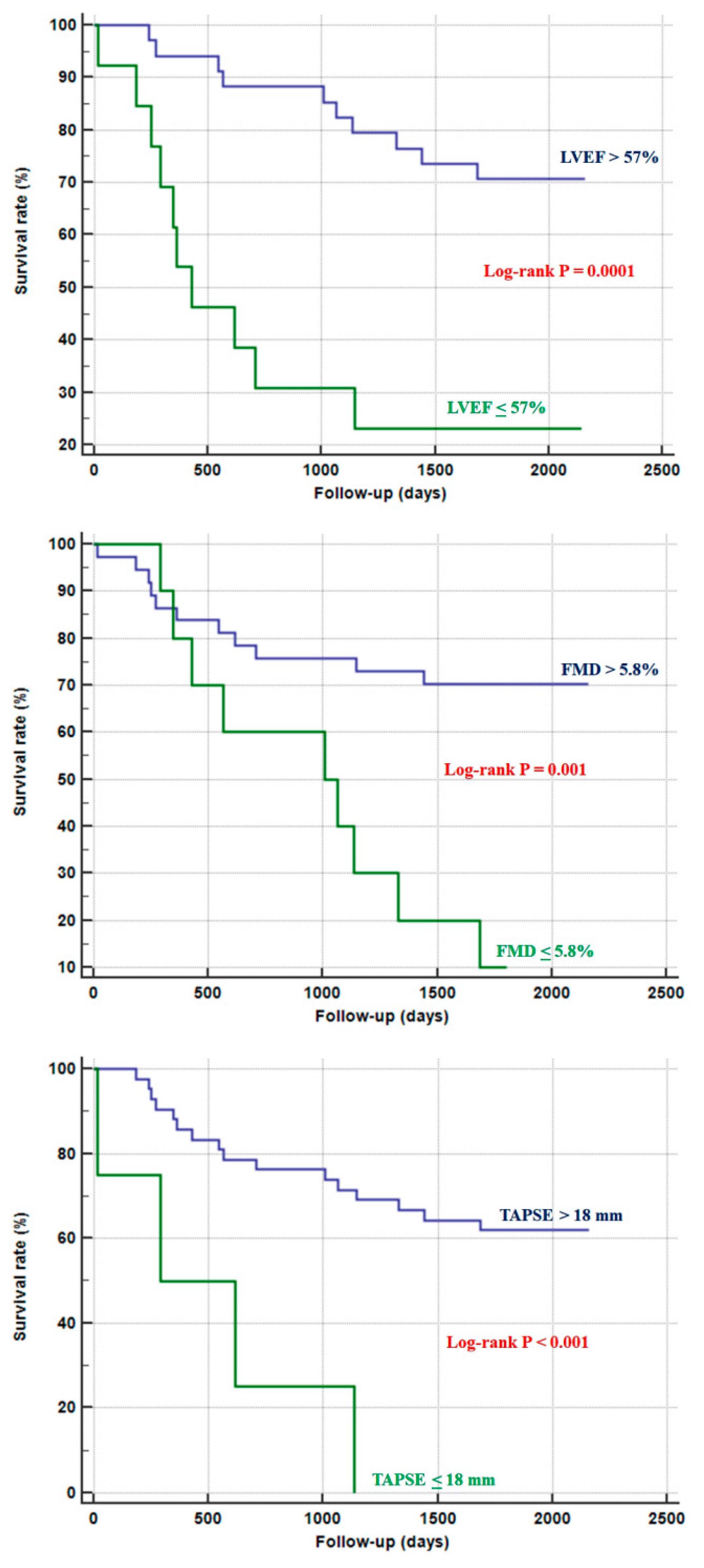
| LVEF (%) | 60.81 ± 3.03 | 57.85 ± 4.45 | ≤57 | 50 | 88.9 | 0.740 | 0.0014 |
| Left E/A ratio | 0.79 ± 0.19 | 1.29 ± 0.85 | >1.16 | 45 | 96.3 | 0.661 | 0.07 |
| Left E/e’ ratio | 9.55 ± 4.11 | 10.72 ± 5.50 | >7.87 | 70 | 51.8 | 0.589 | 029 |
| Left TEI index | 0.46 ± 0.12 | 0.49 ± 0.13 | >0.42 | 65 | 55.6 | 0.562 | 0.47 |
| TAPSE (mm) | 23.08 ± 4.11 | 22.29 ± 4.12 | ≤18 | 20 | 96.3 | 0.534 | 0.69 |
| Right E/A ratio | 0.99 ± 0.27 | 1.12 ± 0.45 | >0.91 | 60 | 66.7 | 0.561 | 0.50 |
| Right E/e’ ratio | 4.84 ± 1.58 | 4.40 ± 1.38 | ≤4.52 | 70 | 59.3 | 0.600 | 0.24 |
| Right TEI index | 0.43 ± 0.14 | 0.44 ± 0.10 | >0.44 | 55 | 63 | 0.547 | 0.58 |
| FMD (%) | 9.71 ± 3.53 | 6.13 ± 2.62 | ≤5.8 | 45 | 96.3 | 0.756 | 0.0003 |
| APAO (mm) | 1.61 ± 0.29 | 1.54 ± 0.23 | ≤1.59 | 70 | 55.6 | 0.570 | 0.40 |
| Mean C-IMT (mm) | 0.68 ± 0.13 | 0.71 ± 0.17 | >0.83 | 25 | 92.6 | 0.533 | 0.70 |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Parameter | β ± SE | p-Value | β ± SE | Wald | p-Value |
| LVEF | −0.228 ± 0.068 | 0.0009 | −0.225 ± 0.089 | 6.379 | 0.0115 |
| FMD | −0.213 ± 0.073 | 0.0037 | −0.249 ± 0.106 | 5.497 | 0.0190 |
| Left E/A ratio | 1.123 ± 0.299 | 0.0002 | 0.714 ± 0.353 | 4.084 | 0.0433 |
| TAPSE | −0.061 ± 0.063 | 0.3302 | |||
| Age | 0.044 ± 0.018 | 0.0163 | |||
| Peripheral artery diseases | 1.703 ± 0.779 | 0.0288 | |||
| Chemotherapy | −1.212 ± 0.627 | 0.0532 | |||
| Surgical intervention | 1.088 ± 0.560 | 0.0522 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scicchitano, P.; Tucci, M.; Ricci, G.; Gesualdo, M.; Carbonara, S.; Totaro, G.; Cecere, A.; Carbonara, R.; Cortese, F.; Loizzi, V.; et al. Vascular and Cardiac Prognostic Determinants in Patients with Gynecological Cancers: A Six-Year Follow-up Study. Appl. Sci. 2021, 11, 6091. https://doi.org/10.3390/app11136091
Scicchitano P, Tucci M, Ricci G, Gesualdo M, Carbonara S, Totaro G, Cecere A, Carbonara R, Cortese F, Loizzi V, et al. Vascular and Cardiac Prognostic Determinants in Patients with Gynecological Cancers: A Six-Year Follow-up Study. Applied Sciences. 2021; 11(13):6091. https://doi.org/10.3390/app11136091
Chicago/Turabian StyleScicchitano, Pietro, Marco Tucci, Gabriella Ricci, Michele Gesualdo, Santa Carbonara, Giuseppe Totaro, Annagrazia Cecere, Rosa Carbonara, Francesca Cortese, Vera Loizzi, and et al. 2021. "Vascular and Cardiac Prognostic Determinants in Patients with Gynecological Cancers: A Six-Year Follow-up Study" Applied Sciences 11, no. 13: 6091. https://doi.org/10.3390/app11136091
APA StyleScicchitano, P., Tucci, M., Ricci, G., Gesualdo, M., Carbonara, S., Totaro, G., Cecere, A., Carbonara, R., Cortese, F., Loizzi, V., Cormio, G., Cicinelli, E., & Ciccone, M. M. (2021). Vascular and Cardiac Prognostic Determinants in Patients with Gynecological Cancers: A Six-Year Follow-up Study. Applied Sciences, 11(13), 6091. https://doi.org/10.3390/app11136091








